Abstract
We have undertaken total synthesis of the Saccharomyces cerevisiae a-factor (NH2-YIIKGVFWDPAC[S-farnesyl]-COOCH3) and several Cys-12 analogs to determine the significance of S-farnesylation and carboxy-terminal methyl esterification to the biological activity of this lipopeptide mating pheromone. Replacement of either the farnesyl group or the carboxy-terminal methyl ester by a hydrogen atom resulted in marked reduction but not total loss of bioactivity as measured by a variety of assays. Moreover, both the farnesyl and methyl ester groups could be replaced by other substituents to produce biologically active analogs. The bioactivity of a-factor decreased as the number of prenyl units on the cysteine sulfur decreased from three to one, and an a-factor analog having the S-farnesyl group replaced by an S-hexadecanyl group was more active than an S-methyl a-factor analog. Thus, with two types of modifications, a-factor activity increased as the S-alkyl group became bulkier and more hydrophobic. MATa cells having deletions of the a-factor structural genes (mfal1 mfa2 mutants) were capable of mating with either sst2 or wild-type MAT alpha cells in the presence of exogenous a-factor, indicating that it is not absolutely essential for MATa cells to actively produce a-factor in order to mate. Various a-factor analogs were found to partially restore mating to these strains as well, and their relative activities in the mating restoration assay were similar to their activities in the other assays used in this study. Mating was not restored by addition of exogenous a-factor to a cross of a wild-type MAT alpha strain and a MATaste6 mutant, indicating a role of the STE6 gene product in mating in addition to its secretion of a-factor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T. Regulation of alpha-factor production in Saccharomyces cerevisiae: a-factor pheromone-induced expression of the MF alpha 1 and STE13 genes. Mol Cell Biol. 1989 Oct;9(10):4507–4514. doi: 10.1128/mcb.9.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Behling R. W., Jelinski L. W. Importance of the membrane in ligand-receptor interactions. Biochem Pharmacol. 1990 Jul 1;40(1):49–54. doi: 10.1016/0006-2952(90)90177-m. [DOI] [PubMed] [Google Scholar]
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):11–20. doi: 10.1128/mcb.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):21–29. doi: 10.1128/mcb.2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell. 1990 Nov 30;63(5):999–1011. doi: 10.1016/0092-8674(90)90503-7. [DOI] [PubMed] [Google Scholar]
- Ciejek E., Thorner J. Recovery of S. cerevisiae a cells from G1 arrest by alpha factor pheromone requires endopeptidase action. Cell. 1979 Nov;18(3):623–635. doi: 10.1016/0092-8674(79)90117-x. [DOI] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F., Hartwell L. H., Jackson C., Konopka J. B. Conjugation in Saccharomyces cerevisiae. Annu Rev Cell Biol. 1988;4:429–457. doi: 10.1146/annurev.cb.04.110188.002241. [DOI] [PubMed] [Google Scholar]
