Abstract
Objectives
Increasing evidence suggests that inflammation contributes to the etiology and progression of schizophrenia. Molecules that initiate inflammation, such as virus- and toxin-induced cytokines, are implicated in neuronal degeneration and schizophrenia-like behavior. Using therapeutic agents with anti-inflammatory or neurotrophic effects may be beneficial for treating schizophrenia.
Methods
One hundred healthy controls and 95 Han Chinese patients with schizophrenia were tested in this double-blind study. Their PANSS scores, plasma interleukin (IL)-1β, TNF-α and brain-derived neurotrophic factor (BDNF) levels were measured before and after pharmacological treatment.
Results
Pretreatment, plasma levels of IL-1β and TNF-α were significantly higher in patients with schizophrenia than in controls, but plasma BDNF levels were significantly lower. Patients were treated with the atypical antipsychotic risperidone (Risp) only or with Risp+add-on dextromethorphan (DM). PANSS scores and plasma IL-1β levels significantly decreased, but plasma TNF-α and BDNF levels significantly increased after 11 weeks of Risp treatment. Patients in the Risp+DM group showed a greater and earlier reduction of symptoms than did those in the Risp-only group. Moreover, Risp+DM treatment attenuated Risp-induced plasma increases in TNF-α.
Conclusion
Patients with schizophrenia had a high level of peripheral inflammation and a low level of peripheral BDNF. Long-term Risp treatment attenuated inflammation and potentiated the neurotrophic function but also produced a certain degree of toxicity. Risp+DM was more beneficial and less toxic than Risp-only treatment.
Keywords: Brain-derived neurotrophic factor (BDNF), Dextromethorphan, Risperidone, Schizophrenia
Introduction
Schizophrenia is a complex and severe brain disorder that causes diverse disturbances in cognition, reality testing, mood, interpersonal relations, and work functions. The etiology and pathophysiology of schizophrenia are poorly understood. While the pharmacological guidelines for schizophrenia treatment are well established, treatment for schizophrenia remains less than ideal. Because many patients with schizophrenia remain symptomatic, even while fully adherent to their medication regimens, the need for greater understanding of the pathogenesis of this illness from the research on the pharmacological mechanisms of schizophrenia medications or definite biomarker is all the more urgent.
Schizophrenia has been associated with a certain degree of central nervous system dysfunction. Imaging studies suggested that ongoing neuronal atrophy accompanies schizophrenia. For example, the significantly decreased grey matter volume (Shepherd, Laurens et al. 2012), enlarged ventricles, decreased cerebral (Rimol, Nesvag et al. 2012), temporal lobe volume, and thalamic anomalies (Sowell, Levitt et al. 2000; Crespo-Facorro, Barbadillo et al. 2007; Galderisi, Quarantelli et al. 2008) had been found in patients with schizophrenia, suggesting a role of neuronal loss and brain cell dysfunction in schizophrenia. Therefore, schizophrenia might belong to a neuronal degeneration disease.
Increasing evidence suggests that abnormal peripheral and central nervous system cytokine levels in psychosis indicate that activation of the cytokine-mediated inflammatory response system may be important in the pathogenesis of neuronal degeneration and highly relevant to the symptoms and progression of schizophrenia. For example, interleukin (IL)-1β and TNF-α are proinflammatory cytokines that modulate synaptic plasticity (Pickering and O'Connor 2007; Viviani, Gardoni et al. 2007) and are important in the neurodegenerative processes in the brain (Grunblatt, Mandel et al. 2000; Barcia, de Pablos et al. 2005). In addition, IL-1β and TNF-α sustain dopaminergic neuron degeneration in the MPTP-induced parkinsonism animal model (Grunblatt, Mandel et al. 2000; Barcia, de Pablos et al. 2005). Overexpressed IL-1β and TNF-α mRNA is found in the peripheral blood mononuclear cells of patients with schizophrenia (Liu, Jia et al. 2010). Moreover, serum IL-1β and IL-6 levels (Schmitt, Bertsch et al. 2005) and cerebral spinal fluid (CSF) IL-1β levels (Soderlund, Schroder et al. 2009) are significantly higher in patients with schizophrenia than in healthy controls. These findings suggest that the activation of inflammation contribute to the neuronal damage and degeneration found in patients with schizophrenia.
Moreover, accumulating evidence also suggests that neurotrophic factors are candidate molecules involved in the pathophysiology of mental illness. For instance, functional alterations in brain-derived neurotrophic factor (BDNF) have recently been associated with the pathophysiology of schizophrenia. BDNF, a basic dimeric protein, is a member of the nerve growth factor family involved in neuronal survival, differentiation (Mizuno, Carnahan et al. 1994), synaptogenesis, and maintenance (Peng, Yang et al. 2003; Carrasco, Castro et al. 2007; Ohira and Hayashi 2009). It has to be maintained throughout life in order to preserve essential functions such as learning and memory. BDNF promotes the survival of a wide range of neuronal cells, such as the dopaminergic neurons of the substania nigra (Hyman, Hofer et al. 1991), cerebellar granule neurons (Segal, Takahashi et al. 1992), motor neurons (Oppenheim, Yin et al. 1992), and retinal ganglion cells (Johnson, Barde et al. 1986). BDNF's influence on dopaminergic neurons may be relevant to the pathogenesis of schizophrenia, which is suggested to be related to dopaminergic dysfunction (Hyman, Hofer et al. 1991). Because schizophrenia is a neuronal developmental disorder, the dysfunction of BDNF might reflect the severity or the neuronal dysfunction and degeneration of schizophrenia. Using animal models, Fumagalli et al. (2004) (Fumagalli, Bedogni et al. 2004) showed a reduction in BDNF expression in the prefrontal cortex and striatum and persistently altered regulation of BDNF in the brain structures in adult rats, and Lipska et al. (2001) (Lipska, Khaing et al. 2001) also showed a decrease in BDNF expression in the hippocampus and prefrontal cortex. Thus, we hypothesized that corticostriatal and hippocampal BDNF dysregulation contribute to permanent alterations in brain function and lead to an increased susceptibility to schizophrenia-like pathology.
