Abstract
In the early years of the molecular biology revolution, cancer research was mainly focused on genetic changes (ie, those that altered DNA sequences). Although this has been extremely useful as our understanding of the pathogenesis and biology of cancer has grown and matured, there is another realm in tumor development that does not involve changing the sequence of cellular DNA. This field is called “epigenetics” and broadly encompasses changes in the methylation of cytosines in DNA, changes in histone and chromatin structure, and alterations in the expression of microRNAs, which control the stability of many messenger RNAs and serve as “master regulators” of gene expression. This review focuses on the epigenetics of colorectal cancer and illustrates the impact epigenetics has had on this field.
Keywords: microRNA, DNA Methylation, Histone Modifications, Genomic Instability
Colorectal cancer (CRC) is a disease caused by genetic alterations. The best known alterations are those that change the DNA sequence: point mutations, insertion-deletion mutations, rearrangements, and so on. These types of mutations typically alter the gene product by changing the amino acid sequence of protein or by altering the quantity of protein produced. Common examples in the context of CRC include point mutations at specific codons of the KRAS gene, which abrogate its ability to be regulated, and deletions of key tumor suppressor genes such as p53 or SMAD4. In other types of cancer, amplifications of oncogenes and the inappropriate splicing of a sequence coding an oncogene so that it is controlled by a highly active promoter are prominent carcinogenic mechanisms. These aberrations dominated cancer research discoveries in the first few decades of the modern era of molecular biology.
Over the past decade, however, attention has been focused on alterations in the regulation of gene expression that do not involve a change in the DNA sequence of the cell. These are referred to as “epigenetic” changes, and the most prominent involves changes in DNA methylation. However, epigenetics can be viewed more broadly to include all of the changes in expression of genes that occur through modified interactions between the regulatory portions of DNA or messenger RNAs (mRNAs) that are not directly caused by a change in the DNA sequence. This usually occurs through changes in the promoters of genes, modification in the stability of transcripts, or alterations in the splicing of transcripts. However, these may be caused by a mutation or a sequence variation in a gene or regulatory element distant from the gene being regulated. Therefore, the borders between genetic and epigenetic alterations may blur, because minor genetic alterations or single nucleotide polymorphisms may modify DNA methylation or mRNA stabilization, change the expression of multiple other genes, and have a profound effect on cell behavior.
