Abstract
Background
Autologous hematopoietic cell transplantation (HCT) improves survival in patients with multiple myeloma, but disease progression remains a challenge. Allogeneic HCT (alloHCT) has the potential to reduce disease progression through graft-versus-myeloma effects. The aim of the BMT CTN 0102 trial was to compare outcomes of autologous HCT (autoHCT) followed by alloHCT with non-myeloablative conditioning (auto-allo) to tandem autoHCT (auto-auto) in patients with standard risk myeloma. Patients in the auto-auto arm were randomized to one year of thalidomide and dexamethasone (Thal-Dex) maintenance therapy or observation (Obs).
Methods
Patients with multiple myeloma within 10 months from initiation of induction therapy were classified as standard (SRD) or high risk (HRD) disease based on cytogenetics and beta-2-microglobulin levels. Assignment to auto-allo HCT was based on availability of an HLA-matched sibling donor. Primary endpoint was three-year progression-free survival (PFS) according to intent-to-treat analysis.
Results
710 patients were enrolled completed a minimum of 3-year follow up. Among 625 SRD patients, 189 and 436 were assigned to auto-allo and auto-auto, respectively. Seventeen percent (33/189) of SR patients in the auto-allo arm and 16% (70/436) in the auto-auto arm did not receive a second transplant. Thal-Dex was not completed in 77% (168/217) of assigned patients. PFS and overall survival (OS) did not differ between the Thal-Dex (49%, 80%) and Obs (41%, 81%) cohorts and these two arms were pooled for analysis. Three year PFS was 43% and 46% (p=0·671) and three-year OS was 77% and 80 % (p=0·191) with auto-allo and auto-auto, respectively. Corresponding progression/relapse rates were 46% and 50% (p=0·402); treatment-related mortality rates were 11% and 4% (p<0·001), respectively. Auto/allo patients with chronic graft-vs-host disease had a decreased risk of relapse. Most common grade 3 to 5 adverse events in auto-allo was hypebilirubenemia (21/189) and in the auto-auto was peripheral neuropathy (52/436). Among 85 HRD patients (37 auto-allo), three PFS was 40% and 33% (p=0·743) and three-year OS was 59% and 67% (p=0·460) with auto-allo and auto-auto, respectively.
Conclusion
Thal-Dex maintenance was associated with poor compliance and did not improve PFS or OS. At three years there was no improvement in PFS or OS with auto-allo compared to auto-auto transplantation in patients with standard risk myeloma. Decisions to proceed with alloHCT after an autoHCT in patients with standard risk myeloma should take into consideration results of the current trial. Future investigation of alloHCT in myeloma should focus to minimize TRM and maximize graft-versus myeloma effects. This trial was registered in Clinicaltrials.gov (NCT00075829) and was funded by the National Heart, Lung and Blood Institute and National Cancer Institute.
Background
High-dose chemotherapy with autologous hematopoietic cell transplantation (autoHCT) improves survival in multiple myeloma (myeloma) patients younger than 65 years compared to conventional chemotherapy1, 2. However, despite high remission rates and improved survival, there is continued risk of disease progression after a single or tandem autoHCT even in patients with “standard risk” myeloma. Investigational options to improve upon these results include tandem autoHCT, post transplant maintenance strategies and allogeneic HCT (alloHCT)3, 9. Planned sequential autoHCT improves responses and survival outcomes compared to single autoHCT4, 6, 10. Maintenance therapy with thalidomide and corticosteroids after autoHCT further prolongs progression-free (PFS) and overall survival (OS)4, 5.
AlloHCT, which provides a tumor-free graft, is an attractive alternative treatment approach as it offers potential additional disease control through a graft-versus-myeloma effect (GVM). Early studies of alloHCT with myeloablative conditioning regimens demonstrated a higher frequency of molecular remissions and lower rates of relapse compared to autoHCT, but overall benefits were offset by high treatment-related mortality (TRM)11–14. Nonmyeloablative conditioning regimens on the other hand, are designed more for immunosuppression than cytoreduction. Furthermore, when used after an autoHCT for cytoreduction, AlloHCT with nomyeloablative conditioning adds a potential for GVM with lower TRM. . The multi-center phase II clinical trial of fifty four patients using this approach demonstrated complete responses (CR) of 57% and TRM of 15% that was far lower than historically alloHCT with myeloablative regimens7. Hence this approach appeared promising to explore in a phase III trial. Tandem autologous transplant (auto-auto) was chosen as the control arm based on data available at that time suggesting that this approach had the highest PFS of the various transplant strategies and potentially offered an OS benefit as well. Herein, we report the results of Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 which compared outcomes of patients with myeloma receiving autoHCT followed by alloHCT (auto-allo) to auto-auto with or without maintenance therapy.
Methods
Study Design
This is a phase III multicenter trial with biologic treatment arm assignment. The trial was conducted in 37 transplant centers of the BMT CTN19 (Appendix 1). Patients who met eligibility criteria were assigned to the auto-allo treatment arm if an HLA-matched sibling donor was identified. Treatment assignment occurred when donor availability status was confirmed; this could occur at any time from enrollment up to 30 days after the first autoHCT. No patient progressed or died prior to treatment assignment. Those without a suitable sibling donor were assigned to the auto-auto arm and randomized to receive one year of maintenance therapy with thalidomide plus dexamethasone (Thal-Dex) or observation (Obs). Block 1:1 randomization was done at time of treatment assignment to auto-auto. Details of the statistical design are described by Logan et al.20. Patients were classified as having standard risk disease after enrollment if their serum β-2 microglobulin was < 4·0 mg/L and no deletion of chromosome 13 was detected by metaphase karyotyping. Karyotype reports were reviewed centrally. Cytogenetic analysis by fluorescence in situ hybridization was not an eligibility prerequisite and not performed routinely at participating centers. Comparison of auto-allo and auto-auto in standard risk patients was the primary objective of the study. Post-enrollment risk stratification was performed to minimize referral bias as described in Logan et al 20. Combined analysis of patients with high and standard risk disease was not pre-specified in the planned analysis.
There were two primary hypotheses to be tested in patients with standard risk myeloma: 1) does maintenance therapy improve outcomes after auto-auto transplantation; and, 2) does an auto-allo approach prolong progression-free survival (PFS) compared to an auto-auto approach.