Taking all these findings together, the increase in cytokine levels in patients with schizophrenia might cause neuronal damage, and a decrease in BDNF levels might indicate a reduction in neurotrophic effects in patients with schizophrenia. Treating schizophrenia with medication that has both anti-inflammatory and neurotrophic effects might be therapeutically beneficial. However, for ethical reasons, we cannot directly monitor the brain cytokine and BDNF levels of human patients.
Cytokines can cross the blood-brain barrier (Simard and Rivest 2005), and high levels of peripheral cytokines induce sustained neuronal inflammation, damage, and degeneration (Qin, Wu et al. 2007). In contrast, BDNF also can cross the blood-brain barrier (Pan, Banks et al. 1998), and BDNF levels in the brain and plasma undergo similar changes during maturation and aging processes in vivo (Karege, Schwald et al. 2002; Cirulli, Francia et al. 2009). Therefore, peripheral cytokine and BDNF levels might be important biomarkers in schizophrenia.
The current therapeutic approach to the treatment of schizophrenia focuses on its symptoms, and the most common therapy is a dopamine antagonist or combined dopamine and serotonin antagonists for positive and negative symptoms. In this study, we used the atypical antipsychotic risperidone (Risp), a dopamine and serotonin antagonist that has been long been used to treat schizophrenia. Risp significantly attenuates the interferon-γ induced production of IL-6 and TNF-α in microglial cells in vitro (Kato, Monji et al. 2007) and in lipopolysaccharide-treated mice (Sugino, Futamura et al. 2009). We also used, as an add-on treatment, the neuroprotective agent dextromethorphan (DM), an antitussive drug with few side effects (Wang, Chou et al. 1977). DM is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist and protects dopamine neurons against inflammation-mediated damage (Liu, Qin et al. 2003; Zhang, Wang et al. 2004). Thus, we hypothesize that combining these two agents might be beneficial for treating schizophrenia. In addition, high doses of DM (240–480 mg) were reported to have a tendency to be abused in human (Boyer 2004). Thus, to preclude DM abuse, we gave participants much lower doses (60 mg).
The aims of the present study were to investigate (1) changes in the plasma levels of inflammatory factors (IL-1β, TNF-α) and plasma BDNF in patients with schizophrenia, (2) whether risperidone has anti-inflammatory and neurotrophic effects, and (3) whether combining risperidone with DM produces greater anti-inflammatory and neurotrophic effects in patients with schizophrenia than does risperidone alone. We hypothesized that Risp+DM treatment would be more efficacious that Risp-only treatment because it targets the biochemical pathology as well as the symptoms of schizophrenia.
Methods
Patient selection
We recruited 10 patients from inpatient acute psychiatric wards and 127 from outpatient psychiatric clinics based on hospitalization status. In all, 100 healthy controls and 137 Han Chinese patients with schizophrenia; age range: 20–59 years) from the Department of Psychiatry at National Cheng Kung University Hospital and the Department of Psychiatry at the National Defense Medical Center were recruited. None of the patients had comorbid psychiatric disorders (depression or anxiety) or used illicit drugs, but about 80% smoked cigarettes; we did not control their smoking. The Chinese version of the Schedule for Affective Disorders and Schizophrenia-Lifetime (SADS-L), which has good reliability and validity (Endicott, Forman et al. 1978; Huang, Lin et al. 2004), was used to screen all participants for psychiatric conditions and to fit DSM-IV diagnostic criteria. The following were the exclusion criteria: 1) women who were pregnant or nursing; 2) women of childbearing potential not using adequate contraception according to investigator judgment, or not willing to use contraception for the duration of the study; 3) having taken dextromethorphan or other selective cyclooxygenase 2 (Cox-2) inhibitors or other anti-inflammatory medication within 1 week before the study began; 4) any major mental illness other than schizophrenia, including alcoholism and illegal substance use disorder; 5) any clinically significant medical condition, e.g., cardiac, hepatic, and renal disease, with current evidence of poor control; 6) having undergone electroconvulsive therapy (ECT) within 4 weeks before the study began; 7) more than 3 times the upper limit of normal aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine levels. The healthy controls were free of major and minor mental illness. The study protocol was reviewed and approved by the National Chung Kung University and National Defense Medical Center Institutional Review Boards (IRB) for the Use of Human Research Subjects before the study began.
