Abstract
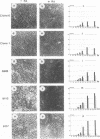
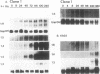
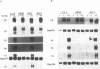
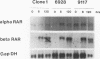
We used a series of cell clones from a human teratocarcinoma cell line, PA-1, to study the effect of transformation by an activated N-ras oncogene on the expression of genes involved in retinoic acid (RA)-induced differentiation and growth regulation. Recently, it has been shown that expression of human HOX 2 genes is sequentially activated by RA beginning from Hox 2.9 at the 3' end of the HOX 2 cluster (A. Simeone, D. Acampora, L. Arcioni, P. W. Andrews, E. Boncinelli, and F. Mavilio, Nature [London] 346:763-766, 1990). We now report that six different genes of the cluster HOX 1 are sequentially induced by RA in a similar temporal pattern, beginning with genes at the 3' end of the cluster. However, in N-ras-transformed cell clones, RA-induced expression of these homeobox genes is delayed. Hox 1.4 and Hox 1.3, genes abundantly induced in nontransformed clones after 3 days of RA treatment, are expressed in N-ras-transformed cells only after 10 days of RA treatment. At this time, the cells' growth is arrested at very high density, and no differentiated morphologic characteristics are observed. Constitutive expression of a transfected Hox 1.4 gene under the control of a simian virus 40 promotor leads to differentiated cell morphology similar to that of the RA-induced phenotype and restores the growth-inhibitory effects of RA in N-ras-transformed cells. These observations provide evidence that enhanced proliferation in N-ras-transformed cells compromises teratocarcinoma cell differentiation by a mechanism that transiently suppresses homeobox gene induction and implies a central role for homeobox genes in RA-induced cell differentiation. We conclude that stimulation of a putative growth factor signal pathway, associated with ras-induced proliferation, transiently suppresses the induction of transcription factors functionally involved in cell growth and differentiation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell. 1989 May 5;57(3):347–349. doi: 10.1016/0092-8674(89)90909-4. [DOI] [PubMed] [Google Scholar]
- Andrews P. W. Human teratocarcinomas. Biochim Biophys Acta. 1988 Aug 3;948(1):17–36. doi: 10.1016/0304-419x(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Nudelman E., Hakomori S., Fenderson B. A. Different patterns of glycolipid antigens are expressed following differentiation of TERA-2 human embryonal carcinoma cells induced by retinoic acid, hexamethylene bisacetamide (HMBA) or bromodeoxyuridine (BUdR). Differentiation. 1990 Apr;43(2):131–138. doi: 10.1111/j.1432-0436.1990.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D. ras proteins: biological effects and biochemical targets (review). Anticancer Res. 1989 Sep-Oct;9(5):1427–1437. [PubMed] [Google Scholar]
- Borasio G. D., John J., Wittinghofer A., Barde Y. A., Sendtner M., Heumann R. ras p21 protein promotes survival and fiber outgrowth of cultured embryonic neurons. Neuron. 1989 Jan;2(1):1087–1096. doi: 10.1016/0896-6273(89)90233-x. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Brockes J. P. Retinoids, homeobox genes, and limb morphogenesis. Neuron. 1989 Apr;2(4):1285–1294. doi: 10.1016/0896-6273(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Snijders A. J., Maassen J. A., van der Eb A. J., Bos J. L. Possible involvement of normal p21 H-ras in the insulin/insulinlike growth factor 1 signal transduction pathway. Mol Cell Biol. 1989 Oct;9(10):4312–4322. doi: 10.1128/mcb.9.10.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao P. J., Kannan P., Yim S. O., Krizman D. B., Wu T. A., Gallick G. E., Tainsky M. A. Susceptibility to ras oncogene transformation is coregulated with signal transduction through growth factor receptors. Oncogene. 1991 May;6(5):713–720. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Voss S. D., Chowdhury K., Gruss P. Structural analysis of murine genes containing homoeo box sequences and their expression in embryonal carcinoma cells. 1985 Apr 25-May 1Nature. 314(6013):713–718. doi: 10.1038/314713a0. [DOI] [PubMed] [Google Scholar]