DNA Methylation
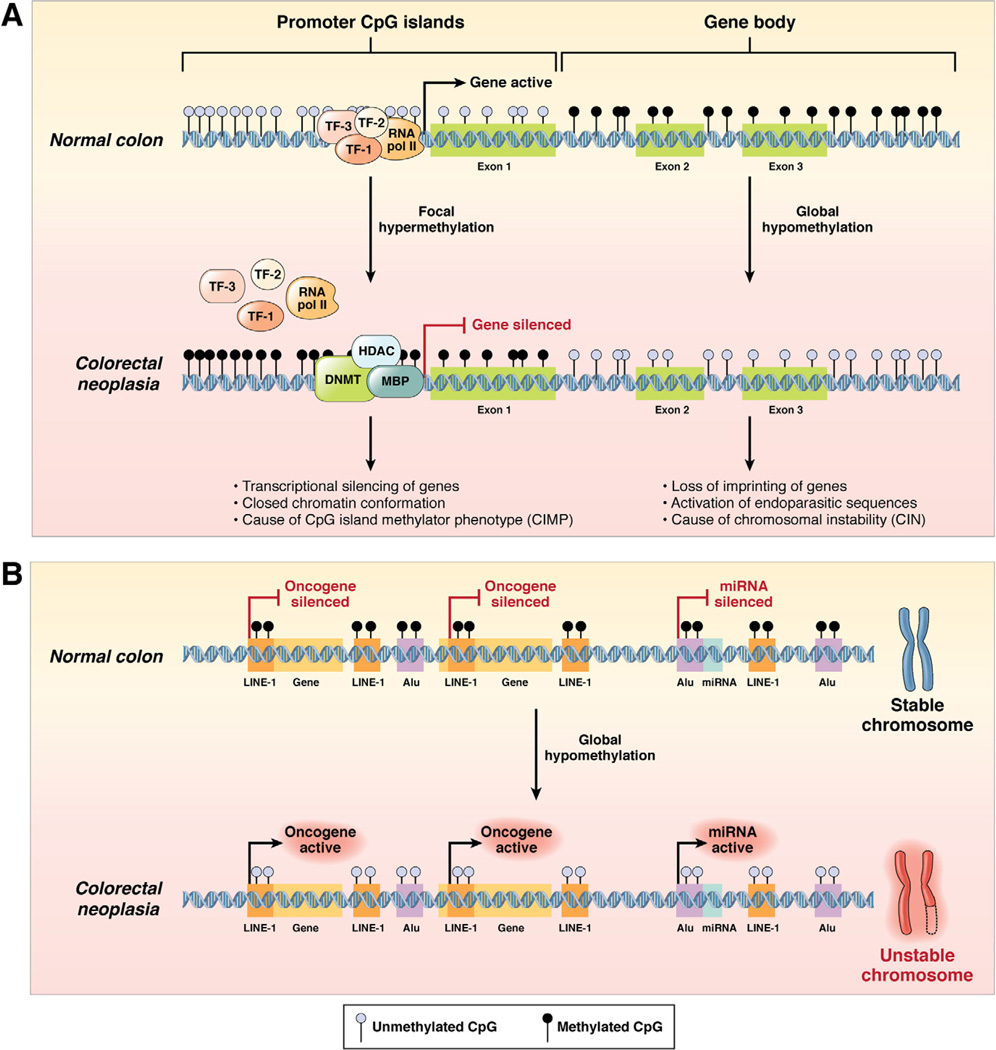
Gene expression is regulated to a considerable degree by interactions between transcription factors and the start codon (ATG) and DNA sequences immediately 5′ upstream or 3′ downstream of the start sequence. In about half of human genes, the promoter is rich in C-G sequences (called CpG sites because of the C-phosphodiester-G bond). CpG sequences are relatively absent from most other places in the genome. If a cluster of CpGs (ie, a CpG island) is encountered in a DNA sequence, it is likely to be in a genetic regulatory element. In CRC, there are examples of both hypermethylation and hypomethylation abnormalities, which are a central focus of this review (Figure 1).
Figure 1.
DNA methylation patterns in the normal colon and colorectal neoplasia. (A) The process of DNA hypermethylation-induced transcriptional silencing of tumor suppressor genes in colorectal neoplasia vis-à-vis normal colonic cells. In this instance, altered DNA methylation occurs at CpG dinucleotides either in the context of CpG-rich “promoter CpG islands” or at the abundant but sparsely distributed CpG sites throughout the “gene body.” In normal cells, CpG sites within the 5′ promoter CpG islands upstream of the transcription start site of a tumor suppressor gene are generally unmethylated (blue circles), whereas the dinucleotide repeats within the gene body are frequently methylated (black circles). This configuration permits easy and uninterrupted access by transcription factors (eg, TF-1, TF-2, TF-3) and RNA polymerase II (RNA pol II) to bind to the gene promoter and facilitate active gene expression. In contrast, in colorectal neoplasia, DNMT in association with HDAC and methyl binding proteins (MBP) catalyze the transfer of methyl groups to cytosines in the promoter CpG sites, resulting in the hypermethylation-induced transcriptional silencing of the associated tumor suppressor gene, which reflects CIMP. Concurrent with hypermethylation of gene promoters, CpG sites within the gene body experience DNA hypomethylation, which causes loss of imprinting of genes, activation of endoparasitic sequences, and chromosomal instability in CRC cells. (B) DNA hypomethylation may also occur at repetitive DNA sequences and within the promoter regions of miRNAs in colorectal neoplasia compared with normal colonic cells (see text). Evolutionarily conserved proto-oncogenes (indicated as “Gene” in the figure) and oncogenic miRNAs (indicated as “miRNA” in the figure) are not expressed in healthy normal colonic cells. Expression of these oncogenes and miRNAs is inhibited by hypermethylation of the promoter CpG sites that occurs within LINE-1 and Alu repeat elements. However, in colorectal neoplasia, LINE-1 and Alu sequences become hypomethylated, which permits the potential activation of previously silenced proto-oncogenes and oncogenic miRNAs as well as chromosomal instability.