Patients
Between December 17, 2003 and March 30, 2007, 710 patients with myeloma were enrolled after completion of initial therapy. Median follow up of the study population is 40 months (inter-quartile range 38–43 months). Entry criteria included: age ≤ 70 years, no disease progression after start of initial therapy, serum bilirubin < 2 x upper limits of normal, liver transaminases < 3 x upper limits of normal, left ventricular ejection fraction > 40%, creatinine clearance > 40ml/min and Karnofsky performance score > 70%. All patients received at least three cycles of any initial systemic anti-myeloma therapy and were within ten months of initiation of this therapy. The type of induction therapy was not specified in the protocol. Patients were required to have ≥ 4 × 106 autologous CD34+ cells/kg available for infusion. Enrollment at each participating center started after review and approval of the protocol and informed consent by the Internal Review Board at each participating center.
Treatment
The study schema is outlined in Figure 1. Enrolled patients received melphalan 200mg/m2 (Mel200) followed by autologous peripheral blood stem cell (PBSC) infusion 48 hours later. The day of first autoHCT was designated as day 0. Filgrastim was started at day +5 and continued until neutrophil recovery.
Figure 1.
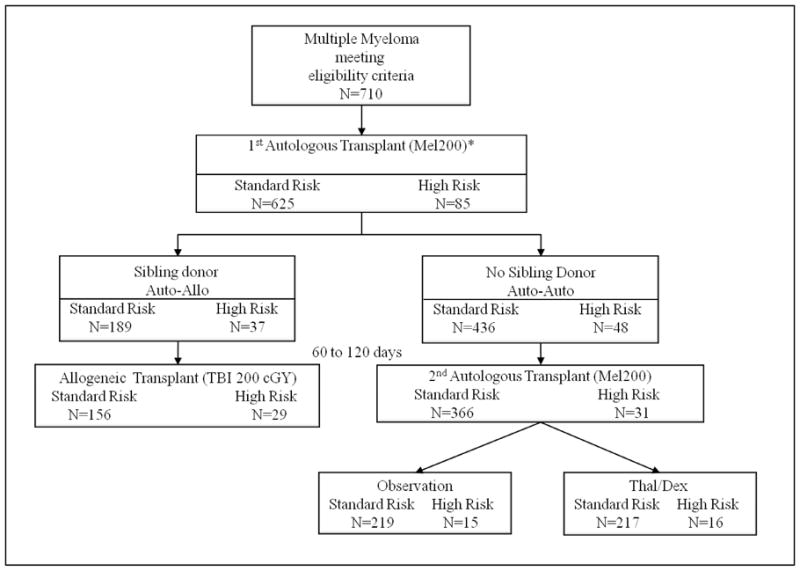
Study schema outlying the number of patients at each step of the trial.
*Primary analysis of the clinical trial focused on patients meeting protocol criteria of standard risk multiple myeloma. Biologic assignment occurred when HLA-typing results were available after enrollment. Randomization to Thal/Dex or Observation occurred once patients were assigned to auto-auto group. Abbreviations: Mel200, melphalan 200mg/m2; TBI; total body irradiation; Thal/Dex, thalidomide and dexamethasone.
Recovery from autografting was defined as hematopoietic recovery, no active infections, resolution of mucositis and gastrointestinal symptoms, and being off hyperalimentation and intravenous hydration. Once recovered, and at least 60 days after the first autoHCT, patients received a second HCT, according to treatment arm. Those assigned to auto-allo received 200 cGy of total body irradiation in a single fraction followed by allogeneic PBSC infusion. The target cell dose for allografts was 2·0 × 106 CD34+ cells/kg. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine and mycophenolate mofetil (MMF). MMF was discontinued on day +28 after alloHCT. In patients without active GVHD, cyclosporine was tapered starting at day +84. Chimerism and engraftment analyses were performed as previously outlined9. Standard infection prophylaxis was administered and CMV reactivation was monitored and treated with ganciclovir per institutional guidelines.
Patients without an HLA-matched sibling donor received a second autoHCT with Mel200 conditioning. Stem cell infusion was the same as with the first autoHCT. For patients randomized to Thal-Dex, thalidomide (200 mg/day) and dexamethasone (40 mg/day for 4 consecutive days, once a month) orally was started at least 60 days after the second HCT, with a goal of one year of therapy.
Endpoints
The primary endpoint of the clinical trial was three-year PFS in patients with standard risk disease. PFS was defined as time to disease relapse or progression, initiation of non-protocol anti-myeloma therapy or death, measured from the time of first auto-HCT, with patients censored at time of last contact. Supporting data for disease status and non-protocol anti-myeloma therapy during the trial were centrally reviewed by an outcome adjudication committee blinded to treatment assignment.
Secondary endpoints were overall survival, incidence of disease relapse or progression, treatment-related mortality, disease response, and incidence of grade 3–5 adverse events (Common Terminology Criteria for Adverse Event v3). Tertiary endpoints included incidence of acute and chronic GVHD, donor-recipient chimerism, and quality of life.
Definition of disease response utilized the International Uniform Response Criteria for myeloma assessment with the addition of a category of near complete response (nCR), defined as evidence of disease by immunofixation electrophoresis without morphologic evidence of bone marrow involvement by myeloma21–23. Disease assessments were performed prior to first autoHCT, at time of second transplantation, and after second transplantation at eight weeks, six months, and every six months up to three years.
Statistical Analysis
Target enrollment was 150 patients with standard risk myeloma assigned to the auto-allo arm. It was estimated that the probability of having an available HLA-matched sibling donor was between 20% and 30%, so that 350 to 600 standard-risk patients would be enrolled on the auto-auto arm if the target auto-allo enrollment were met. This sample size would provide at least 80% power to detect a difference of 15% between auto-allo and auto-auto in the three-year probability of PFS, assuming a baseline probability of 45% PFS in the latter population.
Primary analysis was performed using the intent-to-treat principle, i.e., patients were classified according to their original assigned treatment, even if they did not receive all prescribed interventions. All outcomes were measured from the time of first autoHCT.
Analysis of the primary endpoint was planned to be performed in two steps. First, three-year PFS was compared between Thal-Dex and Obs using the pointwise difference in Kaplan-Meier estimates at three years. If no statistically significant difference was found, the two auto-auto groups were to be pooled and three-year PFS after auto-auto compared with auto-allo. Otherwise the superior arm of Thal-Dex and Obs would be compared with auto-allo. Survival distributions were estimated using the Kaplan-Meier method, while TRM, relapse/progression, acute and chronic GVHD were estimated using cumulative incidence. Groups were compared using pointwise differences in survival at three years or using Gray’s test in cumulative incidence. To address concerns of covariate imbalance in a biologic assignment trial, we conducted a protocol-specified secondary analysis of PFS using a Cox proportional hazards model24. This model was to include some or all of the following prospectively-defined variables, if their distribution differed (p<0·1) between the auto-auto and auto-allo at baseline: β2 microglobulin, Karnofsky Performance Status and age.
