Abstract
Background
Lactobacillus gasseri BNR17 is a type of probiotic strain isolated from human breast milk. A study was reported regarding the fact that BNR17 was an inhibitor of obesity and diabetic activities in the human body through previous animal experiments. This study was furthered to investigate the effect of BNR17, a probiotic strain isolated from human breast milk, on obese and overweight adults.
Methods
Sixty-two obese volunteers aged 19 to 60 with body mass index ≥ 23 kg/m2 and fasting blood sugar ≥ 100 mg/dL participated in a placebo controlled, randomized, and double-blind trial. For 12 weeks, 57 participants were given either placebo or BNR17 and were tested by measuring body fat, body weight, various biochemical parameters, vital signs, and computed tomography at the start of the study and at weeks 4, 8, and 12. The subjects assumed usual daily activities without having to make behavioral or dietary modifications during the course of the study.
Results
At the 12th week, a slight reduction in body weight was noted in the BNR17 group, but there were no significant weight changes between groups. Decrease of waist and hip circumferences in the BNR17 group was more pronounced than those in the placebo group. The two groups had no special or severe adverse reactions.
Conclusion
Despite there being no change in behavior or diet, administration of only the supplement of BNR17 reduced weight and waist and hip circumference. However, there were no significant differences between the two groups. These findings warrant a subsequent longer-term prospective clinical investigation with a large population.
Keywords: Probiotics, Obesity, Metabolic Disorders, Human Breast Milk
INTRODUCTION
Obesity is a major contributor to the global epidemic of type-2 diabetes,1) fatty liver disease,2) and cardiovascular diseases.3) Worldwide, at least 300 million individuals are clinically obese,4) and in Finland, out of those aged 25 to 74 (years), 25% are obese and over half are overweight.5) Obesity, a chronic disease characterized by excessive body weight, results from a complex interplay of multiple factors, including genetic, metabolic, social, behavioral, and cultural factors. Obesity is caused mainly by high energy intake with low energy consumption. Behavioral and cultural influences, represented by the excessive intake of sweet and salty foods combined with insufficient exercise, best explain the rising occurrence of obesity in developed countries.
In recent years, growing awareness that obesity is closely linked to various adult diseases has prompted the rapid development of obesity management programs involving such aspects as drug therapeutics, surgical intervention, and dietary treatment. However, such management strategies often are accompanied by serious side effects or are found ineffective; and even more critically, obese people depend on such programs without changing their eating and exercise habits. In societies where being "underweight" wrongfully equates to being "attractive," many young, socially inept teenagers engage in excessive dietary restrictions that often give rise to anorexia, bulimia, and malnutrition, at a time when balanced nutrition is paramount for healthy growth. Therefore, development of a safe, yet effective dietary supplement to properly manage body weight is a natural calling in this world.
The gut's microbial community has been regarded as one of the critical factors related to obesity and metabolic disorders.6-8) Conventionalization of germ-free mice with a distal gut microbiota harvested from conventionally raised mice leads to an increase in body fat.7) Other reports demonstrated that obese mice have a higher proportion of intestinal Firmicutes communities and extracted calories more efficiently from their diet than lean mice, which have a higher percentage of Bacteroidetes communities.9) It has also been demonstrated that the gut microbiota is involved in high-fat, diet-induced metabolic endotoxemia, adipose tissue inflammation, and metabolic disorders in mice.10-12)
Lactobacillus (Lb.) and Bifidobacteria are major constituents of gut microbiota and include such probiotics as lactic acid bacteria (LAB), which have been shown to benefit to human health, making them natural subjects of investigation in obesity research. Some researchers reported that probiotics caused weight gain of livestock and children with diarrhea;13) meanwhile some did not demonstrate any link.14,15) Despite the arguments for the association between probiotics and obesity, many papers demonstrating various mechanisms of anti-obesity of LAB have been reported, such as regulation of lipid and glucose metabolism,7,16) production of conjugated linoleic acid,17,18) reduction of adipocyte size and increase of numbers of small adipocytes in white adipose tissue,19,20) and regulation of leptin.21)
The Lb. gasseri BNR17 used in this research was isolated from human breast milk. Our previous studies have showed that BNR17 inhibited increase in body weight and white adipose tissue weight in rats fed a high sucrose-diet, and effectively improved various diabetic symptoms, including polydipsia, hyperplasia, polyuria, fasting and postprandial 2-hour blood-glucose levels, and oral glucose tolerance in a type 2 diabetes animal model.22,23)
These results suggest that BNR17 may be a good candidate for probiotic bacteria to prevent obesity and its related disorders. In the present clinical study, we investigated the effects of BNR17 intervention on overweight or obese adults including their primary end point such as a rate of weight reduction, and second end point such as change in body fat, abdominal fat and blood glucose. This is the first clinical study on the anti-obesity effects of the Lb. strain isolated from human breast milk.