Study design
This was a double-blind, stratified randomized, parallel-group, two-center clinical trial from January 2006 to December 2008 conducted by the Department of Psychiatry at National Cheng Kung University and the Department of Psychiatry at the National Defense Medical Center, Taipei. All patients were randomly assigned to add-on treatment groups (Risp+DM 60 mg/day or Risp+Placebo) for 11 weeks (77 days) after they had been screened for eligibility. After the screening phase, all participants were hospitalized to modulate their medication to risperidone and then to measure their psychiatric symptoms, using PANSS, and plasma cytokines again using ELISA, at weeks 0, 2, 4, 8, and 11. The pre-treatment study phase consisted of a 7-day switch-over period—simultaneous titration of open-label risperidone or continuation of existing risperidone dose—followed by a one week open-label risperidone-only (1–6 mg/day) treatment period for hospitalized patients who were taking oral antipsychotic medication other than risperidone or who had been taking depot medication for an entire cycle before the study. Concomitant benzodiazepine medication (preferably up to 8 mg of lorazepam) was used for night-time sedation, agitation, or insomnia during the study.
Positive and Negative Syndrome Scale (PANSS)
The outcome symptom measures were psychopathological changes measured by positive and negative scores on the PANSS; the original PANSS contains 4 subscales: positive, negative, aggressive, and general psychiatric symptoms (Kay, Fiszbein et al. 1987; Kay, Opler et al. 1992). In the present study, we used the 4 components as the outcome variables for PANSS.
Plasma cytokines and BDNF measurements
Ten milliliters of whole blood was drawn from the antecubital vein of all participants at visits, 0, 1, 2, 4, 6, 8, and 11 weeks. After the whole blood had been centrifuged at 3000 g for 15 min at 4°C, the plasma was isolated and immediately stored at −80°C. Plasma cytokines and BDNF levels were quantified using enzyme-linked immunosorbent assays (ELISA). A cytokine kit (Quantikine Human Cytokine Kit; R&D Systems, Minneapolis, MN) and an ELISA reader (SpectraMax-M2; Molecular Devices, Molecular Devices, Sunnyvale, CA) were used to analyze plasma IL-1β, TNF-α, and BDNF levels.
Statistical analysis
Data are means ± SEM (standard error of the mean). A t-test, a one-way analysis of variance (ANOVA), and then a Newman-Keuls test were used for the statistical evaluations. Significance was set at p < 0.05.
Results
We initially enrolled 147 patients with acute schizophrenia, but 42 (30.7%) dropped out during the trial, which finally left us with 95 patients (57 men, 38 women; age: 30.37 ± 0.87 years old) and 100 healthy controls (55 men, 45 women; age: 31.55 ± 0.66 years old) who completed the 11-week study. There were no differences in demographic data between the healthy controls and patients (Table 1).
Table 1.
Demographic data
| Group | Patients with Schizophrenia | Healthy Controls |
P-value | ||
|---|---|---|---|---|---|
| Treatment | Risp+Placebo | Risp+DM | Drop-out | ||
| Number (n) | 45 | 50 | 41 | 100 | |
| Gender (male/female) (n) | 27/18 | 30/20 | 24/17 | 54/46 | 0.861a |
| Age, mean (SE), (years) | 28.60 ± 1.03 | 31.72 ± 1.13 | 30.93 ± 1.36 | 31.55 ± 0.66 | 0.126b |
| PANSS scores Positive scores |
20.43 ± 0.74 | 20.93 ± 0.67 | 21.15 ± 0.65 | None | 0.763b |
| Negative scores | 21.47 ± 0.78 | 23.13 ± 0.86 | 21.71 ± 0.95 | None | 0.251b |
| Aggressive scores | 5.22 ± 0.36 | 4.48 ± 0.24 | 5.56 ± 0.38 | None | 0.062b |
| General scores | 37.56 ± 0.89 | 39.67 ± 0.93 | 39.49 ± 0.86 | None | 0.186b |
| Sum of scores | 84.62 ± 1.96 | 88.22 ± 1.87 | 87.95 ± 2.04 | None | 0.343b |
| Week 0 body weight (kg) | 68.0 ± 2.9 | 65.31 ± 1.9 | 65.7 ± 2.3 | 64.0 ± 1.3 | 0.308b |
| Week 11 body weight (kg) | 70.9 ± 3.1 | 67.10 ± 2.2 | None | None | 0.260c |
| Weight gain after 11 weeks of treatment | 1.7 ± 0.5 | 1.20 ± 0.5 | 0.556c | ||
χ2,
one-way ANOVA,
t-test.
Data are means ± standard error (S.E.).
Efficacy
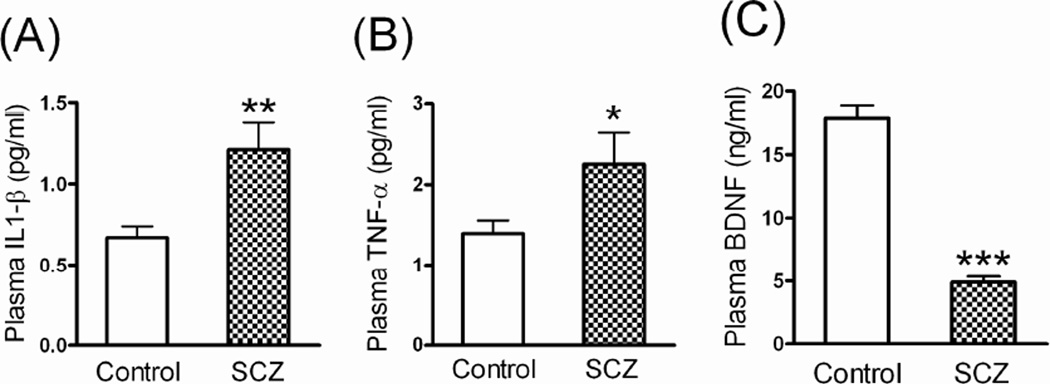
Patients with schizophrenia had significantly higher pretreatment levels of plasma IL-1β (1.21 ± 0.17 vs. 0.19 ± 0.04 pg/ml; p < 0.001) and TNF-α (2.25 ± 0.39 vs. 1.09 ± 0.16 pg/ml; p < 0.05) but lower levels of plasma BDNF (4.88 ± 0.47 vs. 18.32 ± 1.13 ng/ml; p < 0.001) than did healthy controls (Figure 1).