Monitoring for accrual and toxicity according to sequential probability ratio stopping guidelines was performed by the NHLBI-appointed Data and Safety Monitoring Board. First, the proportion of patients assigned to the auto-allo arm at each center was monitored as described in Logan et al.20. Second, TRM was monitored, with the null hypotheses that the rate of six-month TRM was less than 5% after autoHCT and less than 20% after an alloHCT, and that the rate of nine-month TRM was less than 20% in subjects aged 65 years or older. Third, specific toxicities were monitored separately in each treatment arm, including deep vein thrombosis, sensory neuropathy, and cyclosporine-associated renal and hepatic toxicity. No boundaries for safety end points were triggered during the trial.
Unplanned post hoc analysis was performed to assess the impact of GVHD on relapse or progression in patients receiving allo-HCT. Development of chronic GVHD was analyzed as a time dependent covariate in a Cox regression model of disease relapse or progression.
Data were analyzed using statistical software SAS (v9.2, SAS Institute, Cary, NC) and R (v2.10.0).
Findings
Standard Risk Disease
Patients
Demographic information for all patients is depicted in Table 1. Imbalances between the auto-auto and auto-allo arms were found in age and race; patients in the auto-allo group were younger and more often Caucasian. Median times from first to second transplantation were 105 days (61–262 days) for auto-allo and 98 days (58–193 days) for auto-auto (p=0·004). A disease status prior to first autoHCT of very good partial response (VGPR) or better was observed in 41% (78/189) and 42% (185/436) of patients assigned to auto-allo and auto-auto, respectively (p=0·678). Compliance with second transplantation was 83% (156/189) and 84% (366/436) for auto-allo and auto-auto, respectively (Figure 1). Reasons for not undergoing second transplantation are shown in Table 2.
Table 1.
Demographic characteristics of patients with multiple myeloma with standard risk disease enrolled in the BMT CTN 0102.
| Standard Risk | High Risk | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Auto-Auto N (%) (n=436) |
Auto-Allo N (%) (n=189) |
P-value1 | Auto-Auto N (%) (n=48) |
Auto-Allo N (%) (n=37) |
P-value1 | |
|
| ||||||
| Male | 260 (63) | 111 (59) | 0·833 | 27 (56) | 21 (57) | 0·963 |
|
| ||||||
| Median Age | 55 (22–70) | 53 (29–68) | 0·006 | 57 (32–70) | 51 (32–66) | 0·012 |
|
| ||||||
| Race | 0·032 | 0·458 | ||||
| African American | 77 (18) | 18 (10) | 8(17) | 3 (8) | ||
| Caucasian | 335 (77) | 161 (85) | 38 (79) | 33 (89) | ||
| Other | 24 (5) | 10 (5) | 2 (4) | 1(3) | ||
|
| ||||||
| Performance Score ≥ 90 | 334 (77) | 148 (78) | 0·642 | 27 (56) | 26 (70) | 0·186 |
|
| ||||||
| β-2 Microglobulin, median | 2.0 | 2.0 | 0·949 | 4.4 | 3.7 | 0·035 |
|
| ||||||
| Durie-Salmon | 0·739 | 0·702 | ||||
| Stage I-II | 142 (33) | 59 (31) | 10 (21) | 9 (24) | ||
| Stage III | 294 (67) | 130 (69) | 38 (79) | 28(76) | ||
|
| ||||||
| Interval from diagnosis to transplantation, median in months (range) | 7 (3–55) | 7 (4–35) | 0·925 | 7 (5–22) | 7 (3–38) | 0·683 |
|
| ||||||
| Disease Status2.3 | 0·678 | 0·028 | ||||
| CR | 41 (10) | 24 (13) | 1 (2) | 3 (8) | ||
| Near CR | 65 (15) | 22 (12) | 1 (2) | 2 (5) | ||
| Very Good PR | 79 (19) | 32 (17) | 1 (2) | 7 (19) | ||
| PR | 158 (37) | 76 (40) | 21 (44) | 14 (38) | ||
| MR | 31 (7) | 17 (9) | 10 (21) | 3 (8) | ||
| SD | 21 (5) | 6 (3) | 6 (13) | 4 (11) | ||
| Not Evaluable4 | 30 (7) | 12(6) | 8 (17) | 4 (11) | ||
|
| ||||||
| Interval from first to second transplantation, median in days (range) | 98 (58–193) | 105 (61–262) | 0·004 | 101 (63–155) | 111 (61–155) | 0·397 |
P-values of treatment arm characteristics shown after biologic assignment.
Disease status at time of first autologous transplantation represents the result from initial multiple myeloma therapy received prior to trial enrollment.
Details of induction regimens were not collected.
Not evaluable are cases without available comprehensive disease assessment at time immediately prior to initial multiple myeloma therapy. Abbreviations: auto-allo, autologous followed by allogeneic hematopoietic cell transplantation; auto-auto, double autologous hematopoietic cell transplantation; CR, complete response; MR, minimal response; PR, partial response; SD, stable disease.
Table 2.
Reasons for not proceeding to second transplantation or maintenance therapy among patients with standard risk disease enrolled in the BMT CTN 0102 trial.
| Auto-Auto | Auto-Allo | |
|---|---|---|
|
| ||
| Total assigned | 436 | 189 |
|
| ||
| Total not receiving 2nd transplantation | 70* | 33 |
|
| ||
| Reasons for no 2nd transplantation | ||
| Adverse Event (grade 3–5) | 4 (6%) | 1 (3%) |
| Death | 4 (6%) | 1 (3%) |
| Progression/Relapse | 10 (14%) | 6 (18%) |
| Insurance Coverage denied | 2 (3%) | 0 |
| Inadequate physical recovery from first transplant | 2 (3%) | 2 (6%) |
| Patient refused/withdrew consent | 43 (61%) | 19 (58%) |
| Other reasons1 | 4 (6%) | 4 (12%) |
| Reason Unknown | 1 (<1%) | 0 |
One patients assigned to auto-auto group did not receive the first transplant due to disease progression
Auto-auto: Nocardia infection, physician decision (n=3). Auto-Allo: Donor failed to mobilized sufficient cell dose according to protocol, cardiac amyloidosis diagnosed after the first transplant, pyschosocial issues, donor deemed not medically eligible to donate.
Maintenance Therapy
Fifty-nine patients (27%) randomized to Thal-Dex refused to start maintenance and 168 patients (77%) did not complete Thal-Dex. The cumulative incidence of premature maintenance discontinuation was 84%. Probabilities of three-year PFS and OS after first autoHCT of patients randomized to Thal-Dex maintenance were 49% (95% confidence interval [CI], 43–57%) and 80% (95% CI, 75–86%), respectively, compared with 43% (95% CI, 37–50%, p=0·080) and 81% (95% CI, 75–86%, p=0·817) in the Obs group. Thal-Dex and Obs groups were combined into a single auto-auto arm for comparison with auto-allo. Among patients who tolerated Thal-Dex (N=116) the median time of maintenance therapy was 256 days (range 3 to 466 days).