METHODS
1. Study Design
This study was a single center, randomized, double-blinded, and placebo-controlled clinical trial to examine the efficacy and safety of BNR17 in obese or overweight adults. The trial was approved by the institutional review board in Yeungnam University Medical Center. Of 83 registered obese adults screened, 62 were enrolled. The subjects included men and non-pregnant women aged between 19 and 60 with body mass index (BMI) ≥ 23 kg/m2 and fasting blood sugar (FBS) ≥ 100 mg/dL, and were divided into two groups, each with 31 subjects.
Subjects were excluded if they were taking drugs that could affect weight change, including anti-diabetic drugs, lipid-lowering drugs, orantiphycotic drugs, or if they had endocrine, cardiovascular, thyroid, or chronic liver disease. Also subjects were excluded if they had received surgery to reduce weight, were taking probiotics or antibiotics within one month, or if their weight had changed over five percent within three months.
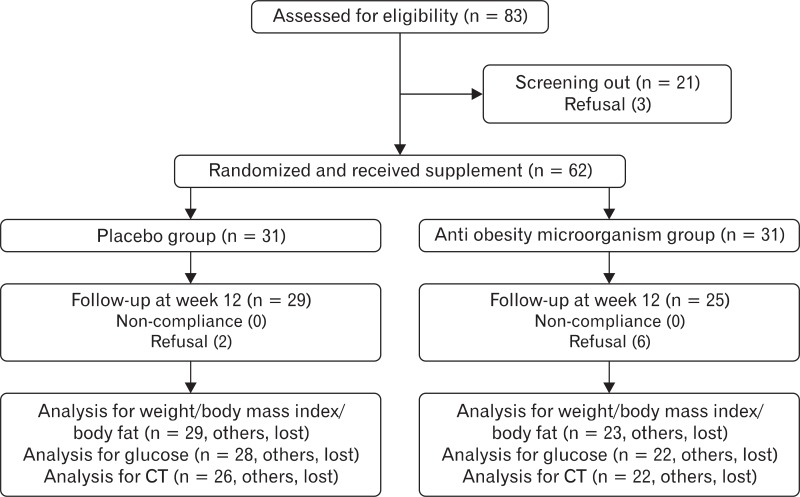
During the 2nd week of the screening period, each subject was analyzed for the eligibility criteria after consenting to test, including demographic data, past medical history, medication use, vital signs, blood sugar rate, and body weight. On the last day of the 2nd week of the screening period, eligibility of the subjects was evaluated based on the collected information. Subject recruitment was stopped when all 62 subjects were recruited. No wash-out period was required. Sixty-two eligible subjects were randomly assigned to the BNR17 or the placebo group and prescribed supplements according to a random code (Figure 1).
Figure 1.
Diagram showing the flow of participants in the BNR17 and placebo groups. CT: computed tomography.
2. Preparation of Samples and Treatment
BNR17 capsules were composed of 1010 cfu of Lb. gasseri BNR17 and filler powder (50% trehalose, 25% skim milk, and 25% fructooligosaccharide) and placebo capsules were packaged only with the filler produced by the pharmaceutical factory. BNR17 or placebo capsules were dispensed by the investigational pharmacist. Subjects were instructed to take 6 capsules per day (2 capsules 30 minutes before breakfast, lunch, and dinner) for 12 weeks. If the subject withdrew from the study, measurements were taken at the time of the last administration, and follow-up measurements were made within a month. We investigated subjects' diary of administration and pill count at every visit to improve compliance.
3. Measurements
Subjects returned to clinic at 0, 4, 8, and 12 weeks post randomization, and at each visit, vital signs such as blood pressure and pulse rate were measured after sitting comfortably for 5 minutes. We collected health-related behaviors (smoking, alcohol intake, and dietary patterns such as calory intake and regular exercise) at every visit by the dietitian. Subjects were recommended to engage in the same health-related behaviors during the study. Bowel habits were examined on the second and fifth visit. For common blood and urinary tests, subjects were asked to fast for at least 8 hours prior to the clinic visit. Electrocardiogram was obtained at screening and on the last visit. X-ray was taken only during screening. Abdominal obesity was determined by measuring waist (navel line) and hip circumferences using a measuring tape. Also body composition including body fat and muscle amount was determined by a bioelectrical impedance test (In-Body 3.0, Biospace, Seoul, Korea).