Fig 1.
The plasma (A) TNF-α, (B) IL-1β, and (C) BDNF levels of healthy controls (n = 100) and patients with schizophrenia (SCZ) (n = 95) before treatment. *p < 0.05, **p < 0.01, ***p < 0.001 vs. healthy controls (t-test).
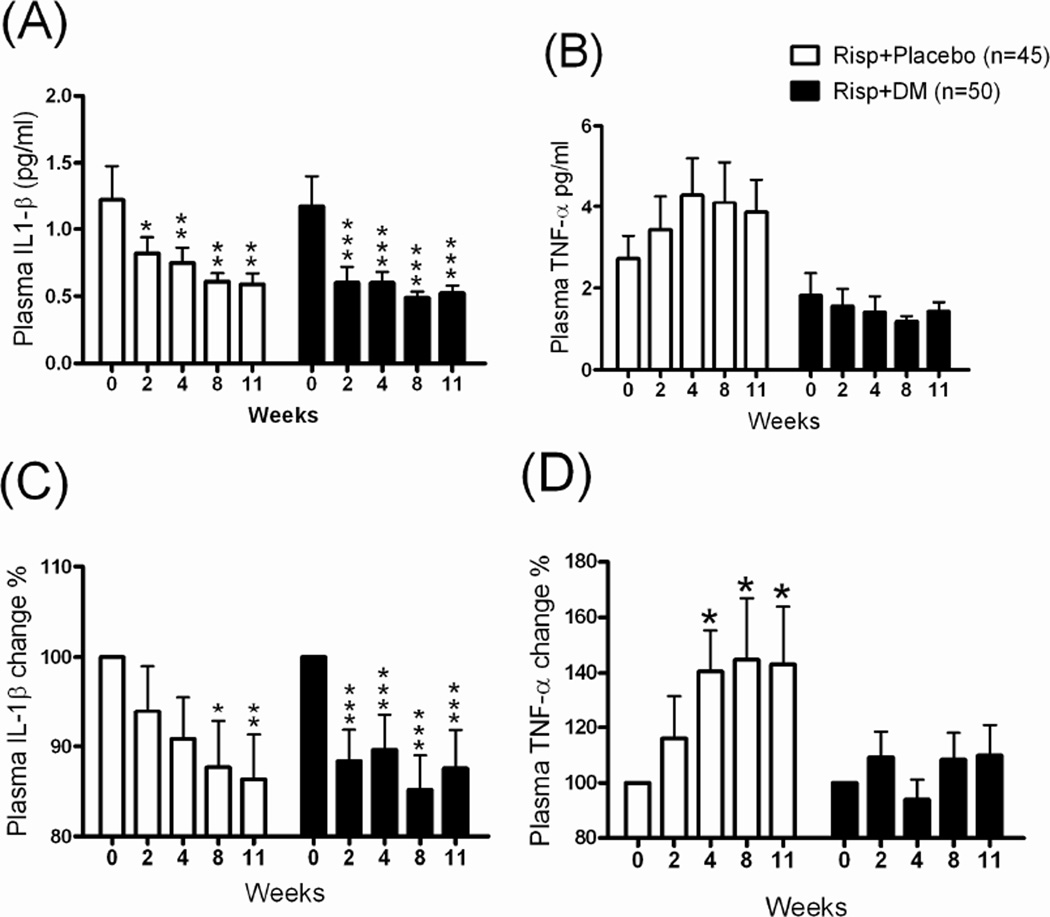
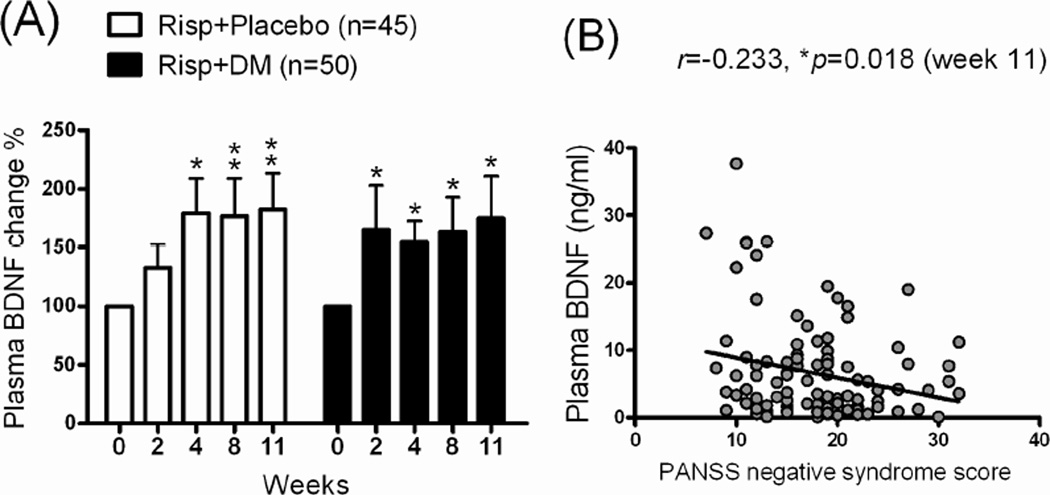
There was no significant difference in the dose of Risp (mean dose: 3–4 mg/day) between the Risp+Placebo and Risp+DM groups from the initial visit to the end of the study (data not shown). In the inflammatory factors analysis, a significant decrease in plasma IL-1β levels and percentage was found in both the Risp+Placebo and Risp+DM groups after 11 weeks of treatment (Figure 2A, 2C). Although plasma TNF-α levels were not significantly different between groups, they tended to be higher in the Risp+Placebo after 11 weeks (Fig 2B). We then normalized the plasma TNF-α level by the pretest (week 0 as 100 %) data and found that plasma TNF-α was significantly higher (from 100% to 143.1 ± 20.1%) after 11 weeks of Risp+Placebo treatment (Fig 2D). After 11 weeks of treatment, plasma BDNF levels were higher in both patient groups: Risp+Placebo (from 100% to 182.5 %) and Risp+DM (from 100% to 176%) (Figure 3A). There was no significant correlation of plasma IL-1β/TNF-α/BDNF levels with the PANSS scores (data not shown); however, the plasma BDNF level was significantly negatively correlated with PANSS negative syndrome scores (Figure 3B).
Fig 2.