Progression-Free and Overall Survival
Probabilities of three-year PFS were 43% (95% CI, 36–51%) and 46% (95%CI, 42–51%, p=0·671) after auto-allo and auto-auto, respectively (Figure 2A). Among the three pre-specified baseline variables, only age differed significantly between the arms and was included in a multivariate Cox regression analysis. The hazard ratio (HR) of treatment failure (death or relapse/progression), adjusted for age, for patients assigned to auto-allo versus auto-auto was 1.17 (95% CI, 0.94–1.46, p=0·17).
Figure 2.

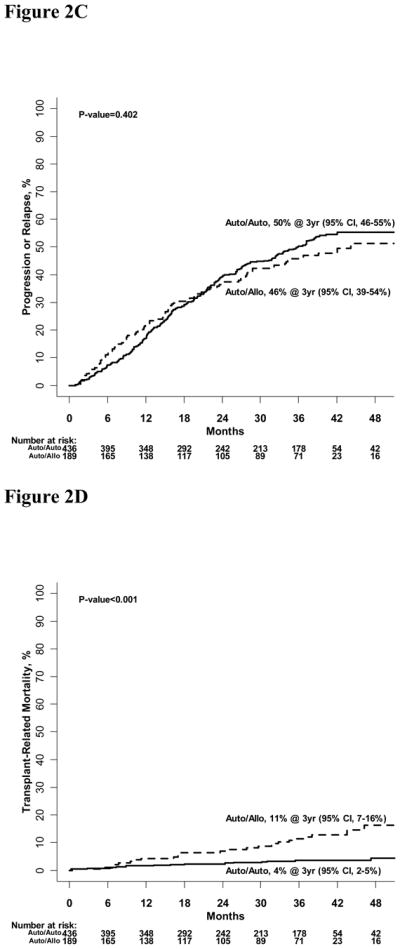
Figure 2A: Progression-free survival of standard risk auto-auto and auto-allo groups
Figure 2B: Overall survival of standard risk auto-auto and auto-allo groups.
Figure 2C: Cumulative incidence of disease progression or relapse for standard risk auto-auto and auto-allo groups.
Figure 2D: Cumulative incidence of transplant-related mortality for standard risk auto-auto and auto-allo groups.
Probabilities of one, two and three-year OS were 91% (95% CI, 87–95 %), 85% (95% CI, 80–91%) and 77% (95% CI, 72–84%) after auto-allo; and 95% (95% CI,93–97%), 93% (95% CI, 86–92%) and 80% (95% CI, 77–84%, p=0·191) after auto-auto, respectively (Figure 2B).
Disease Progression/Relapse and Treatment-Related Mortality
Cumulative incidences of disease relapse or progression at three years were 46% (95% CI, 39–54%) and 50% (95% CI, 46–55%, p=0·402) after auto-allo and auto-auto, respectively (Figure 2C). Corresponding incidences of three-year TRM were 11% (95% CI, 7–16%) and 4% (95% CI, 2–5%, p<0·001) (Figure 2D).
Disease Response
Overall CR rates were 50% and 40% for the auto-allo and auto-auto groups, respectively (p=0·03) (Table 3). Rates of VGPR or better disease responses were 62% with auto-allo and 65% with auto-auto (p=0·482). Among patients not in CR prior to second transplantation (n=415), 48% and 35% of auto-allo and auto-auto patients upgraded their disease status to CR, respectively (p=0·009).
Table 3.
Best disease responses in patient with standard risk disease by treatment arm.
| Time | Population | Complete Responses | Very Good Partial Responses and Better* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Auto/Auto N (%) | N | Auto/Allo N (%) | P-values | N | Auto/Auto N (%) | N | Auto/Allo N (%) | P-values | ||
| 1st HCT | All Patients (N=625)1 | 436 | 175 (40%) | 189 | 94 (50%) | 0·026 | 436 | 285 (65%) | 189 | 118 (62%) | 0·482 |
| Patients not in CR (N=560)2 | 395 | 142 (36%) | 165 | 74 (45%) | 0·049 | 395 | 252 (64%) | 165 | 98 (59%) | 0·327 | |
| 2nd HCT | All Patients (N=522)3 | 366 | 164 (45%) | 156 | 90 (58%) | 0·007 | 366 | 272 (74%) | 156 | 113 (72%) | 0·655 |
| Patients not in CR (N=415)4 | 295 | 102 (35%) | 120 | 58 (48%) | 0·009 | 295 | 210 (71%) | 120 | 81 (68%) | 0·457 | |
Include all patients with standard risk disease enrolled in the trial.
Subset of patients with less than complete response (CR) at study entry.
Subset of patients who received a second transplant.
Subset of patients who received a second transplant and who were not in complete remission immediately prior to the second transplant.
Very good partial response (VGPR) includes VGPR near complete response and complete responses.
Overall Toxicities
Grade 3 to 5 toxicities were reported in 46% (87/189, grade 3, 41%; grade 4, 15%; grade 5, 6%) and 42% (185/436, grade 3, 36%; grade 4, 9%; grade 5, 5%) of auto-allo and auto-auto patients, respectively. Specific grade 3–5 toxicities at 1 and 3 years after the second transplant are shown in table 4. Grade 3 to 5 toxicities among patients in the auto-auto arm were similar except for grade 3 and 4 neuropathy of 18% (39/217) and 11% (24/219) reported in patients assigned to Thal-Dex and Obs, respectively.
Table 4.