One of the mechanisms by which a cell can completely silence gene expression is to modify the promoter region and prevent transcription factors from interacting with the DNA, diminishing gene expression. This is mediated by DNA methyltransferases (DNMTs), which catalyze the covalent addition of a methyl group to the 5′ carbon of cytosine, creating 5-methyl-cytosine, which some have called the “fifth base” of DNA. CpG islands are defined as sequences of at least 200 bases in length (usually >500) with >50% CG content and “a ratio of observed to expected CpGs that is >60%.”1, 2
Most CpG sites are maintained in a methylated state, especially those that are not in the promoter region of a gene, whereas the CpGs are not methylated in actively expressed genes. Methylation starts at one end of the CpG island and extends through the promoter and start site of the gene, which alters the 3-dimensional configuration of the DNA, inhibiting interaction with transcription factors, which silences gene expression.3 Importantly, this epigenetic modification is stably passed to the progeny of the parent cell, maintained by “histone marking” at the sites of methylation.4 Permanent silencing of genes is a prominent part of normal development, because certain genes are no longer used after the embryonic stage; moreover, maladaptive cellular behaviors may occur if certain developmental genes are inappropriately expressed in differentiated tissues. The X chromosome needs to inactivate one of its 2 copies in female mammalian cells, and it does so by completely methylating CpG sites throughout the chromosome, leading to the formation of the chromatin Barr body.5
Promoter Hypermethylation in CRC
Altered methylation of DNA was among the first of the genetic aberrations found in CRC, but the initial observations led to confusion that took nearly 2 decades to understand. The earliest study of abnormal methylation in CRC was published in 1983 by Feinberg and Vogelstein.6 The presence of 5-methylcytosine in DNA had recently been discovered, and they hypothesized that there may be differences in DNA methylation between colon cancer tissues and normal tissues from which the tumor arose. They used the restriction enzymes HpaI and HpaII, which can discriminate between methylated and unmethylated CpG sites. After digestion, the DNA from 4 CRC samples harbored a significantly larger number of hypomethylated fragments compared with the normal colon at 3 different genes (γ-globin, α-globin, and human growth hormone). They found a similar phenomenon in one case of lung cancer and noted that hypomethylation was more pronounced in the metastatic tumor from that individual. Although they did not propose that these genes were directly involved in tumorigenesis, they proposed that these 3 genes, located in 3 different genomic locations, were substantially hypomethylated and that the process was possibly progressive in metastasis. They later demonstrated that the hypomethylation was present in adenomatous polyps, indicating that it occurred in the early stages of colorectal neoplasia7 and that there was an 8% to 10% reduction in 5-methylcytosine content in human colorectal adenomas and cancers compared with normal mucosa.8
Hoping to find specific “cancer-related” genes altered by this type of epigenetic alteration, in 1986, Baylin et al examined methylation of the promoter of the calcitonin gene in lung and other cancers and found quite the opposite: hypermethylation.9 The functional significance of this was unclear at the time. It did not take long to discover that hypermethylation occurred in the promoter sequences of many genes in cancers, including critical tumor suppressor genes, which resulted in loss of gene expression.10–13 A catalogue of genes silenced by promoter hypermethylation accumulated. In 1999, Baylin, Issa, and others coined the term the “CpG island methylator phenotype,” or CIMP, for the phenotype in which tumor suppressor genes were methylated, and tumorigenesis occurred— at least in theory—through progressive genetic silencing, possibly even in the absence of any genetic mutations.14 Methylation is common at the PTEN, RUNX3, and UNC5C loci in CRC, making these key genes “targets” for silencing in the evolution of a CRC with CIMP.15–17 The recognition that a diverse spectrum of genes with an important role in CRC are targets of aberrant methylation (as reviewed in Supplementary Table 1) represented a significant step forward in the understanding of colorectal tumor formation and a paradigm shift for a broader understanding of how cancer evolves.