The plasma (A) IL-1β and (B) TNF-α levels and their change percentages (%) (C), (D) in patients with schizophrenia after 11 weeks of treatment with risperidone (Risp)+Placebo or Risp+add-on dextromethorphan (DM) (60 mg/day). Plasma samples were collected and analyzed at 0, 2, 4, 8, and 11 weeks. *p < 0.05, **p < 0.01, ***p < 0.001 vs. week 0 data within same group (repeated-measures ANOVA with Newman-Keuls post test).
Fig 3.
(A) The plasma BDNF change percentages (%) after 11 weeks of treatment with risperidone (Risp)+Placebo or Risp+add-on dextromethorphan (DM) (60 mg/day). Plasma samples were collected and analyzed at 0, 2, 4, 8, and 11 weeks. *p < 0.05, **p < 0.01 vs. week 0 data within same group (repeated-measures ANOVA with Newman-Keuls post test) and (B) scatter plots of plasma BDNF levels with PANSS negative symptom scores in patients with schizophrenia (Pearson r = −0.233, p = 0.018). Linear regression and the Pearson product-moment correlation coefficient were used to analyze the correlation.
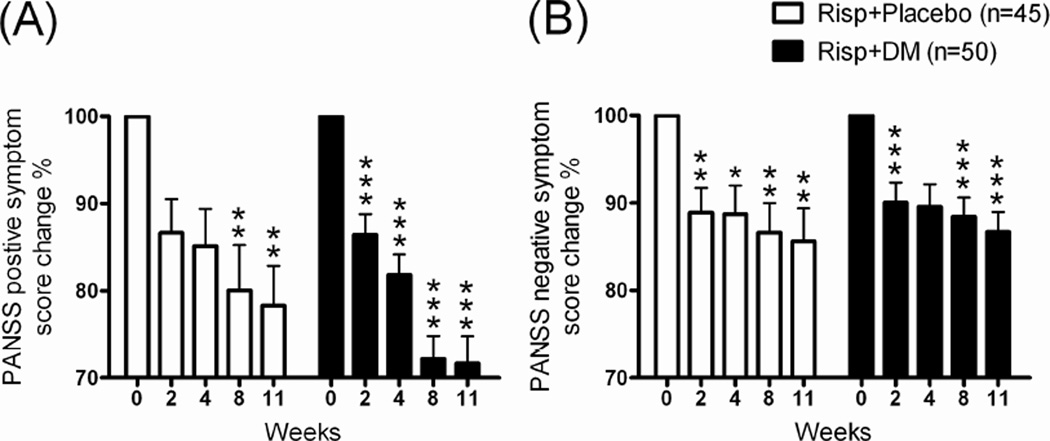
PANSS general (data not shown), positive (Figure 4A), and negative symptom scores (Figure 4B) were significantly decreasing in both Risp+Placebo and Risp+DM groups after 11 weeks of treatment. In addition, the Risp+DM group showed early and more positive symptom scores decreasing profile after 11 weeks of treatment than did the Risp+Placebo group (Figure 4A).
Fig 4.
The change percentages (%) of (A) PANSS positive symptom scores and (B) PANSS negative symptom scores in patients with schizophrenia after 11 weeks of risperidone (Risp)+Placebo or Risp+add-on dextromethorphan (DM) (60 mg/day) treatment. *p < 0.05, **p < 0.01, **p < 0.001 vs. week 0 data within same group (repeated-measures ANOVA with Newman-Keuls post test).