Specific Grade 3–5 toxicities by treatment arm after second transplant1.
| 1 year | 3 years | |||
|---|---|---|---|---|
| Auto-Auto (%) | Auto-Allo (%) | Auto-Auto (%) | Auto-Allo (%) | |
| Abnormal Liver Enzymes | 11 (2·5) | 9 (4·8) | 25 (5·7) | 17 (9·0) |
| Ataxia | 1 (0·2) | 3 (1·6) | 1 (0·2) | 3 (1·6) |
| Capillary Leak Syndrome2 | 0 (0) | 4 (2·1) | 0 (0) | 4 (2·1) |
| Cardiac Arrhythmias | 7 (1·6) | 2 (1·1) | 7 (1·6) | 2 (1·1) |
| Deep Vein Thrombosis | 14 (3·2) | 7 (3·7) | 14 (3·2) | 7 (3·7) |
| Dyspnea | 25 (5·7) | 17 (9·0) | 56 (12·8) | 35 (18·5) |
| Hemorrhage | 1 (0·2) | 1 (0·5) | 1 (0·2) | 1 (0·5) |
| Hemorrhagic Cystitis | 2 (0·5) | 3 (1·6) | 7 (1·6) | 4 (2·1) |
| Hyperbilirubenemia2 | 9 (2·1) | 20 (10·6) | 14 (3·2) | 21 (11·1) |
| Hyperglycemia2 | 20 (4·6) | 20 (10·6) | 20 (4·6) | 20 (10·6) |
| Hypertension2 | 10 (2·3) | 17 (9·0) | 10 (2·3) | 17 (9·0) |
| Hypotension | 12 (2·8) | 8 (4·2) | 12 (2·8) | 8 (4·2) |
| Hypoxia | 19 (4·4) | 12 (6·4) | 41 (9·4) | 23 (12·2) |
| Left Ventricular Dysfunction | 2 (0·5) | 3 (1·6) | 2 (0·5) | 3 (1·6) |
| Mucositis2 | 32 (7·3) | 4 (2·1) | 32 (7·3) | 4 (2·1) |
| Peripheral Motor Neuropathy | 10 (2·3) | 6 (3·2) | 23 (5·3) | 17 (9·0) |
| Peripheral Sensory Neuropathy2 | 33 (7·6) | 4 (2·1) | 52 (11·9) | 11 (5.8) |
| Seizures | 1 (0·2) | 0 (0) | 1 (0·2) | 0 (0) |
| Somnolence | 6 (1·4) | 7 (3·7) | 6 (1·4) | 7 (3·7) |
| Thrombotic Microangiopathy | 1 (0·2) | 2 (1·1) | 1 (0·2) | 2 (1·1) |
Toxicities observed from day 0 of second transplant to 1 year and 3 years.
Indicates significant difference between the two treatment arms (p<0·05).
Auto-Allo Chimerism Analyses
Ten patients (6·4%, 10/156) had graft failure after alloHCT. Two patients failed to engraft and eight had secondary graft failure. Six secondary failures occurred prior to day 100 and two by day 365. The median percentages of overall donor chimerism were 91%, 97%, 98%, 100%, and 100% at days 28, 56, 84, 180, and 365 after allo-HCT, respectively.
Auto-Allo Graft-versus-host Disease
Cumulative incidences of grades II-IV and III-IV acute GVHD at 100 days were 26% (95% CI, 19–33%) and 9% (95% CI, 4–14%), respectively. Cumulative incidences of chronic GVHD at one and two years were 47% (95% CI, 39–56%) and 54% (95% CI, 46–63%), respectively. The relative risk of disease relapse or progression was 0·44 (95% CI, 0·24–0·81, p=0·008) for patients with versus those without chronic GVHD. Among auto-allo recipients alive at six months post allo-HCT, those who developed chronic GVHD (n=29) in the first six months had a cumulative incidence of disease relapse or progression of 17% at three years post allo-HCT compared to 39% for patients who did not develop chronic GVHD by six months (n=99; p=0·012).
Causes of Death
Sixty (32%,60/189) auto-allo patients and 108 (25%,108/436) auto-auto patients died. The leading cause of death was myeloma, accounting for 37% (22/60) of auto-allo deaths and 72% (78/108) of auto-auto deaths. Other causes in the auto-allo group were organ failure (20%), infection (17%), GVHD (13%), idiopathic pneumonia syndrome (5%), acute respiratory distress syndrome (3%), graft failure (2%), and other causes (3%). In the auto-auto group, other causes were organ failure (14%), infection (2%), other causes (8%), and unknown causes (4%).
High Risk Disease
Table 1 depicts demographics of patients with HR disease. Patients in the auto-allo group were younger. PR was the most frequent disease status at study entry in both treatment arms. Probabilities of three-year PFS were 40% (95% CI, 27–60%) and 33% (95%CI, 22–50%, p=0·743); and corresponding probabilities of OS were 59% (95% CI, 45–78%) and 67% (95% CI, 54–82%, p=0·460) after auto-allo and auto-auto, respectively (Figure 3A,3B). Cumulative incidences of disease relapse or progression at three years were 38% (95% CI, 22–54%) and 57% (95% CI, 42–71%, p=0·079) after auto-allo and auto-auto, respectively. Corresponding incidences of three-year TRM were 22% (95% CI, 8–35%) and 11% (95% CI, 2–19%, p=0.311).
Figure 3.
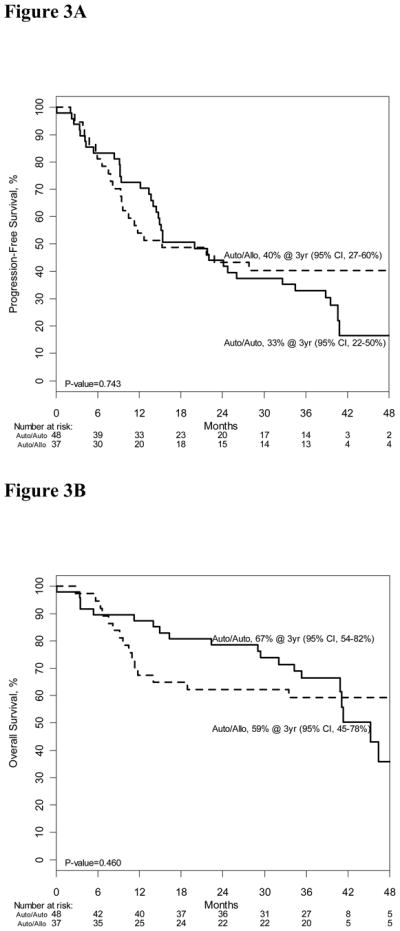
Figure 3A: Progression-free survival of high risk auto-auto and auto-allo groups
Figure 3B: Overall survival of high risk auto-auto and auto-allo groups.
Interpretation
In this trial of autoHCT followed by alloHCT using a non-myeloablative conditioning regimen in patients with SRD, we demonstrate no benefit of this approach in three-year PFS or OS compared to auto-auto. There was indirect evidence of a graft-vs.-myeloma effect with a 60% reduction in the risk of relapse in patients diagnosed with chronic GVHD. TRM was significantly higher in the auto-allo group (11% vs. 4%), despite use of a non-myeloablative conditioning regimen. This was largely due to GVHD and infections. There was no statistically significant difference in the risks of progression or relapse between the two groups. Although VGPR and better responses were similar in both arms, a larger proportion of patients in the auto-allo group upgraded their response to a CR. Differences in toxicities and causes of death are inherant to the transplant approach. AlloHCT recipients experienced more complications related to organ dysfunction and immune deregulation as a result of the use of chronic immunosuppression and the development or treatment of GVHD. AutoHCT recipients experienced more neuropathy related to thalidomide use. Additional analysis comparing quality of life assessments between treatment arms is planned for a subsequent publication of this study.