- Deschenes R. J., Stimmel J. B., Clarke S., Stock J., Broach J. R. RAS2 protein of Saccharomyces cerevisiae is methyl-esterified at its carboxyl terminus. J Biol Chem. 1989 Jul 15;264(20):11865–11873. [PubMed] [Google Scholar]
- Farnsworth C. C., Gelb M. H., Glomset J. A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science. 1990 Jan 19;247(4940):320–322. doi: 10.1126/science.2296721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher G., Perry K., Thorner J. Cell-cell recognition in Saccharomyces cerevisiae: regulation of mating-specific adhesion. J Bacteriol. 1978 Jun;134(3):893–901. doi: 10.1128/jb.134.3.893-901.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold A. A., Schafer W. R., Rine J., Whiteway M., Tamanoi F. Common modifications of trimeric G proteins and ras protein: involvement of polyisoprenylation. Science. 1990 Jul 13;249(4965):165–169. doi: 10.1126/science.1695391. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Sprague G. F., Jr Induction of the yeast alpha-specific STE3 gene by the peptide pheromone a-factor. J Mol Biol. 1984 Oct 5;178(4):835–852. doi: 10.1016/0022-2836(84)90314-0. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone. J Cell Biol. 1980 Jun;85(3):811–822. doi: 10.1083/jcb.85.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
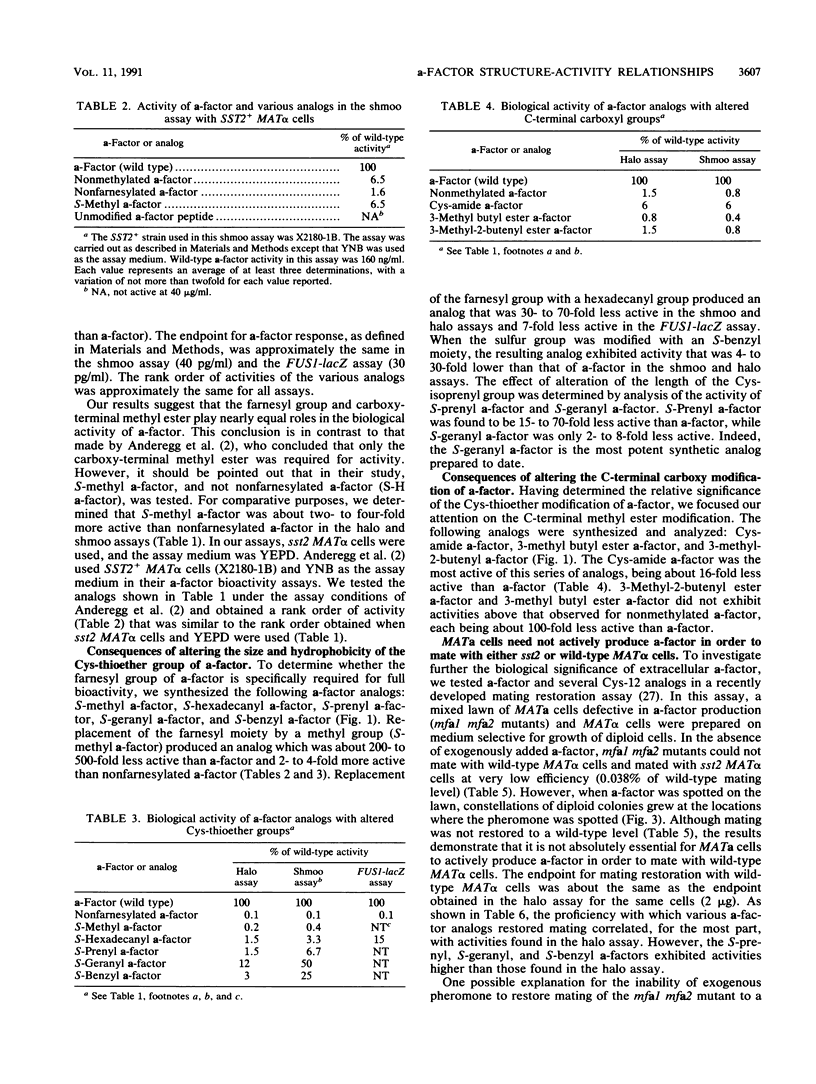
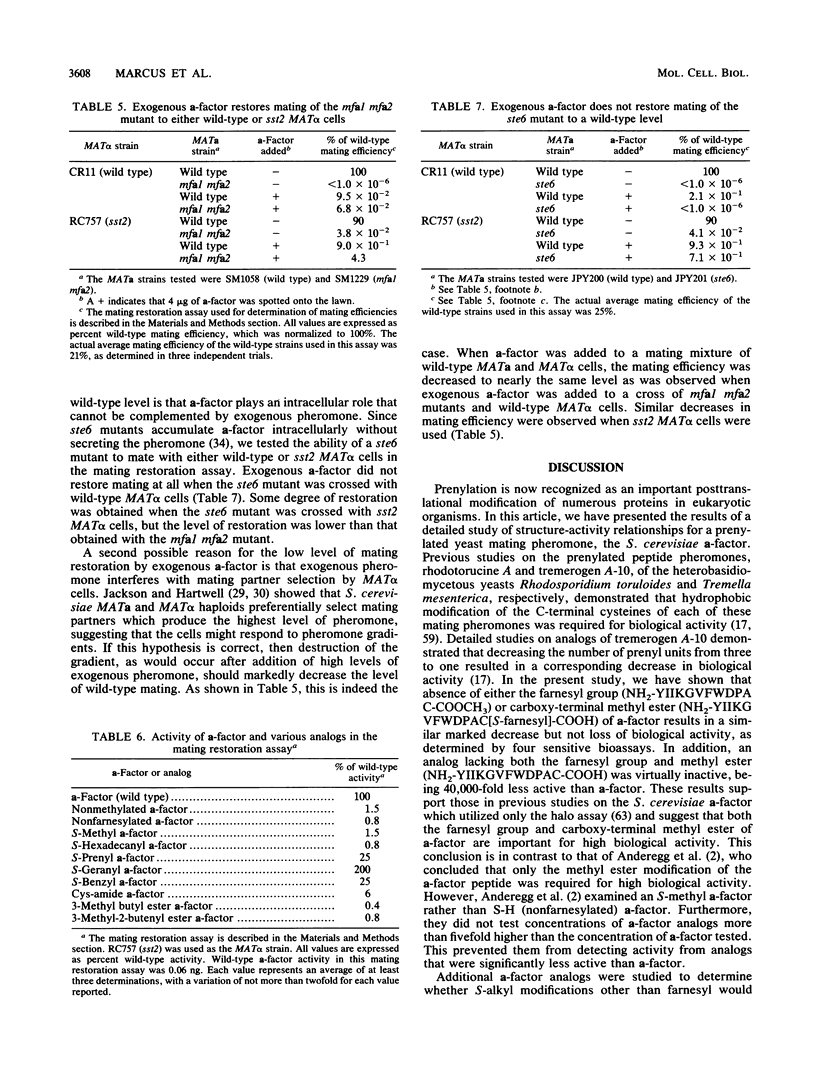
- Hartwell L. H. Synchronization of haploid yeast cell cycles, a prelude to conjugation. Exp Cell Res. 1973 Jan;76(1):111–117. doi: 10.1016/0014-4827(73)90425-4. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989 Dec 14;342(6251):749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Dec;52(4):536–553. doi: 10.1128/mr.52.4.536-553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycyna C. A., Clarke S. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Oct;10(10):5071–5076. doi: 10.1128/mcb.10.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. L., Hartwell L. H. Courtship in S. cerevisiae: both cell types choose mating partners by responding to the strongest pheromone signal. Cell. 1990 Nov 30;63(5):1039–1051. doi: 10.1016/0092-8674(90)90507-b. [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Hartwell L. H. Courtship in Saccharomyces cerevisiae: an early cell-cell interaction during mating. Mol Cell Biol. 1990 May;10(5):2202–2213. doi: 10.1128/mcb.10.5.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y., Sakurai A., Tamura S., Takahashi N. Structure of rhodotorucine A, a novel lipopeptide, inducing mating tube formation in Rhodosporidium toruloides. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1077–1083. doi: 10.1016/0006-291x(78)91505-x. [DOI] [PubMed] [Google Scholar]
