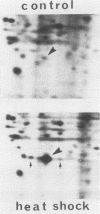
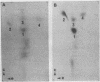
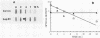Abstract
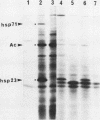
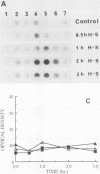
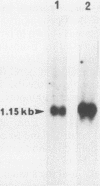
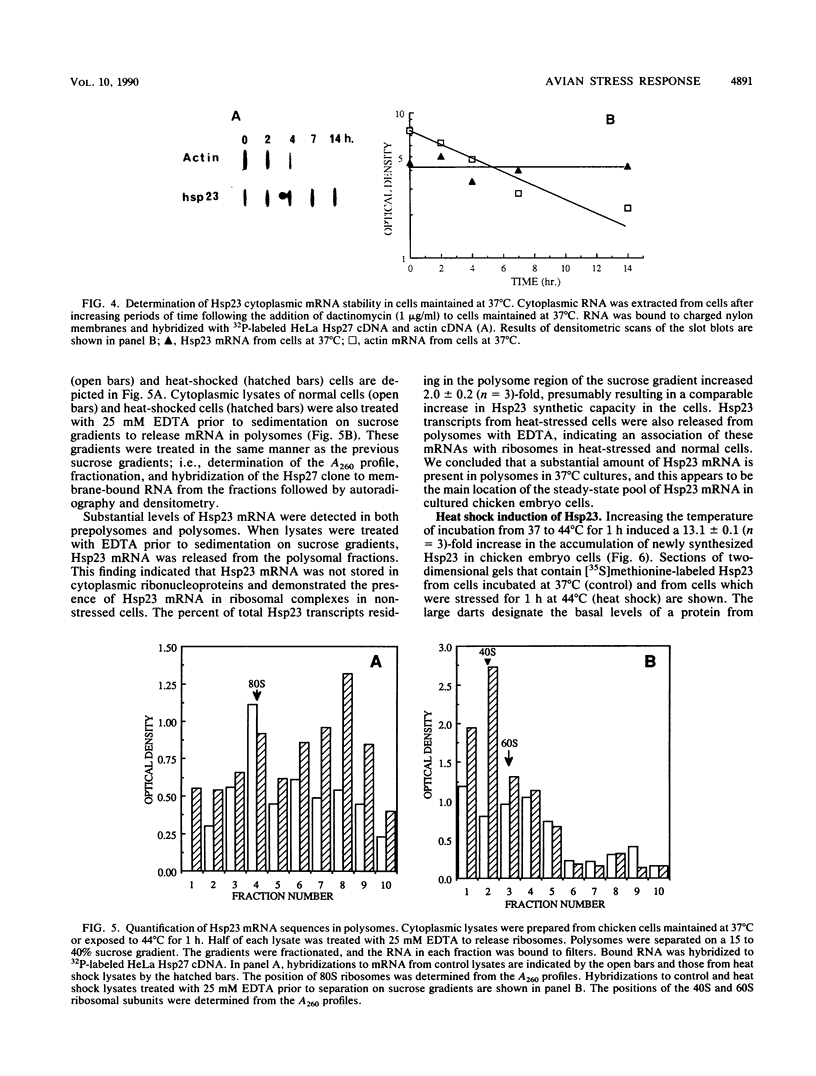
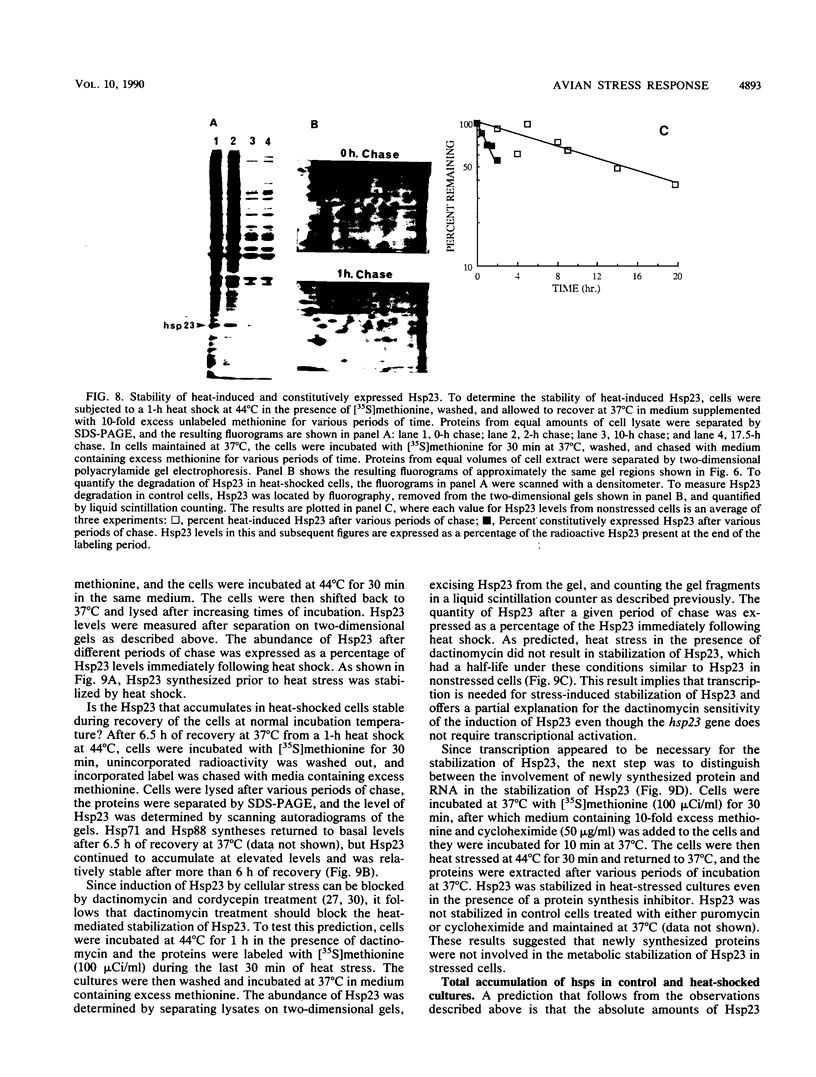
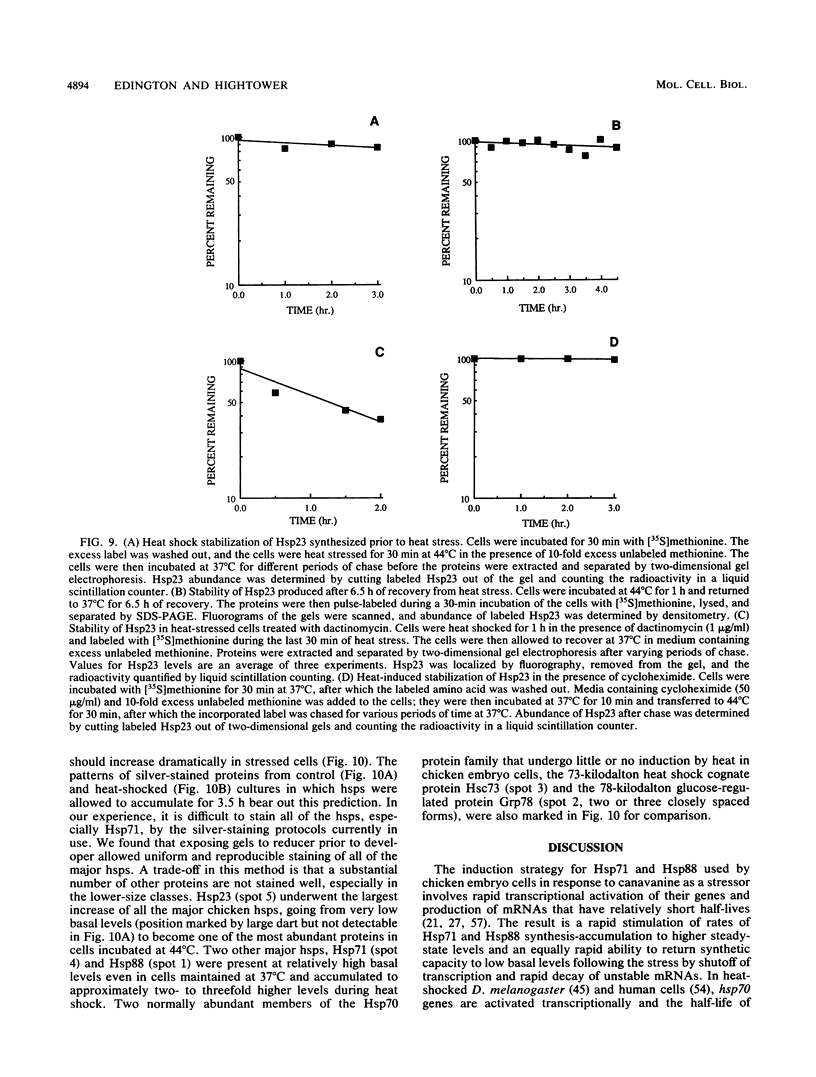
A novel form of regulation of expression of a vertebrate heat shock gene is described. A cDNA clone encoding human Hsp27 was shown to specifically recognize chicken Hsp23 RNA by Northern (RNA) blot analysis and hybrid-select translation. This probe was then used to measure chicken hsp23 gene activity in control and heat-stressed cells. The hsp23 gene(s) was transcriptionally active in non-heat-stressed cells, and its rate of transcription did not increase significantly upon heat shock. Cytoplasmic Hsp23 mRNA, which was metabolically very stable in nonstressed cells, underwent a fourfold increase in amount after a 1-h heat shock, resulting in a twofold increase in Hsp23 mRNA in polysomes. Hsp23 mRNA was relatively abundant and translationally active even in non-heat-shocked cells. Taken together, these data implicated posttranscriptional nuclear events as an important control point for induction of Hsp23 RNA transcripts. The protein half-life of Hsp23 increased from approximately 2 h in control cultures to 13 h in heat-shocked cells, revealing a second major control point. Hsp23 which was synthesized prior to heat shock also increased in stability and contributed to the overall accumulation of Hsp23 in heat-shocked cells. Cycloheximide had no effect on this change in Hsp23 half-life, while dactinomycin blocked the stabilization of Hsp23, suggesting a need for newly synthesized RNA. These data indicated that stabilization of Hsp23 protein and posttranscriptional nuclear events resulting in increased production of Hsp23 mRNA were primarily responsible for a 13-fold increase in the accumulation of newly synthesized Hsp23 after 1 h of heat shock. The regulation of the hsp23 gene is discussed in comparison with several other posttranscriptionally regulated genes, including the proto-oncogene c-fos, the developmentally regulated chicken delta-crystallin gene, and regulation of cellular gene expression by the proto-oncogene c-myc.