We observed no benefit to maintenance therapy in this trial, but since compliance with Thal-Dex was poor, definitive conclusions about the role of Thal-Dex maintenance cannot be drawn. Earlier trials demonstrated benefit of thalidomide maintenance on survival outcomes4, 25, 26, but compliance with maintenance was also a challenge in those trials. In fact, the IFM 99-02 trial in which most patients were not exposed to thalidomide at induction, 39% of patients discontinued thalidomide due to toxicity. The median time of thalidomide maintenance in the IFM99-02 was 15 months, similar to BMT CTN 0102 4. Hence, presumable prior thalidomide related neuropathy could in part be a reason for a higher rate of noncompliance in the BMT CTN 0102.
This was a biologic assignment rather than a randomized trial and several measures were used to minimize selection bias20 including: separation of the cohort by disease risk; planned multivariate analysis adjusting for pre-specified demographic characteristics; and, active monitoring of auto-allo enrollment at each center. Despite limitations of biologic assignment trials, both groups were comparable, and adjusted and unadjusted comparisons of the primary endpoint yielded similar results. It is noteworthy that separation according to disease risk after enrollment indeed resulted in a more balanced ratio of donor vs. no donor cohorts in the SRD compared to HRD.
Three similar biologic assignment trials have compared auto-allo with auto-auto HCT approaches in myeloma15, 16. Bruno et al. demonstrated superior event-free survival (EFS) and OS in 58 and 46 recipients of auto-allo and auto-auto, respectively15, 17. Biologic assignment in this trial was performed in patients with siblings. However, of note, there was a fairly high drop out rate with ultimately only 46 of 82 patients in the auto-auto arm completing two transplants. Superiority of the auto-allo arm in this trial could be attributed to small number of patients at risk beyond 2 years. In addition, the second transplant conditioning regimen consisted of varying doses of melphalan down to as low as 100mg/m2. This may in part be the reason for the low CR rate of 26% in the auto-auto arm. In the Spanish Group trial (PETHEMA), biologic assignment occurred after the first transplant in patients not in CR or nCR (n=110) and 25 patients received an alloHCT16. Median EFS and OS were similar in the allo-auto and auto-auto arms. Bjorkstrand et al18 showed longer PFS and OS with auto-allo approach using a combination of fludarabine and TBI 200cGy in patients followed beyond three years. Outcomes in autoHCT arm appear inferior to the BMT CTN 0102 trial at 48 months, possibly due to a combination of single and tandem autoHCT as the control arm, and inclusion of patients with high risk disease as defined in the BMT CTN 0102 trial. Additionally, in the “as treated” analysis among 91 recipients of auto-allo, overall survival was similar to the autoHCT arm.
Differences in EFS observed in the Italian Group trial occurred after 30 months. CR rates were higher in the auto-allo arm in both the Italian Group15 and BMT CTN trial; and thus longer follow-up of patients treated on BMT CTN 0102 is important in order to assess whether the observed differences in CR rates will translate into better long-term survival, as shown in other myeloma trials1, 3, 18.
The French Group (IFM) ran two parallel phase II trials in patients with high-risk myeloma, defined by deletion of chromosome 13 or β-2 microglobulin > 3 mg/L. IFM99-03 analyzed patients with available HLA-match sibling donors who received an alloHCT with reduced intensity conditioning (n=65) after an autoHCT. Outcomes of these patients were compared to patients enrolled in IFM99-04 (n=219), an auto-auto trial. Similar to BMT CTN 0102, EFS and OS were not significantly different for patients receiving auto-allo and auto-auto in the IFM trials27. The transplant related mortality of 10.9% was also similar to BMT CTN0102, however, the relapse or progression rate was strikingly high at 56.5% in the auto allo group. Despite the definition of HRD in the BMT CTN 0102 not including most recent cytogenetic markers of poor prognosis, patients with HRD experienced worse outcomes than patients with SRD. Although limited by small number of patients, the comparison in HRD group demonstrated similar survival outcomes.
In conclusion, with three years of follow-up, we observed no improvement in PFS or OS in patients with standard-risk myeloma assigned to auto-allo HCT vs. auto-auto HCT. Disease relapse remains a major problem in both groups suggesting that additional pre and post transplant therapies to reduce the risk of relapse are warranted28–30. The results of the BMT CTN 0102 trial is the largest trial to address the role of alloHCT in standard risk myeloma and lack of superiority compared to an auto-auto approach should be part of the treatment decision discussions with patients. Nonetheless, this trial offers glimpses of the anti-tumor activity of the alloHCT approach as seen in the reduced risk of relapse in patients with chronic graft GVHD. Further improvements in alloHCT to reduce TRM and/or augment GVM will prompt future investigation.
Research in Context
The main objective of this trial was to assess the role of an auto-allo as part of upfront treatment of myeloma in patients with standard risk disease. The investigational auto allo arm was demonstrated to be feasible and demonstrated initial promising results (Maloney et al, Blood 2003). The most appropriate comparative treatment arm was the auto-auto based on the data from the French Group (IFM) which demonstrated an improvement in progression free survival with tandem transplants using melphalan 200mg/m2 as a conditioning regimen compared to a single auto transplant. (Attal et al, NEJM 2003). During the same period, data on the use of thalidomide was maturing, which suggested a role of maintenance of this drug. Early observation from investigators from the IFM group demonstrated that it was feasible to administer thalidomide post transplant and that it was well tolerated in the maintenance setting (Attal et al, Blood 2006). Additionally, during clinical trial development, data from the Center for International Blood and Marrow Transplant Research (CIBMTR) was used to assess the number of transplants performed in the most active US centers to target participation and analyze the expected outcomes of patients who would fulfill the anticipated trial eligibility criteria.