On the second and fifth visit, abdominal obesity was directly measured using a computed tomography scan. Body weight was measured using the same scale throughout the study period. BMI was calculated by dividing body weight (kg) by height (m2, barefoot). Oxygen consumption and basal metabolic rate (BMR) were measured by indirect calorimetry (Sensorimedics Co., Yorba Linda, CA, USA) using a ventilated hood system while the subject was resting comfortably lying down on a bed. Insulin, hemoglobin A1c and FBS were measured at each visit. Two hour postprandial blood sugar (2PPBS) was examined only on the second and fifth visit. A pregnancy test was conducted on women of reproductive age on every visit.
After measurements, subjects were given a supply of the test foods to permit dosing until the time of the next scheduled visit. Adverse reactions were observed for by monitoring anthropometric parameters and biochemical indices before the intake of test food and after completion of the 12 week trial.
4. Sample Size Estimation
The intended sample of 62 recruited subjects provided a power of approximately 80%, assuming a significance level < 0.05, to detect a 2.5 kg placebo-adjusted treatment effect in body weight (change from baseline to week 12th) with a standard deviation of 3.5 kg. The formula for the sample size was as follows: n = 2(za/2 + zβ)2/{(µ0 - µ1)/σ}2 where two population means (µ0 and µ1), σ2 = common variance.
5. Statistical Analysis
The data were analyzed using intention-to-treat (ITT) and per-protocol (PP) analysis. For ITT analysis, all data from the subjects who received the test food at least once were included. For missing or incomplete data, the most recently available data were used (last observation carried forward analysis). For the PP analysis, only the data obtained from the subjects who completed the study on schedule were included, and the analysis was performed only when the data were available at weeks 0 and 12. Of the 62 subjects enrolled in the study, 8 withdrew (2 from the placebo group and 6 from the BNR17 group), and none were omitted due to noncompliance. Efficacy-related data received ITT analysis while safety and side effect-related data underwent ITT & PP analysis. All side effects, regardless of their relevance to ingestion of the test food, were reported and noted in detail.