- Deschamps J., de Laaf R., Joosen L., Meijlink F., Destrée O. Abundant expression of homeobox genes in mouse embryonal carcinoma cells correlates with chemically induced differentiation. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1304–1308. doi: 10.1073/pnas.84.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature. 1985 Dec 19;318(6047):630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dony C., Gruss P. Expression of a murine homeobox gene precedes the induction of c-fos during mesodermal differentiation of P19 teratocarcinoma cells. Differentiation. 1988;37(2):115–122. doi: 10.1111/j.1432-0436.1988.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Galliot B., Dollé P., Vigneron M., Featherstone M. S., Baron A., Duboule D. The mouse Hox-1.4 gene: primary structure, evidence for promoter activity and expression during development. Development. 1989 Oct;107(2):343–359. doi: 10.1242/dev.107.2.343. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Jetten A. M. Multistep process of squamous differentiation in tracheobronchial epithelial cells in vitro: analogy with epidermal differentiation. Environ Health Perspect. 1989 Mar;80:149–160. doi: 10.1289/ehp.8980149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Boncinelli E., Andrews P. W. Activation of four homeobox gene clusters in human embryonal carcinoma cells induced to differentiate by retinoic acid. Differentiation. 1988;37(1):73–79. doi: 10.1111/j.1432-0436.1988.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Ohkuma Y., Horikoshi M., Roeder R. G., Desplan C. Binding site-dependent direct activation and repression of in vitro transcription by Drosophila homeodomain proteins. Cell. 1990 May 4;61(3):475–484. doi: 10.1016/0092-8674(90)90529-n. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Spizz G., Tainsky M. A. The oncogenic forms of N-ras or H-ras prevent skeletal myoblast differentiation. Mol Cell Biol. 1987 Jun;7(6):2104–2111. doi: 10.1128/mcb.7.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Sherman M. I. Differentiation of embryonal carcinoma cells: commitment, reversibility, and refractoriness. Curr Top Dev Biol. 1986;20:345–356. doi: 10.1016/s0070-2153(08)60674-2. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Nigro V., Faiella A., D'Esposito M., Stornaiuolo A., Mavilio F., Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991 Mar;33(3):215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Tainsky M. A., Cooper C. S., Giovanella B. C., Vande Woude G. F. An activated rasN gene: detected in late but not early passage human PA1 teratocarcinoma cells. Science. 1984 Aug 10;225(4662):643–645. doi: 10.1126/science.6740333. [DOI] [PubMed] [Google Scholar]
- Tainsky M. A., Krizman D. B., Chiao P. J., Yim S. O., Giovanella B. C. PA-1, a human cell model for multistage carcinogenesis: oncogenes and other factors. Anticancer Res. 1988 Sep-Oct;8(5A):899–913. [PubMed] [Google Scholar]
- Tainsky M. A., Shamanski F., Blair D., Giovanella B. C. Causal role for an activated N-ras oncogene in the induction of tumorigenicity acquired by a human cell line. Cancer Res. 1987 Jun 15;47(12):3235–3238. [PubMed] [Google Scholar]
- Thaller C., Eichele G. Characterization of retinoid metabolism in the developing chick limb bud. Development. 1988 Jul;103(3):473–483. doi: 10.1242/dev.103.3.473. [DOI] [PubMed] [Google Scholar]
- Tournier-Lasserve E., Odenwald W. F., Garbern J., Trojanowski J., Lazzarini R. A. Remarkable intron and exon sequence conservation in human and mouse homeobox Hox 1.3 genes. Mol Cell Biol. 1989 May;9(5):2273–2278. doi: 10.1128/mcb.9.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya T. B., Rhodes S. J., Taparowsky E. J., Konieczny S. F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989 Aug;9(8):3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T., Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature. 1990 Jun 28;345(6278):819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- Williams T., Admon A., Lüscher B., Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988 Dec;2(12A):1557–1569. doi: 10.1101/gad.2.12a.1557. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Cho K. W., Oliver G., De Robertis E. M. Vertebrate homeodomain proteins: families of region-specific transcription factors. Trends Biochem Sci. 1989 Feb;14(2):52–56. doi: 10.1016/0968-0004(89)90043-1. [DOI] [PubMed] [Google Scholar]
- Zeuthen J., Nørgaard J. O., Avner P., Fellous M., Wartiovaara J., Vaheri A., Rosén A., Giovanella B. C. Characterization of a human ovarian teratocarcinoma-derived cell line. Int J Cancer. 1980 Jan 15;25(1):19–32. doi: 10.1002/ijc.2910250104. [DOI] [PubMed] [Google Scholar]