Other trials addressing the role of auto-allo were recently reported. The Italian group randomized all patients with available sibling donors but there was a significant drop out of patients from enrollment to time of starting intervention. This trial demonstrated an event free and overall survival benefit of the auto-allo approach, although the overall number of patients who received both interventions was small (Bruno B. et al NEJM 2005). The Spanish group also published their results of auto-allo approach compared to auto-auto and despite improvement in time to progression, no survival benefit was identified in with the auto-allo approach. Again the number of patients was small, , in the Spanish trial only 25 patients were assigned to the auto-allo arm (Rosignol et al, Blood 2008). Most recently the European Blood and Marrow Transplant group (EBMT) trial updated their long term results, which with a median follow up of 61 months demonstrated a superiority of the auto-allo approach over single or tandem auto. (Bjorkstrand B et al, JCO 2011). This study analyzed patients irrespective of disease risk and differences in survival outcomes did not emerge till beyond 24 months. Although, the overall survival benefit in the auto-allo arm was minimized in the as treated analysis there was a highly significant benefit in risk reduction over time in the auto allo arm. Thus this question remains controversial. However, the current BMT CTN trial is the largest to date to address this question, and it shows that an auto-allo approach is not different from an auto-auto one among patients with standard risk myeloma. The BMT CTN 0102 trial is the definitive trial which demonstrates that the auto-allo approach is not different than the auto-auto approach for patients with standard risk myeloma. Clinicians should discuss BMT CTN 0102 results with patients and the decision to proceed with allogeneic transplant in patients with standard risk disease should be carefully considered. Additional implications of these results include development of studies with focus to explore GVM and reduction of TRM.
Supplementary Material
Acknowledgments
Funding
The Blood and Marrow Transplant Clinical Trials Network is supported in part by grant #U01HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute. The Clinicaltrials.gov identifier is NCT00075829.
*The role of the sponsors in the study design.
NHLBI statisticians are members of the protocol team and assisted in the development of the study design.
*The role of the sponsors in the collection, analysis, or interpretation of the data.
NHLBI and NCI had access to data presented to the Data and Safety Monitoring Board.
*The role of the sponsors in the writing of the report.
NHLBI revised and offered in input to this manuscript.
*Those who had access to the raw data (by author initials).
MCP, BL, and JW
The corresponding author had full access to all of the data and the final responsibility to submit for publication.
APPENDIX
A-1 – List of participating transplant centers
| Baylor College of Medicine/The Methodist Hospital |
| Blood & Marrow Transplant Program at North side Hospital |
| City of Hope National Medical Center |
| City of Hope Samaritan |
| Dana Farber Cancer Institute/Brigham & Women’s Hospital |
| Duke University Medical Center - Adult BMT |
| Emory University |
| Fox Chase - Temple University - BMT Program |
| Fred Hutchinson Cancer Research Center |
| Hackensack University Medical Center |
| Indiana BMT at Beech Grove |
| Jewish Hospital BMT Program |
| Loyola University Medical Center |
| Medical College of Wisconsin |
| Memorial Sloan-Kettering Cancer Center |
| Oregon Health & Science University |
| Rocky Mountain BMT Program |
| Scripps Clinic/Green Hospital |
| Stanford Hospital and Clinics |
| Texas Transplant Institute |
| Tufts Medical Center |
| University of California San Diego Medical Center |
| University Hospitals of Cleveland/University Hospitals Case Medical Center |
| University of Alabama at Birmingham |
| University of Florida College of Medicine (Shands) |
| University of Kansas Hospital |
| University of Michigan Medical Center |
| University of Minnesota |
| University of Nebraska Medical Center |
| University of Oklahoma Medical Center |
| University of Pennsylvania Abramson Cancer Center |
| University of Texas/MD Anderson Cancer Research Center |
| University of Wisconsin Hospital & Clinics |
| University of Utah Medical School |
| Vanderbilt University Medical Center/VA Tennessee Valley Healthcare System |
| Virginia Commonwealth University MCV Hospitals |
| Wichita CCOP |
Footnotes
Author Contribution:
Protocol Development: DHV, FS, EAS, ME, NLG, SC, MMH, SGi, DGM
Study Conduct: AK, MCP, EAS, DVH, ME, SC, MMH, SGi, DGM
Patient Enrollment: AK, EA, JHA, RC, SGo, PH, GL, MHQ, SR, DTV, DW, SGi, DGM
Endpoint Review Committee: AK, MCP, DVH, EAS, NLG, SC, JW, DGM
Analysis: MCP, BL, SC, JW
Analysis Interpretation: AK, MCP, DVH, EAS, NLG, SC, MMH, DGM, DTV.
Manuscript preparation: AK, MCP, JHA, EAS, DVH, DTV, MMH, NLG, DGM, Literature Search: AK, MCP, DVH, DGM
Figures and Tables: MCP, JW, BL
Study protocol is available at http://bmtctn.net
Conflict of interest statement:
Authors declared no conflict of interest: MCP, BL, EAS, ME, JW, SC, NLG, MMH, DTV, DW
Potential conflict of interest declared:
Celgene Pharmaceutics: Speakers Bureau and Consultant - AK
Celgene Consultant - DGM
Millenium Pharmaceutics: Speakers Bureau - AK
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amrita Krishnan, City of Hope Cancer Center, 1500 East Duarte Road, Heme BMT, Los Angeles, CA 91010.
Marcelo C. Pasquini, Medical College of Wisconsin, 9200 W. Wisconsin Avenue, Milwaukee, WI 53226.
Brent Logan, Medical College of Wisconsin, 9200 W. Wisconsin Avenue, Milwaukee, WI 53226.
Edward A. Stadtmauer, University of Pennsylvania Abramson Cancer Center, 3400 Spruce Street, 16 Penn Tower, Philadelphia, PA 19104.
David H. Vesole, Hackensack University Medical Center, 92 Second Street, Hackensack, NJ 07601.
Edwin Alyea, III, Dana-Farber Cancer Institute, 44 Binney Street, Boston, MA 02114.
Joseph H. Antin, Dana-Farber Cancer Institute, 44 Binney Street, 1B58, Boston, MA 02114.
Raymond Comenzo, Tufts University and Medical Center, 800 Washington Street, Boston, MA 02111.
Stacey Goodman, Vanderbilt University Medical Center/VA Tennessee Valley Healthcare System, 1500 21st Avenue South, Suite 3500 Village at Vanderbilt, Nashville, TN 37232-8210.
Parameswaran Hari, Medical College of Wisconsin, 9200 W. Wisconsin Avenue, Milwaukee, WI 53226.
Ginna Laport, Stanford University, 300 Pasteur Dr. Room H3249, Stanford, CA 94305-5623.
Muzaffar H. Qazilbash, MD Anderson Cancer Research Center, 1515 Holcombe Blvd., Box 423, Houston, TX 77030.
Scott Rowley, Hackensack University Medical Center, 20 Prospect Avenue, Ste 400, Hackensack, NJ 07601.
Firoozeh Sahebi, City of Hope Cancer Center, 1500 East Duarte Road, Los Angeles, CA 91010 and Southern California Kaiser Permanente Medical Group, Los Angeles, CA.
George Somlo, City of Hope Cancer Center, 1500 East Duarte Road, Los Angeles, CA 91010.
Dan T. Vogl, University of Pennsylvania Abramson Cancer Center, 3400 Spruce Street, 16 Penn Tower, Philadelphia, PA 19104.