Discussion
We found that plasma cytokine levels were significantly higher in patients with schizophrenia than in healthy controls, which implied the activation of an immunological response in these patients. Higher levels of cytokines in plasma and CSF have been found in different mental illness and are hypothesized to be both causal and exacerbating factors in these diseases (Hope, Melle et al. 2009; Hu, Chen-Plotkin et al. 2010; Soderlund, Olsson et al. 2010). Other studies have speculated that plasma cytokines may access the brain through certain carriers or a damaged blood brain barrier (Qin, Wu et al. 2007; Quan and Banks 2007). Qin et al. (2007) reported that a systemically administered single dose of lipopolysaccharide which could not readily reach the brain induced both early (1–9 h) serum and liver TNF-α expression and long-lasting (7–30 days) brain TNF-α expression, and, subsequently, activated microglia and caused neuronal damage and degeneration; therefore, peripheral inflammation may initiate or contribute to neuroinflammation and neurodegeneration in the central nervous system (Simard and Rivest 2005; Qin, Wu et al. 2007). Thus, although we did not find a very high level of peripheral cytokines or a significant association between cytokines and symptom scores in schizophrenia, an increase in the level of peripheral cytokines might represent a certain degree of sustained brain inflammation in schizophrenia, which, in turn, might indicate a certain degree of cytokine induced-neuronal damage.
In the present study, we found that Risp treatment reduced elevated plasma IL-1β levels in patients with schizophrenia, and this might be the major therapeutic benefit of Risp in schizophrenia. This agrees with the finding of Song et al. (2009) that 4 weeks of Risp treatment significantly reduced IL-1β serum levels in patients with schizophrenia (Song, Lv et al. 2009). Moreover, in the present study, we found that Risp treatment induced a significant increase in plasma TNF-α levels, which suggested that Risp would have a toxic effect after long-term treatment. Thus, we hypothesized that adding on the DM might provide beneficial anti-inflammatory adjuvant therapy when using Risp for extended treatment. Our finding that combined Risp+DM treatment attenuated Risp-induced TNF-α production supports that hypothesis. Moreover, we found an early and more positive decrease in schizophrenia symptoms in the Risp+DM group than in the Risp+Placebo group, which indicated Risp+DM might be therapeutically more beneficial for treating schizophrenia. DM protects dopaminergic neurons against endotoxicity (Liu, Qin et al. 2003; Zhang, Wang et al. 2004; Li, Cui et al. 2005; Zhang, Qin et al. 2005; Zhang, Shin et al. 2006). Liu et al. (2003) and Zhang et al. (2005a) reported that DM protected dopamine neurons against inflammation-mediated damage. The mechanism of DM’s neuroprotective effect is associated with inhibiting microglial activation (Liu, Qin et al. 2003). Thus, the above evidence that DM protects dopamine neurons in inflammation-related neurodegenerative models supports the notion that add-on DM might be more therapeutically effective than atypical antipsychotics alone in schizophrenia.
Ketamine and PCP, both NMDA antagonists, exacerbate the symptoms of schizophrenia (Jentsch and Roth 1999; Hashimoto and Iyo 2002; Javitt 2007). In our study, we used the NMDA receptor antagonist DM and found the more beneficial in schizophrenia treatment. DM at a high dose (10 mg/kg) is an NMDA receptor antagonist (Ribeiro Do Couto, Aguilar et al. 2004; Ribeiro Do Couto, Aguilar et al. 2005). However, in the present study, patients took 60 mg/day (about 1 mg/kg), and their plasma DM concentrations ranged from 10–100 ng/ml (28–280 nM) (data not shown). Because DM is a weak NMDA receptor antagonist (ED50: 5–50 µM) (Church, Sawyer et al. 1994), DM in such low doses might be insufficient to block NMDA receptors. However, because low dose DM has an alternative mechanism of anti-inflammation and neurotrophic effects, using low-dose DM seems to be an ideal adjuvant strategy for treating schizophrenia.
In addition, we showed a significantly (73.4%) lower plasma BDNF level in patients with schizophrenia. BDNF can cross the blood brain barrier (Pan, Banks et al. 1998), and BDNF levels in the brain and plasma undergo similar changes during the maturation and aging processes in rats (Karege, Schwald et al. 2002) and rhesus macaques (Cirulli, Francia et al. 2009), which suggests that plasma BDNF levels may reflect BDNF levels in the brain. Thus, a significantly lower plasma BDNF level might reflect the dysfunction of the neurotrophic system and a certain degree of neuronal degeneration in schizophrenia. Although some studies have reported negative findings of BDNF expression in patients with schizophrenia—Shimizu et al. (2003) (Shimizu, Hashimoto et al. 2003) reported unaltered levels in 40 patients, and Takahashi et al. (2000) (Takahashi, Shirakawa et al. 2000) reported elevated levels in the anterior cingulated cortex and hippocampus in postmortem brains—our findings agreed with those of Buckley et al. (2007) and Toyooka et al. (2002), who reported significantly lower serum BDNF levels in patients with schizophrenia (Toyooka, Asama et al. 2002; Buckley, Pillai et al. 2007). Lower BDNF mRNA levels in the prefrontal cortex and hippocampus of patients with schizophrenia have also been reported (Thompson, Weickert et al. 2011), as have lower levels of BDNF in the dorsolateral frontal cortex (Weickert, Hyde et al. 2003), anterior cingulated cortex (Iritani, Niizato et al. 2003), and hippocampus (Durany, Michel et al. 2001).