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. K., Harding M. A., Calvet J. P., Adamson E. D. The heat shock response in HeLa cells is accompanied by elevated expression of the c-fos proto-oncogene. Mol Cell Biol. 1987 Oct;7(10):3452–3458. doi: 10.1128/mcb.7.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Darlix J. L., Khandjian E. W., Simon M., Spahr P. F. Characterization of the prosome from Drosophila and its similarity to the cytoplasmic structures formed by the low molecular weight heat-shock proteins. EMBO J. 1985 Feb;4(2):399–406. doi: 10.1002/j.1460-2075.1985.tb03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G. Synthesis of heat-shock proteins by cells undergoing myogenesis. J Cell Biol. 1981 Jun;89(3):666–673. doi: 10.1083/jcb.89.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag J. Free messenger ribonucleoprotein complexes of chicken primary muscle cells following modification of protein synthesis by heat-shock treatment. Eur J Biochem. 1983 Sep 15;135(2):187–196. doi: 10.1111/j.1432-1033.1983.tb07636.x. [DOI] [PubMed] [Google Scholar]
- Banerji S. S., Laing K., Morimoto R. I. Erythroid lineage-specific expression and inducibility of the major heat shock protein HSP70 during avian embryogenesis. Genes Dev. 1987 Nov;1(9):946–953. doi: 10.1101/gad.1.9.946. [DOI] [PubMed] [Google Scholar]
- Berger E. M., Woodward M. P. Small heat shock proteins in Drosophila may confer thermal tolerance. Exp Cell Res. 1983 Sep;147(2):437–442. doi: 10.1016/0014-4827(83)90225-2. [DOI] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Bond U. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 1988 Nov;7(11):3509–3518. doi: 10.1002/j.1460-2075.1988.tb03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Caizergues-Ferrer M., Amalric F., Zalta J. P., Banville D., Simard R. Synthesis and behaviour of small RNA species of CHO cells submitted to a heat chick. Nucleic Acids Res. 1981 Apr 10;9(7):1615–1625. doi: 10.1093/nar/9.7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Feramisco J. R., Helfman D. M. Cloning of the chick hsp 90 cDNA in expression vector. Nucleic Acids Res. 1985 Sep 11;13(17):6035–6047. doi: 10.1093/nar/13.17.