Table 1.
MicroRNAs and Their Target Genes in the Pathogenesis of CRC
| miRNA | Up-regulated or down-regulated in CRC |
Gene target | Reference |
|---|---|---|---|
| miR-16 | Up-regulated | p53 | 117 |
| miR-17 | Up-regulated | PTEN, DLC1, ZBP1 | 91 |
| miR-17-92 cluster | Up-regulated | E2F1a | 108 |
| miR-18a | Up-regulated | KRASa | 168 |
| miR-20a | Up-regulated | PTEN, RUNX1, TP53INP1 | 91 |
| miR-21 | Up-regulated | PTENa, PDCD4a,RECKa,TPM1a, SPRY2a, TIMP3a | 90,113–115,169–171 |
| miR-29a | Up-regulated | DNMT3a, DNMT3ba,MCL1a | 91,172 |
| miR-31 | Up-regulated | BMP2 | 91 |
| miR-32 | Up-regulated | BTG2a | 173,174 |
| miR-92 | Up-regulated | p63 | 91 |
| miR-93 | Up-regulated | AID | 173 |
| miR-96 | Up-regulated | KRAS | 87 |
| miR-106a/b | Up-regulated | E2F1, p21 | 90 |
| miR-122a | Up-regulated | MSH2, APCa | 175 |
| miR-135a/b | Up-regulated | MSH2, APCa | 106 |
| miR-155 | Up-regulated | MLH1a,MSH2a,MSH6a | 176 |
| miR-181b | Up-regulated | CyclinD, VSNL1 | 113 |
| miR-182 | Up-regulated | IGFR1 | 91 |
| miR-183 | Up-regulated | EZRIN | 91 |
| miR-203 | Up-regulated | SOX2, KLF4, BMI1 | 90,110 |
| miR-223 | Up-regulated | PTEN, RECK, ST5 | |
| miR-224 | Up-regulated | SMAD4 | 109 |
| miR-196b | Up-regulated | HOXB8 | 91 |
| miR-451 | Up-regulated | MIFa | 178 |
| miR-675 | Up-regulated | pRB | 111 |
| let-7 | Down-regulated | KRASa, cMYC | 179 |
| miR-9 | Down-regulated | TCF4, MSH2 | 96,97 |
| miR-34a/b/c | Down-regulated | CDK4a,CDK6a,E2F3a,CyclinE2a | 97,180 |
| miR-101 | Down-regulated | COX2a | 181 |
| miR-126 | Down-regulated | PIK3C2A, CRK, PGR | |
| miR-129 | Down-regulated | NOTCH1, CAMTA1 | 87 |
| miR-137 | Down-regulated | LSD1a, MITF, CDC42 | 87,101 |
| miR-139 | Down-regulated | β-CATENIN | 183 |
| miR-141 | Down-regulated | TGFβ1, SIP1 | 184 |
| miR-143 | Down-regulated | DNMT3aa,KRASa,ERK5a,p53 | 88,89,117,118,122,123 |
| miR-145 | Down-regulated | IRS-1a, cMYC, YES1, STAT1, OCT4, SOX, KLF4, p53 | 88,89,116–121 |
| miR-192 | Down-regulated | DHFR, TS, TYMS | 110,185,186 |
| miR-195 | Down-regulated | BCL2 | 187 |
| miR-200a/b/c | Down-regulated | ZEB1a,ZEB2a | 125,188 |
| miR-215 | Down-regulated | DHFR, TS, TYMS | 186 |
| miR-320 | Down-regulated | PTENa | 88,189 |
| miR-342 | Down-regulated | DNMT1 | 190 |
| miR-491 | Down-regulated | BClXL | 191 |
A genetic target that has been validated for the specific miRNA.