Low plasma BDNF protein levels in humans might reflect the degree of neuronal degeneration in Alzheimer's disease (Laske, Stransky et al. 2006). Thus, low plasma BDNF levels might be associated with the neuronal degeneration of schizophrenia, and neurotrophic medications might be beneficial in treating schizophrenia. In our study, the plasma BDNF level was significantly higher after 11 weeks of Risp+Placebo or Risp+DM treatment. Studies (Yoshimura, Hori et al. 2007; Rizos, Papadopoulou et al. 2008) have reported no increases in plasma BDNF in patients with schizophrenia treated with Risp. However, our results agreed with those of a study (Lee and Kim 2009) which showed that Risp treatment significantly increased plasma BDNF levels.
In conclusion, we found an increase in inflammation and a decrease in neurotrophic response in patients with schizophrenia. Medications that are both anti-inflammatory and neurotrophic might provide therapeutic benefits for treating schizophrenia. In this study, we also found that long-term treatment with the atypical antipsychotic Risp attenuated inflammatory activity and augmented BDNF levels, and that it also produced a certain degree of toxicity. Risp+DM seemed to be more beneficial and to prevent the toxicity produced by long-term Risp-only treatment.
Acknowledgments
This study was supported in part by the grant DOH95-TD-I-111-004 from the Taiwan Ministry of Health (to RBL), the Taiwan National Science Council NSC98-2627-B-006-017 (to RBL) and by a grant from the National Cheng Kung University Project to Promote Academic Excellence and Develop a World-Class Research Center, Taiwan.
Footnotes
Conflicts of interest
None.
Author contributions
Shiou-Lan Chen wrote the first draft of the manuscript. Ru-Band Lu and Jau-Shyong Hong designed the study and wrote the protocol. Shiou-Lan Chen, Shih-Heng Chen, Chun-Hsien Chu, and Yun-Hsuan Chang managed the laboratory work and the data analyses. Shiou-Lan Chen, Ru-Band Lu, and Yun-Hsuan Chang did the literature search. Po-See Chen, I-Hui Lee, Tzung-Lieh Yeh, Yen-Kuang Yang, and Ru-Band Lu recruited the participants. All authors contributed to and have approved the final manuscript.
References
- Barcia C, de Pablos V, et al. Increased plasma levels of TNF-alpha but not of IL1-beta in MPTP-treated monkeys one year after the MPTP administration. Parkinsonism Relat Disord. 2005;11(7):435–439. doi: 10.1016/j.parkreldis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care. 2004;20(12):858–863. doi: 10.1097/01.pec.0000148039.14588.d0. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Pillai A, et al. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91(1–3):1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MA, Castro P, et al. Regulation of glycinergic and GABAergic synaptogenesis by brain-derived neurotrophic factor in developing spinal neurons. Neuroscience. 2007;145(2):484–494. doi: 10.1016/j.neuroscience.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Church J, Sawyer D, et al. Interactions of dextromethorphan with the N-methyl-D-aspartate receptor-channel complex: single channel recordings. Brain Res. 1994;666(2):189–194. doi: 10.1016/0006-8993(94)90771-4. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, et al. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34(2):172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Barbadillo L, et al. Neuropsychological functioning and brain structure in schizophrenia. International Review of Psychiatry. 2007;19(4):325–336. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- Durany N, Michel T, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52(1–2):79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- Endicott J, Forman JB, et al. Research approaches to diagnostic classification in schizophrenia. Birth Defects Orig Artic Ser. 1978;14(5):41–57. [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, et al. Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. Eur J Neurosci. 2004;20(5):1348–1354. doi: 10.1111/j.1460-9568.2004.03592.x. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Quarantelli M, et al. Patterns of structural MRI abnormalities in deficit and nondeficit schizophrenia. Schizophrenia Bulletin. 2008;34(2):393–401. doi: 10.1093/schbul/sbm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunblatt E, Mandel S, et al. Neuroprotective strategies in Parkinson's disease using the models of 6-hydroxydopamine and MPTP. Ann N Y Acad Sci. 2000;899:262–273. doi: 10.1111/j.1749-6632.2000.tb06192.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M. Glutamate hypothesis of schizophrenia and targets for new antipsychotic drugs. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002;22(1):3–13. [PubMed] [Google Scholar]
- Hope S, Melle I, et al. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disorders. 2009;11(7):726–734. doi: 10.1111/j.1399-5618.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- Hu WT, Chen-Plotkin A, et al. Novel CSF biomarkers for Alzheimer's disease and mild cognitive impairment. Acta Neuropathologica. 2010;119(6):669–678. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SY, Lin WW, et al. Possible interaction of alcohol dehydrogenase and aldehyde dehydrogenase genes with the dopamine D2 receptor gene in anxiety-depressive alcohol dependence. Alcohol Clin Exp Res. 2004;28(3):374–384. doi: 10.1097/01.alc.0000117832.62901.61. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Iritani S, Niizato K, et al. Immunohistochemical study of brain-derived neurotrophic factor and its receptor, TrkB, in the hippocampal formation of schizophrenic brains. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):801–807. doi: 10.1016/S0278-5846(03)00112-X. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Barde YA, et al. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Schwald M, et al. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neuroscience Letters. 2002;328(3):261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Kato T, Monji A, et al. Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr Res. 2007;92(1–3):108–115. doi: 10.1016/j.schres.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, et al. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler IA, et al. Positive and negative syndrome scale (PANSS) rating manual. Toronto, Ontario, Canada: Multihealth System; 1992. [Google Scholar]