Differences in baseline characteristics between groups were analyzed using a chi-square test or Fisher's exact test. For efficacy analysis, a paired t-test and independent t-test were used to detect the differences within and between groups in the ITT dataset, and a repeated measures analysis of variance was used in the PP dataset. The same statistical methods used in efficacy analysis for the ITT dataset were performed in safety analysis.
RESULTS
1. Baseline Characteristics of the Subjects
The subjects' baseline characteristics are summarized in Table 1. Although the overall number of males was less than that of females, there were no significant differences in the sex ratio between the two groups. Seventy-eight point nine percent of the subjects were nonsmokers, and 38.6% were nondrinkers. Smoking, drinking, dietary, and exercise patterns were similar in both groups. Past medical history and drug use history revealed no significant differences between the two groups. There were no non-compliance subjects except 2 persons in the placebo group and 6 persons in the anti obesity microorganism group who refused study procedures during the study.
Table 1.
Baseline characteristics of the subjects (n = 57)
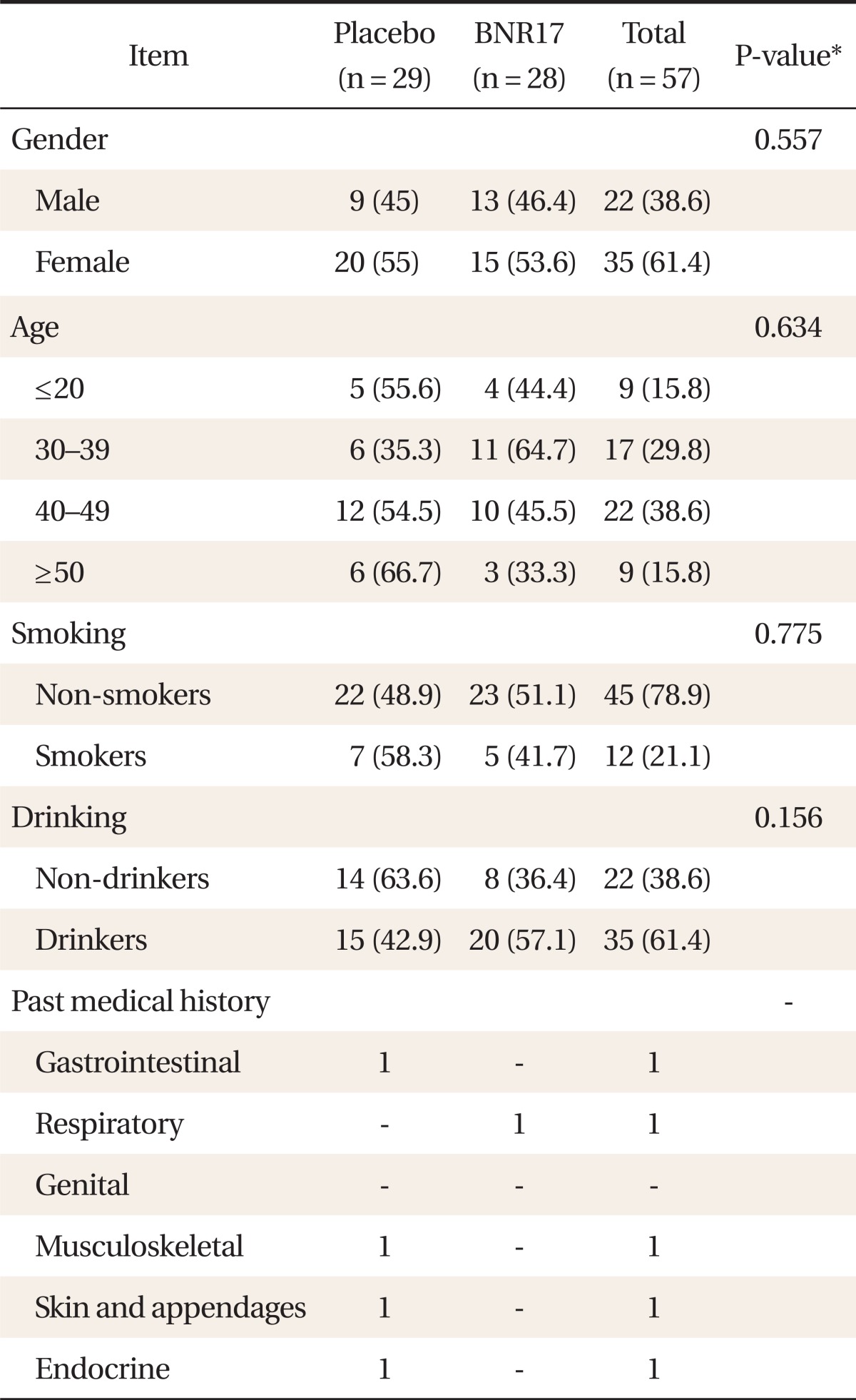
Values are presented as number (%).
*χ2-test, Fisher's exact test result.
2. Efficacy Analyses
1) Reduction of body weight
In the placebo group, body weight showed little change after the 12th week (Table 2). In contrast, within-group comparisons showed a slight decrease in body weight (-1.1 ± 2.2 kg) in the BNR17 group. In the between group, however, there were no significant changes in body weight by ITT analysis.
Table 2.
Clinical characteristics of the subjects (n = 57)
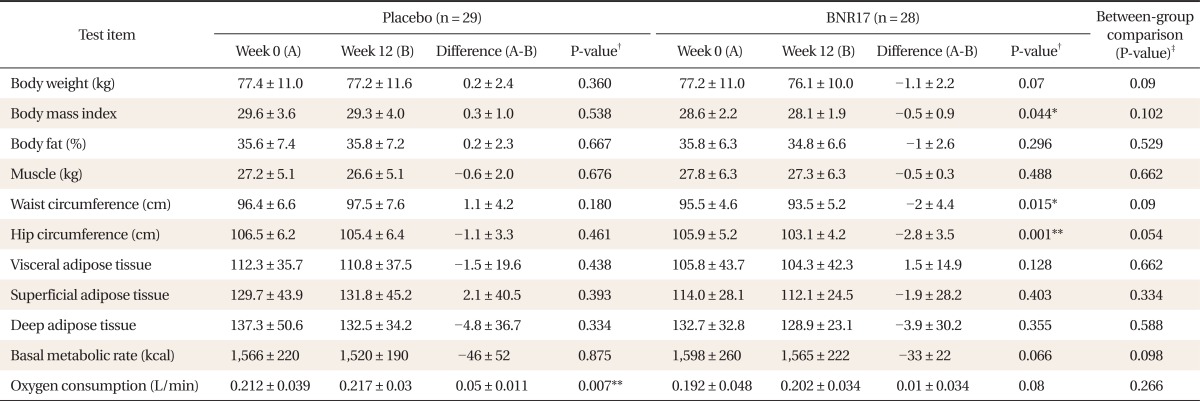
Values are presented as mean ± SD.
*P < 0.05. **P < 0.01. †t-test result for both groups compared at weeks 0 and 12. ‡Paired t-test result for between-group comparison.