- King K., Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Control of yeast mating signal transduction by a mammalian beta 2-adrenergic receptor and Gs alpha subunit. Science. 1990 Oct 5;250(4977):121–123. doi: 10.1126/science.2171146. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Holly J. A., MacKay V. L. A yeast operator overlaps an upstream activation site. Cell. 1987 Jul 31;50(3):369–377. doi: 10.1016/0092-8674(87)90491-0. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J. Alpha-factor structural gene mutations in Saccharomyces cerevisiae: effects on alpha-factor production and mating. Mol Cell Biol. 1985 Apr;5(4):787–796. doi: 10.1128/mcb.5.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke P. N., Taylor A., Ballou C. E. Morphogenic effects of alpha-factor on Saccharomyces cerevisiae a cells. J Bacteriol. 1976 Jul;127(1):610–618. doi: 10.1128/jb.127.1.610-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A., Erdman R. A. Characterization of isoprenoid involved in the post-translational modification of mammalian cell proteins. J Biol Chem. 1989 Oct 25;264(30):18168–18172. [PubMed] [Google Scholar]
- Maltese W. A., Robishaw J. D. Isoprenylation of C-terminal cysteine in a G-protein gamma subunit. J Biol Chem. 1990 Oct 25;265(30):18071–18074. [PubMed] [Google Scholar]
- Marcus S., Caldwell G. A., Xue C. B., Naider F., Becker J. M. Total in vitro maturation of the Saccharomyces cerevisiae a-factor lipopeptide mating pheromone. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1310–1316. doi: 10.1016/0006-291x(90)91592-g. [DOI] [PubMed] [Google Scholar]
- Marcus S., Xue C. B., Naider F., Becker J. M. Degradation of a-factor by a Saccharomyces cerevisiae alpha-mating-type-specific endopeptidase: evidence for a role in recovery of cells from G1 arrest. Mol Cell Biol. 1991 Feb;11(2):1030–1039. doi: 10.1128/mcb.11.2.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr R. S., Blair L. C., Thorner J. Saccharomyces cerevisiae STE14 gene is required for COOH-terminal methylation of a-factor mating pheromone. J Biol Chem. 1990 Nov 25;265(33):20057–20060. [PubMed] [Google Scholar]
- McCaffrey G., Clay F. J., Kelsay K., Sprague G. F., Jr Identification and regulation of a gene required for cell fusion during mating of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 Aug;7(8):2680–2690. doi: 10.1128/mcb.7.8.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Herskowitz I. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol Cell Biol. 1988 Mar;8(3):1309–1318. doi: 10.1128/mcb.8.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Nishihara M., Tsuchiya E., Fukui S. Role of metabolism of the mating pheromone in sexual differentiation of the heterobasidiomycete Rhodosporidium toruloides. J Bacteriol. 1982 Sep;151(3):1184–1194. doi: 10.1128/jb.151.3.1184-1194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naider F., Becker J. M. Structure-activity relationships of the yeast alpha-factor. CRC Crit Rev Biochem. 1986;21(3):225–248. doi: 10.3109/10409238609113612. [DOI] [PubMed] [Google Scholar]