- Laske C, Stransky E, et al. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm. 2006;113(9):1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Increased plasma brain-derived neurotropic factor, not nerve growth factor-Beta, in schizophrenia patients with better response to risperidone treatment. Neuropsychobiology. 2009;59(1):51–58. doi: 10.1159/000205518. [DOI] [PubMed] [Google Scholar]
- Li G, Cui G, et al. Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB J. 2005;19(6):489–496. doi: 10.1096/fj.04-2555com. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Khaing ZZ, et al. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14(1):135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Jia F, et al. Tyrosine hydroxylase, interleukin-1beta and tumor necrosis factor-alpha are overexpressed in peripheral blood mononuclear cells from schizophrenia patients as determined by semi-quantitative analysis. Psychiatry Res. 2010;176(1):1–7. doi: 10.1016/j.psychres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, et al. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J Pharmacol Exp Ther. 2003;305(1):212–218. doi: 10.1124/jpet.102.043166. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, et al. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165(1):243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Ohira K, Hayashi M. A New Aspect of the TrkB Signaling Pathway in Neural Plasticity. Curr Neuropharmacol. 2009;7(4):276–285. doi: 10.2174/157015909790031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, Yin QW, et al. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992;360(6406):755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Peng HB, Yang JF, et al. Differential effects of neurotrophins and schwann cell-derived signals on neuronal survival/growth and synaptogenesis. The Journal of Neuroscience. 2003;23(12):5050–5060. doi: 10.1523/JNEUROSCI.23-12-05050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M, O'Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, et al. Effects of NMDA receptor antagonists (MK-801 and memantine) on the acquisition of morphine-induced conditioned place preference in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(6):1035–1043. doi: 10.1016/j.pnpbp.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, et al. NMDA glutamate but not dopamine antagonists blocks drug-induced reinstatement of morphine place preference. Brain Res Bull. 2005;64(6):493–503. doi: 10.1016/j.brainresbull.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71(6):552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Rizos EN, Papadopoulou A, et al. Reduced serum BDNF levels in patients with chronic schizophrenic disorder in relapse, who were treated with typical or atypical antipsychotics. World J Biol Psychiatry. 2008:1–5. doi: 10.3109/15622970802182733. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Bertsch T, et al. Increased serum interleukin-1beta and interleukin-6 in elderly, chronic schizophrenic patients on stable antipsychotic medication. Neuropsychiatr Dis Treat. 2005;1(2):171–177. doi: 10.2147/nedt.1.2.171.61048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Takahashi H, et al. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992;9(6):1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, et al. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36(4):1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, et al. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci Lett. 2003;351(2):111–114. doi: 10.1016/j.neulet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Do pathogen exposure and innate immunity cause brain diseases? Neurol Res. 2005;27(7):717–725. doi: 10.1179/016164105X49526. [DOI] [PubMed] [Google Scholar]
- Soderlund J, Olsson SK, et al. Elevation of cerebrospinal fluid interleukin-1ss in bipolar disorder. Journal of Psychiatry & Neuroscience. 2010;35(6):100080. doi: 10.1503/jpn.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund J, Schroder J, et al. Activation of brain interleukin-1beta in schizophrenia. Mol Psychiatry. 2009;14(12):1069–1071. doi: 10.1038/mp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XQ, Lv LX, et al. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65(6):481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Levitt J, et al. Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. The American Journal of Psychiatry. 2000;157(9):1475–1484. doi: 10.1176/appi.ajp.157.9.1475. [DOI] [PubMed] [Google Scholar]
- Sugino H, Futamura T, et al. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):303–307. doi: 10.1016/j.pnpbp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shirakawa O, et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry. 2000;5(3):293–300. doi: 10.1038/sj.mp.4000718. [DOI] [PubMed] [Google Scholar]
- Thompson RM, Weickert CS, et al. Decreased BDNF, trkB-TK+ and GAD(67) mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36(1) doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Asama K, et al. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110(3):249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, et al. Cytokines and neuronal ion channels in health and disease. Int Rev Neurobiol. 2007;82:247–263. doi: 10.1016/S0074-7742(07)82013-7. [DOI] [PubMed] [Google Scholar]
- Wang SC, Chou DT, et al. Studies on the potency of various antitussive agents. Agents Actions. 1977;7(3):337–340. doi: 10.1007/BF01969565. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, et al. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Hori H, et al. Treatment with risperidone for 4 weeks increased plasma 3-methoxy-4-hydroxypnenylglycol (MHPG) levels, but did not alter plasma brain-derived neurotrophic factor (BDNF) levels in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1072–1077. doi: 10.1016/j.pnpbp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang W, Qin L, et al. 3-hydroxymorphinan is neurotrophic to dopaminergic neurons and is also neuroprotective against LPS-induced neurotoxicity. FASEB J. 2005;19(3):395–397. doi: 10.1096/fj.04-1586fje. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shin EJ, et al. 3-Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro. FASEB J. 2006;20(14):2496–2511. doi: 10.1096/fj.06-6006com. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, et al. Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase. FASEB J. 2004;18(3):589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]