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrétien P., Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol. 1988 Oct;137(1):157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Collier N. C., Heuser J., Levy M. A., Schlesinger M. J. Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J Cell Biol. 1988 Apr;106(4):1131–1139. doi: 10.1083/jcb.106.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcription and translation of Newcastle disease virus mRNA's in vitro. J Virol. 1978 Oct;28(1):324–336. doi: 10.1128/jvi.28.1.324-336.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcriptional map for Newcastle disease virus. J Virol. 1980 Sep;35(3):682–693. doi: 10.1128/jvi.35.3.682-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington B. V., Whelan S. A., Hightower L. E. Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: additional support for the abnormal protein hypothesis of induction. J Cell Physiol. 1989 May;139(2):219–228. doi: 10.1002/jcp.1041390202. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Bendena W. G., Pardue M. L. Unusual behavior of the cytoplasmic transcript of hsr omega: an abundant, stress-inducible RNA that is translated but yields no detectable protein product. J Cell Biol. 1989 Jun;108(6):2045–2057. doi: 10.1083/jcb.108.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Yeast thermotolerance does not require protein synthesis. J Bacteriol. 1983 Dec;156(3):1363–1365. doi: 10.1128/jb.156.3.1363-1365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Sadis S., Smale G., Weber L. A. Molecular cloning of sequences encoding the human heat-shock proteins and their expression during hyperthermia. Gene. 1986;43(1-2):147–154. doi: 10.1016/0378-1119(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Horrell A., Shuttleworth J., Colman A. Transcript levels and translational control of hsp70 synthesis in Xenopus oocytes. Genes Dev. 1987 Jul;1(5):433–444. doi: 10.1101/gad.1.5.433. [DOI] [PubMed] [Google Scholar]