2) Changes of clinical parameters
There were no significant changes in fat (%) or muscle amount (kg) (Table 2). BMI in the BNR17 group decreased after the 12th week compared with week 0 (-0.5 ± 0.9 kg; P < 0.05). A within-group comparison showed a significant reduction in waist circumference in the BNR17 group (-2 ± 4.4 cm; P < 0.05). But there were no significant changes based on the between-group differences. In both groups, there were no significant differences in visceral adipose tissue and deep adipose tissue based on between- and within-group comparisons. The placebo groups showed an increase (0.05 ± 0.011 L/min; P < 0.01) in oxygen consumption and a decrease in BMR of borderline significance (-33 ± 22; P > 0.05), respectively; however, they were not of clinical significance.
3) Changes in metabolic related parameters
The placebo group exhibited an increased level of 2PPBS of borderline significance (16.3 ± 34.1 mg/dL; P = 0.05) at week 12 (Table 3) compared with week 0; however the increase was not clinically significant. All serum lipids including total cholesterol, high density lipoprotein, low density lipoprotein (LDL), and triglyceride showed no significant clinical differences between the two groups.
Table 3.
Blood glucose, insulin, and lipid profiles between BNR17 and placebo groups at baseline (week 0) and after intervention (week 12)
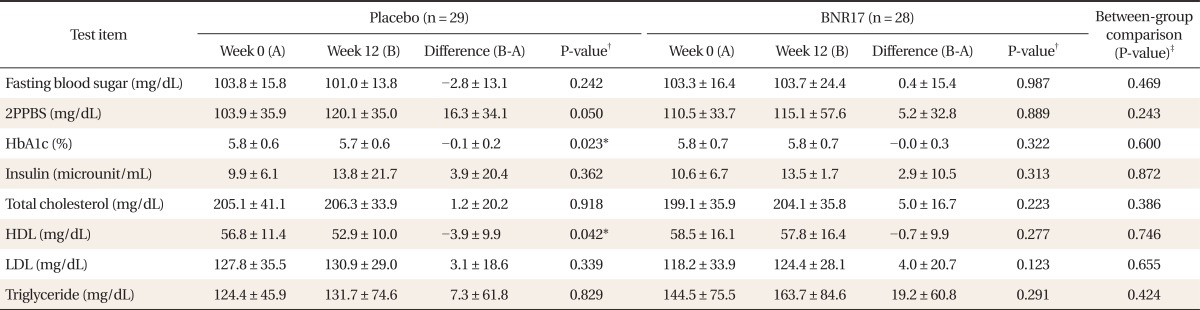
Values are presented as mean ± SD.
2PPBS: 2 hour postprandial blood sugar, HDL: high density lipoprotein, LDL: low density lipoprotein.
*P < 0.05. †t-test result for both groups compared at weeks 0 and 12. ‡Paired t-test result for between-group comparison.
3. Safety Analyses
1) Changes in blood pressure and pulse rate
There were no significant changes in systolic or diastolic blood pressure or pulse rate between groups during the study period (Table 4).
Table 4.
Blood pressure and pulse rate difference between BNR17 and placebo groups
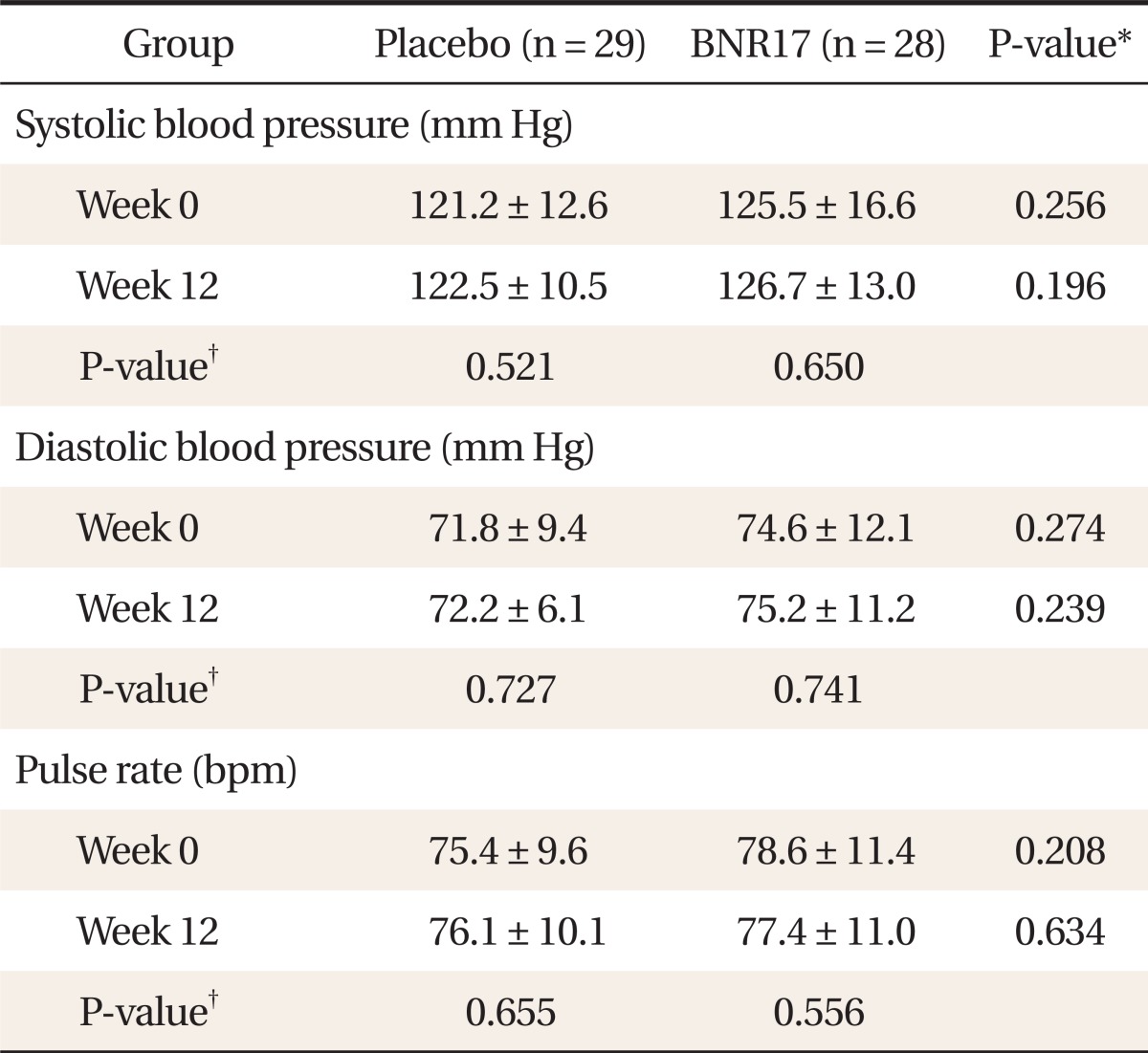
Values are presented as mean ± SD.
*t-test result for Placebo group and anti obesity microorganism group. †Paired t-test result for both groups at weeks 0 and 12.