Daniel Weisdorf, University of Minnesota, 420 Delaware Street, SE, Box 8905, B-553, Minneapolis, MN 55455.
Marian Ewell, The EMMES Corporation, 401 N. Washington Street, Ste. 700, Rockville, MD 20850.
Juan Wu, The EMMES Corporation, 401 N. Washington Street, Ste. 700, Rockville, MD 20850.
Nancy L. Geller, National Heart, Lung, and Blood Institute, 6701 Rockledge Drive, Rockledge 2, Ste 9202, Bethesda, MD 20892.
Mary M. Horowitz, Medical College of Wisconsin, 9200 W. Wisconsin Avenue, Milwaukee, WI 53226.
Sergio Giralt, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 235, New York, NY 10065.
David G. Maloney, Fred Hutchinson Cancer Research Center, 1100, Fairview Ave North, D1-100, PO Box 19024, Seattle, WA 98109-1024.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996 Jul 11;335(2):91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003 May 8;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003 Dec 25;349(26):2495–502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 4.Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006 Nov 15;108(10):3289–94. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 5.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006 Mar 9;354(10):1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 6.Barlogie B, Tricot GJ, van Rhee F, Angtuaco E, Walker R, Epstein J, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006 Oct;135(2):158–64. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- 7.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003 Nov 1;102(9):3447–54. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 8.Spencer A, Prince HM, Roberts AW, Prosser IW, Bradstock KF, Coyle L, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009 Apr 10;27(11):1788–93. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 9.Lokhorst HM, van der Holt B, Zweegman S, Vellenga E, Croockewit S, van Oers MH, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010 Feb 11;115(6):1113–20. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 10.Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007 Jun 10;25(17):2434–41. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 11.Bensinger WI, Buckner CD, Anasetti C, Clift R, Storb R, Barnett T, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996 Oct 1;88(7):2787–93. [PubMed] [Google Scholar]
- 12.Gahrton G, Tura S, Ljungman P, Blade J, Brandt L, Cavo M, et al. Prognostic factors in allogeneic bone marrow transplantation for multiple myeloma. J Clin Oncol. 1995 Jun;13(6):1312–22. doi: 10.1200/JCO.1995.13.6.1312. [DOI] [PubMed] [Google Scholar]
- 13.Gahrton G, Tura S, Ljungman P, Belanger C, Brandt L, Cavo M, et al. Allogeneic bone marrow transplantation in multiple myeloma. European Group for Bone Marrow Transplantation. N Engl J Med. 1991 Oct 31;325(18):1267–73. doi: 10.1056/NEJM199110313251802. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds C, Ratanatharathorn V, Adams P, Braun T, Silver S, Ayash L, et al. Allogeneic stem cell transplantation reduces disease progression compared to autologous transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2001 Apr;27(8):801–7. doi: 10.1038/sj.bmt.1703006. [DOI] [PubMed] [Google Scholar]
- 15.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007 Mar 15;356(11):1110–20. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 16.Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008 Nov 1;112(9):3591–3. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 17.Bruno B, Storer B, Patriarca F, Rotta M, Sorasio R, Allione B, et al. Long-Term Follow up of a Comparison of Non-Myeloablative Allografting with Autografting for Newly Diagnosed Myeloma. ASH Annual Meeting Abstracts; 2010. Nov 19, p. 525. [Google Scholar]
- 18.Bjorkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, et al. Tandem Autologous/Reduced-Intensity Conditioning Allogeneic Stem-Cell Transplantation Versus Autologous Transplantation in Myeloma: Long-Term Follow-Up. Journal of Clinical Oncology. 2011 Jul;5:2011. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 19.Weisdorf D, Carter S, Confer D, Ferrara J, Horowitz M. Blood and marrow transplant clinical trials network (BMT CTN): addressing unanswered questions. Biol Blood Marrow Transplant. 2007 Mar;13(3):257–62. doi: 10.1016/j.bbmt.2006.11.017. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 20.Logan B, Leifer E, Bredeson C, Horowitz M, Ewell M, Carter S, et al. Use of biological assignment in hematopoietic stem cell transplantation clinical trials. Clin Trials. 2008;5(6):607–16. doi: 10.1177/1740774508098326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 22.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine. 2005;352(24):2487–98. doi: 10.1056/NEJMoa043445. [see comment] [DOI] [PubMed] [Google Scholar]
- 23.Lahuerta JJ, Martinez-Lopez J, Serna Jdl, Bladé J, Grande C, Alegre A, et al. Remission status defined by immunofixation vs. electrophoresis after autologous transplantation has a major impact on the outcome of multiple myeloma patients. British Journal of Haematology. 2000;109(2):438–46. doi: 10.1046/j.1365-2141.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- 24.Cox D. Regression Models and Life Tables. J R Stat Soc B. 1972;4:187–200. [Google Scholar]
- 25.Barlogie B, Shaughnessy JD, Crowley J. Duration of Survival in Patients with Myeloma Treated with Thalidomide. New England Journal of Medicine. 2008;359(2):210–2. doi: 10.1056/NEJMc0801352. [DOI] [PubMed] [Google Scholar]
- 26.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and Hematopoietic-Cell Transplantation for Multiple Myeloma. New England Journal of Medicine. 2006;354(10):1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 27.Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006 May 1;107(9):3474–80. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 28.Kroger N, Shimoni A, Zagrivnaja M, Ayuk F, Lioznov M, Schieder H, et al. Low-dose thalidomide and donor lymphocyte infusion as adoptive immunotherapy after allogeneic stem cell transplantation in patients with multiple myeloma. Blood. 2004 Nov 15;104(10):3361–3. doi: 10.1182/blood-2004-05-2031. [DOI] [PubMed] [Google Scholar]
- 29.Lioznov M, El-Cheikh J, Jr, Hoffmann F, Hildebrandt Y, Ayuk F, Wolschke C, et al. Lenalidomide as salvage therapy after allo-SCT for multiple myeloma is effective and leads to an increase of activated NK (NKp44(+)) and T (HLA-DR(+)) cells. Bone Marrow Transplant. 2010 Feb;45(2):349–53. doi: 10.1038/bmt.2009.155. [DOI] [PubMed] [Google Scholar]
- 30.Minnema MC, van der Veer MS, Aarts T, Emmelot M, Mutis T, Lokhorst HM. Lenalidomide alone or in combination with dexamethasone is highly effective in patients with relapsed multiple myeloma following allogeneic stem cell transplantation and increases the frequency of CD4+Foxp3+ T cells. Leukemia. 2009 Mar;23(3):605–7. doi: 10.1038/leu.2008.247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