- Jones D., Dixon D. K., Graham R. W., Candido E. P. Differential regulation of closely related members of the hsp16 gene family in Caenorhabditis elegans. DNA. 1989 Sep;8(7):481–490. doi: 10.1089/dna.1.1989.8.481. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. c-myc and c-myb protein degradation: effect of metabolic inhibitors and heat shock. Mol Cell Biol. 1988 Jun;8(6):2504–2512. doi: 10.1128/mcb.8.6.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Morimoto R. I., Hunt C., Huang S. Y., Berg K. L., Banerji S. S. Organization, nucleotide sequence, and transcription of the chicken HSP70 gene. J Biol Chem. 1986 Sep 25;261(27):12692–12699. [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989 Mar;9(3):1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D. Synthesis, modification and structural binding of heat-shock proteins in tomato cell cultures. Eur J Biochem. 1984 Mar 1;139(2):303–313. doi: 10.1111/j.1432-1033.1984.tb08008.x. [DOI] [PubMed] [Google Scholar]
- Petersen R. B., Lindquist S. Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul. 1989 Nov;1(1):135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Cole M. D. Posttranscriptional regulation of cellular gene expression by the c-myc oncogene. Mol Cell Biol. 1989 Jan;9(1):124–134. doi: 10.1128/mcb.9.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. M., Lindquist S. The intracellular location of yeast heat-shock protein 26 varies with metabolism. J Cell Biol. 1989 Feb;108(2):425–439. doi: 10.1083/jcb.108.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadis S., Hickey E., Weber L. A. Effect of heat shock on RNA metabolism in HeLa cells. J Cell Physiol. 1988 Jun;135(3):377–386. doi: 10.1002/jcp.1041350304. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Banerji S. S., Morimoto R. I. HSP70 mRNA translation in chicken reticulocytes is regulated at the level of elongation. J Biol Chem. 1988 Oct 5;263(28):14579–14585. [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R., Bromley P. A. Massive heat-shock polypeptide synthesis in late chicken embryos: convenient system for study of protein synthesis in highly differentiated organisms. Mol Cell Biol. 1982 May;2(5):479–483. doi: 10.1128/mcb.2.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. N., Hightower L. E. Stress mRNA metabolism in canavanine-treated chicken embryo cells. Mol Cell Biol. 1984 Aug;4(8):1534–1541. doi: 10.1128/mcb.4.8.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]