2) Changes in hematology and blood chemistry
There were no significant changes in hematological or blood chemistry parameters except hematocrit changes in the BNR17 group (Table 5). Other adverse reactions were monitored during the course of the study. Of the 57 subjects, none reported adverse reactions directly related to the intervention. One subject in the BNR17 group reported diarrhea, and one in the placebo group had gastrointestinal symptoms (nausea) that were unrelated to the test food. No serious adverse reactions were reported.
Table 5.
Changes of blood biochemistry between BNR17 and placebo groups at baseline (week 0) and after intervention (week 12)
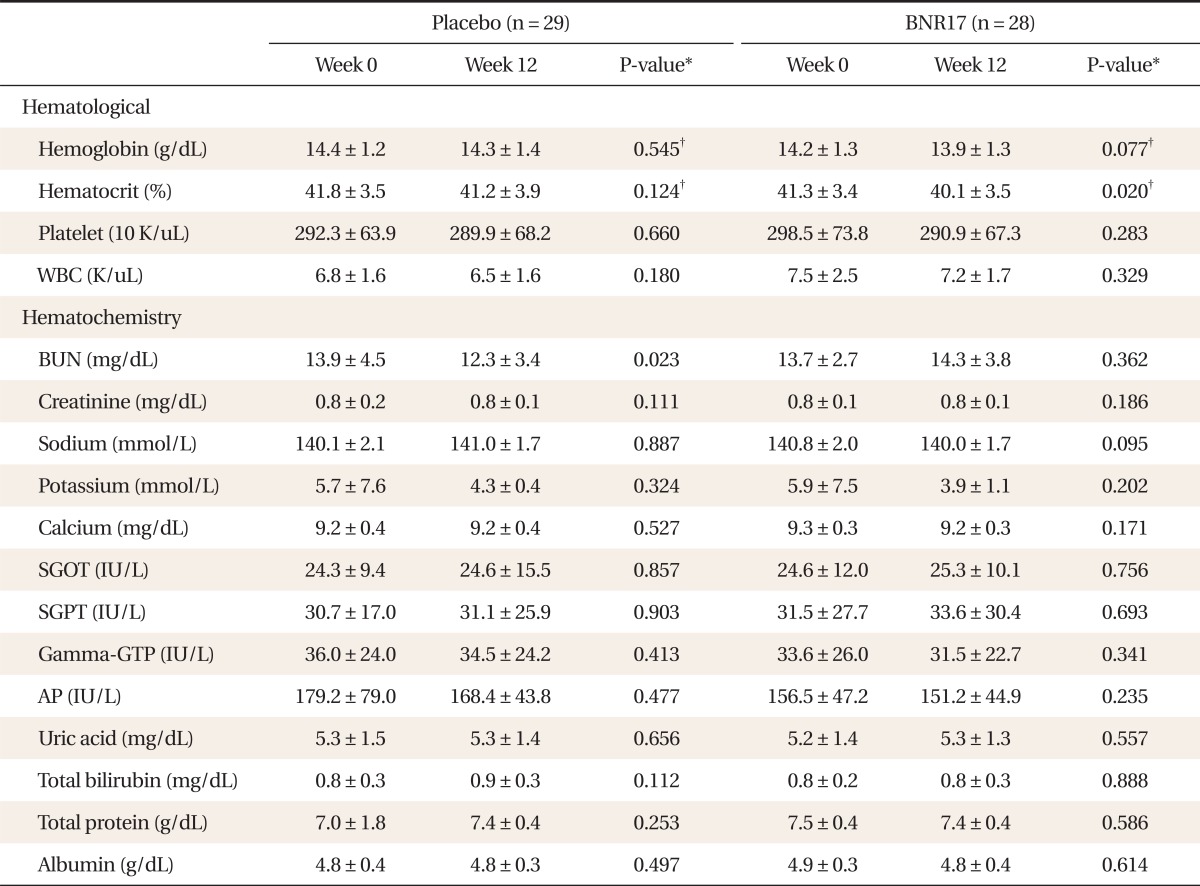
Values are presented as mean ± SD.
WBC: white blood cell, BUN: blood urea nitrogen, SGOT: alanine transaminase, SGPT: aspartic acid transaminase, Gamma-GTP: gamma-glutamyl transferase, AP: alkaline phosphatase.
*Paired t-test result for both groups at weeks 0 and 12. †Significance probability in paired t-test for change over 12 weeks.
DISCUSSION
In this study, 1010 cfu of Lb. gasseri BNR17 were given daily before meals for 12 weeks and effects on obesity-related factors were investigated. There were no significant differences in ratio of gender, age, and history of personal habits including smoking, drinking, medical treatment, or medication use between the placebo and BNR17 group.
Lb. gasseri SBT2055 also showed lowering effects on abdominal adiposity, body weight, and waist and hip circumferences in a randomized controlled human trial,19) which was similar with our results. The reduction in waist circumference is meaningful because it is a useful indicator of visceral and subcutaneous fat distribution, and is strongly correlated with atherogenic lipid profiles.24,25) However, there were no changes in visceral or superficial fat in this study.
There were no changes in diabetic indices such as FBS or insulin. In animal experiments using db/db mouse,23) however, we observed more remarkable results than in the present study; administration of BNR17 not only reduced FBS and 2PPBS but also improved tolerance to oral glucose.