The earliest reports suggested that there were 2 types of CIMP: one associated with aging (CIMP-A) and one associated with cancer (CIMP-C).18, 19 Although there is a progressive increase in methylation of the promoters of the estrogen receptor and other genes as we age, there is not a sharp distinction between the genes methylated in aging and those involved in cancer.20 There is a close relationship between mutations in the BRAF gene (particularly the V600E mutation) and CIMP, but it has not been shown that BRAF mutations can cause CIMP. Importantly, the sporadic cases of microsatellite instability (MSI) are usually associated with BRAF mutations, whereas this is almost never the case with Lynch syndrome CRCs.21–23 Most CRCs are believed to begin as sporadic adenomas, but some proportion of CRCs with CIMP begin as sessile serrated adenomas, suggesting that there is a novel “pathway” for the evolution of colorectal neoplasia associated with CIMP.24, 25 There is no understanding of what causes BRAF mutations or CIMP, but there is an association between CRCs with CIMP and both older age and smoking.26, 27 Moreover, both CIMP and BRAF mutations are associated with poorer clinical outcomes and a poorer response to 5-fluorouracril (5-FU)–based adjuvant chemotherapy.28, 29 Although the final analysis is still evolving, methylation of the MLH1 gene is associated with resistance to 5-FU, which is reversed on demethylation of that gene in vitro.30 Clinical analyses suggest that patients with MSI CRCs are not candidates for 5-FU–based adjuvant chemotherapy.31–33
It has been shown that CRCs with CIMP are distinct from those with chromosomal instability and that these 2 forms of nuclear derangement represent 2 alternative pathways for the development of CRC.34, 35 There is a degree of pathway overlap, because hypermethylation can occur in the APC gene, which is part of the chromosomal instability pathway,36 or the MLH1 gene, triggering MSI.37 Furthermore, methylation of the MGMT gene occurs during progression of CRC in either pathway and may facilitate the accumulation of point mutations as tumors evolve.38
MSI, Hypermethylation of the MLH1 Promoter, and Lynch Syndrome
The recognition that CIMP occurred in CRC and other tumors did nothing to contradict the genetic processes previously attributed to colorectal carcinogenesis; it simply made it more complicated. Interestingly, one of these “targets” of hypermethylation—the MLH1 gene—at once simplified and complicated the picture.
The MLH1 gene is one of the DNA mismatch repair genes, and germline mutations in MLH1 account for approximately 40% of cases of the hereditary CRC disease called Lynch syndrome.39 Loss of DNA mismatch repair (which occurs when there is biallelic inactivation of MLH1, for example) creates the distinctive mutational signature in DNA called MSI. Approximately 15% of all CRCs have MSI, but only about 3% of all CRCs are attributable to Lynch syndrome. It was discovered that almost all of the CRCs with MSI that are not due to Lynch syndrome have hypermethylation of the promoter of the MLH1 gene.21, 40 These tumors occur in patients who are, on average, much older than patients with Lynch syndrome. The mutational signature in Lynch syndrome CRCs is overwhelmingly dominated by MSI. CRCs with acquired methylation of MLH1 have a background epimutational signature dominated by CIMP (ie, numerous genes have been silenced by methylation); when the MLH1 gene is silenced, the tumors develop MSI secondarily. Not surprisingly, the clinical features of these 2 types of tumors, both characterized by MSI and loss of MLH1 expression, have some degree of overlap but are fundamentally distinct.29
The methylation of MLH1 in CRC appears to occur randomly as a consequence of CIMP. Most CRCs with CIMP seem to occur through the serial silencing of tumor suppressor genes, although details about the sequence and time course by which this occurs are still incomplete. 41
Methylation of MSH2 in CRC