- Powers S., Michaelis S., Broek D., Santa Anna S., Field J., Herskowitz I., Wigler M. RAM, a gene of yeast required for a functional modification of RAS proteins and for production of mating pheromone a-factor. Cell. 1986 Nov 7;47(3):413–422. doi: 10.1016/0092-8674(86)90598-2. [DOI] [PubMed] [Google Scholar]
- Rilling H. C., Breunger E., Epstein W. W., Crain P. F. Prenylated proteins: the structure of the isoprenoid group. Science. 1990 Jan 19;247(4940):318–320. doi: 10.1126/science.2296720. [DOI] [PubMed] [Google Scholar]
- Sargent D. F., Schwyzer R. Membrane lipid phase as catalyst for peptide-receptor interactions. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5774–5778. doi: 10.1073/pnas.83.16.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Schafer W. R., Trueblood C. E., Yang C. C., Mayer M. P., Rosenberg S., Poulter C. D., Kim S. H., Rine J. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science. 1990 Sep 7;249(4973):1133–1139. doi: 10.1126/science.2204115. [DOI] [PubMed] [Google Scholar]
- Steden M., Betz R., Duntze W. Isolation and characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by the mating hormone a-factor. Mol Gen Genet. 1989 Nov;219(3):439–444. doi: 10.1007/BF00259617. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., MacKay V. L. Sexual conjugation in yeast. Cell surface changes in response to the action of mating hormones. J Cell Biol. 1979 Feb;80(2):326–333. doi: 10.1083/jcb.80.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueheart J., Boeke J. D., Fink G. R. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol Cell Biol. 1987 Jul;7(7):2316–2328. doi: 10.1128/mcb.7.7.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya E., Fukui S., Kamiya Y., Sakagami Y., Fujino M. Requirements of chemical structure of hormonal activity of lipopeptidyl factors inducing sexual differentiation in vegetative cells of heterobasidiomycetous yeasts. Biochem Biophys Res Commun. 1978 Nov 14;85(1):459–463. doi: 10.1016/s0006-291x(78)80064-3. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Stetler G. L., Thorner J. The yeast repeated element sigma contains a hormone-inducible promoter. Mol Cell Biol. 1987 Feb;7(2):749–759. doi: 10.1128/mcb.7.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorburger K., Kitten G. T., Nigg E. A. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J. 1989 Dec 20;8(13):4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C. B., Caldwell G. A., Becker J. M., Naider F. Total synthesis of the lipopeptide a-mating factor of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1989 Jul 14;162(1):253–257. doi: 10.1016/0006-291x(89)91989-x. [DOI] [PubMed] [Google Scholar]
- Xue C. B., Ewenson A., Becker J. M., Naider F. Solution phase synthesis of Saccharomyces cerevisiae a-mating factor and its analogs. Int J Pept Protein Res. 1990 Oct;36(4):362–373. doi: 10.1111/j.1399-3011.1990.tb01295.x. [DOI] [PubMed] [Google Scholar]