It has been reported that obesity is accompanied by increased concentrations of serum total cholesterol.26,27) In many studies, reduction of serum total cholesterol or LDL-cholesterol by consumption of fermented milk containing probiotic strains was described, suggesting a hypocholesterolaemic effect.13,28) However, no reduction of cholesterol level was observed in the BNR17 group in this study, thus, it appears unlikely that BNR17 exerts a hypocholesterolaemic effect.
Administration of BNR17 reduced coliform bacterial counts in the feces of subjects. This result indicates that BNR17 suppresses the proliferation of harmful bacteria in the gut, confirming its probiotic activity. It was reported that Lb. plantarum No. 14 may exert a beneficial effect on the onset of diet-induced obesity by reducing the cell size of white adipose tissues in mice.20) They considered that the inhibition of lipid absorption is a possible mechanism for those effects. We also observed the reduction of white adipose tissue weight by the administration of BNR17 in high-fat diet-fed rats.22) Adipose tissue serves as a metabolic buffer, sequestering fatty acids in the postprandial state, and releasing them under fasting conditions.28)
It has been reported that gut microbiota have an essential role in obesity development. The mechanisms that gut microbiota may influence which affect weight gain include regulation of energy harvest from the gut,9) digestion of indigestible polysaccharides in diet,10) production or activation of signaling molecules involved in host metabolism,18) modification of gut permeability,10) and release of hormones and inflammation.21,29,30) However, whether typical probiotics such as Lb. and Bifidobacteria, which are members of gut microbiota, exert a similar effect in the human intestine is largely unknown. In a study that determined the influence of an obesity treatment program on gut microbiota and body weight of overweight adolescents,31) an increase in Lb. group counts in feces was correlated with weight loss and BMI z-score reductions in the high weight loss group, suggesting the role of this bacterial group in body weight management.
Another researcher reported that high numbers of Bifidobacteria and low numbers of Staphylococcus aureus in infancy may provide protection against obesity development.9) The feeding of mice with a high-fat diet led to increased serum lipopolysaccharides (LPS) levels and metabolic endo toxemia, resulting in an increase in proinflammatory cytokine concentration in various tissues.4) Supplementation of the high-fat diet with oligofructose resulted in increased concentration of Bifidobacteria and normalized concentration of LPS and consequently improved glucose tolerance and insulin sensitivity.10) Supplementation with probiotic strains has the same effect as although endogenous bacteria is not completely clear,8) however it seems that BNR17 may have a similar effect on energy metabolism and inflammation, because Lb. gasseri is one of the major constituents of gut microbiota.
This will be the first clinical study on the slight weight-reducing effects of Lb. gasseri BNR17, a probiotic strain originating from human breast milk, and could provide a new and safe means to manage body weight. Even though this study had limitations such as a short trial period and unevaluated indices, it still suggests that BNR17 is effective in reducing body weight in obese or overweight patients.
ACKNOWLEDGMENTS
This work was supported by a grant from the Solomon Contract Research Organization and funded by Bioneer Cooperation.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 3.van Dis I, Kromhout D, Geleijnse JM, Boer JM, Verschuren WM. Body mass index and waist circumference predict both 10-year nonfatal and fatal cardiovascular disease risk: study conducted in 20,000 Dutch men and women aged 20-65 years. Eur J Cardiovasc Prev Rehabil. 2009;16:729–734. doi: 10.1097/HJR.0b013e328331dfc0. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Report of a WHO consultation. Geneva: World Health Organization; 2000. [Google Scholar]
- 5.Saaristo TE, Barengo NC, Korpi-Hyovalti E, Oksa H, Puolijoki H, Saltevo JT, et al. High prevalence of obesity, central obesity and abnormal glucose tolerance in the middle-aged Finnish population. BMC Public Health. 2008;8:423. doi: 10.1186/1471-2458-8-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 8.Sanz Y, Santacruz A, Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc. 2010;69:434–441. doi: 10.1017/S0029665110001813. [DOI] [PubMed] [Google Scholar]
- 9.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 10.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 13.Agerbaek M, Gerdes LU, Richelsen B. Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur J Clin Nutr. 1995;49:346–352. [PubMed] [Google Scholar]
- 14.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich SD. Probiotics - little evidence for a link to obesity. Nat Rev Microbiol. 2009;7:901. doi: 10.1038/nrmicro2209-c1. [DOI] [PubMed] [Google Scholar]
- 16.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 17.Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. 2006;1761:736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobesity effect of trans-10,cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol. 2007;103:1140–1146. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 19.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 20.Takemura N, Okubo T, Sonoyama K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp Biol Med (Maywood) 2010;235:849–856. doi: 10.1258/ebm.2010.009377. [DOI] [PubMed] [Google Scholar]
- 21.Sousa R, Halper J, Zhang J, Lewis SJ, Li WI. Effect of Lactobacillus acidophilus supernatants on body weight and leptin expression in rats. BMC Complement Altern Med. 2008;8:5. doi: 10.1186/1472-6882-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48:712–714. doi: 10.1007/s12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 23.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107:1681–1686. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 24.Dourmashkin JT, Chang GQ, Gayles EC, Hill JO, Fried SK, Julien C, et al. Different forms of obesity as a function of diet composition. Int J Obes (Lond) 2005;29:1368–1378. doi: 10.1038/sj.ijo.0803017. [DOI] [PubMed] [Google Scholar]
- 25.Lu B, Zhou J, Waring ME, Parker DR, Eaton CB. Abdominal obesity and peripheral vascular disease in men and women: a comparison of waist-to-thigh ratio and waist circumference as measures of abdominal obesity. Atherosclerosis. 2010;208:253–257. doi: 10.1016/j.atherosclerosis.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Terry RB, Wood PD, Haskell WL, Stefanick ML, Krauss RM. Regional adiposity patterns in relation to lipids, lipoprotein cholesterol, and lipoprotein subfraction mass in men. J Clin Endocrinol Metab. 1989;68:191–199. doi: 10.1210/jcem-68-1-191. [DOI] [PubMed] [Google Scholar]
- 27.Ghibaudi L, Cook J, Farley C, van Heek M, Hwa JJ. Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes Res. 2002;10:956–963. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- 28.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10:306–315. doi: 10.1007/s11892-010-0122-6. [DOI] [PubMed] [Google Scholar]
- 29.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9:737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santacruz A, Marcos A, Wärnberg J, Marti A, Martin-Matillas M, Campoy C, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009;17:1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]