In 2006, it was first reported that heritable methylation of the promoter of MSH2 could also cause Lynch syndrome.42 This was a difficult concept, because it is generally noted that methylation is “erased” during embryogenesis, and it was not clear how this might occur. In 2009, Ligtenberg et al found that Alu-mediated deletions of the stop codon of the EPCAM gene, which is immediately 5′ upstream of the start of MSH2, result in the somatic methylation and silencing of MSH2 in those tissues that express EPCAM.43 Moreover, a deletion that removes the stop codon in EPCAM while retaining the promoter and start regions of MSH2 creates a disease that is clinically a CRC-predominant familial cancer syndrome that will look like Lynch syndrome–MSH2 type but in which a genetic alteration in the MSH2 gene cannot be detected.44 Additionally, methylation of MSH2 is not uncommon in Lynch syndrome tumors and appears to represent the second hit at that locus in some instances.45
Soma-wide or Constitutional Hypermethylation of MLH1
Patients with a CIMP CRC are more likely to have a positive family history of CRC.46 In 2002, an individual from a familial CRC cluster was found to have a methylated promoter of MLH1, but no inheritance of the epimutation was found.47 Hitchins et al studied a cohort of 160 probands from familial clusters of CRC and found one individual who had monoallelic methylation of MLH1 in the blood, buccal mucosa, and hair follicle DNA, and the methylated allele was silenced.48 The methylated allele was maternally inherited, but the patient’s mother and the maternal allele in a sister did not show promoter methylation, indicating that the aberrant methylation was acquired, not inherited. The same group reported that this “epimutation” (ie, the hypermethylation of MLH1) had been passed from mother to son and was detected in the son’s spermatozoa.49 The inheritance of a rare single nucleotide polymorphism in the 5′ untranslated region of the MLH1 promoter (c.−27 C>A) has been linked to constitutional silencing of MLH1.50 This has not been confirmed by other laboratories and, in any event, is quite uncommon.
The presence of soma-wide or constitutional hypermethylation of MLH1 has been reported in a series of 6 affected individuals with 13 tumors.51 The methylated allele can be inherited from either parent (even though the parents do not have the same epimutation) and is present in endodermal, mesodermal, and ectodermal cell lineages, and the affected patients act as though they have Lynch syndrome with early-onset CRCs and recurrent independent tumors.
CIMP High, CIMP Low, and CIMP Negative
The promoters of more than half of human genes are in CpG islands, so the frequency of CIMP might depend on which promoters are examined for methylation. Some promoters are more valuable than others for identifying CIMP. It is possible to classify tumors according to the proportion of promoters that exceed a threshold degree of DNA methylation (reviewed by Ogino and Goel52). CRCs with mutations in BRAF and methylation in a panel of CIMP-specific markers can be used to identify CRCs that are CIMP high, CIMP low, and CIMP 0.53 CIMP-high CRCs are significantly associated with BRAF mutations and female sex, CIMP-low CRCs with KRAS mutations and male sex, and CIMP-0 CRCs with wild-type BRAF and KRAS genes. This classification needs additional refinement, but it is reminiscent of the process by which CRCs were divided into groups according to high, low, and absent degrees of MSI.39
Global Hypomethylation of DNA in CRC
Much of the attention from the mid-1980s until after 2000 was focused on promoter hypermethylation and CIMP in the context of CRC. However, the initial observation of global hypomethylation, which is unrelated to CIMP, remained a more pervasive problem in the biology of CRC. Cells cultured over an extended period in the presence of a demethylating agent such as 5-azacytidine will develop aneuploidy.54 CRCs with chromosomal instability tend to be hypomethylated, and most of those with MSI are hypermethylated (due to methylation of MLH1); a third group without chromosomal instability or MSI has a unique methylation pattern and clinical features, including a better prognosis. This group may represent yet another “pathway” for the development of CRC.55 In addition, tumors from familial clusters of CRC that are not Lynch syndrome and do not have MSI show a significant degree of LINE-1 hypomethylation, which is a marker of global hypomethylation56 (Figure 1).
Histone Modifications in CRC
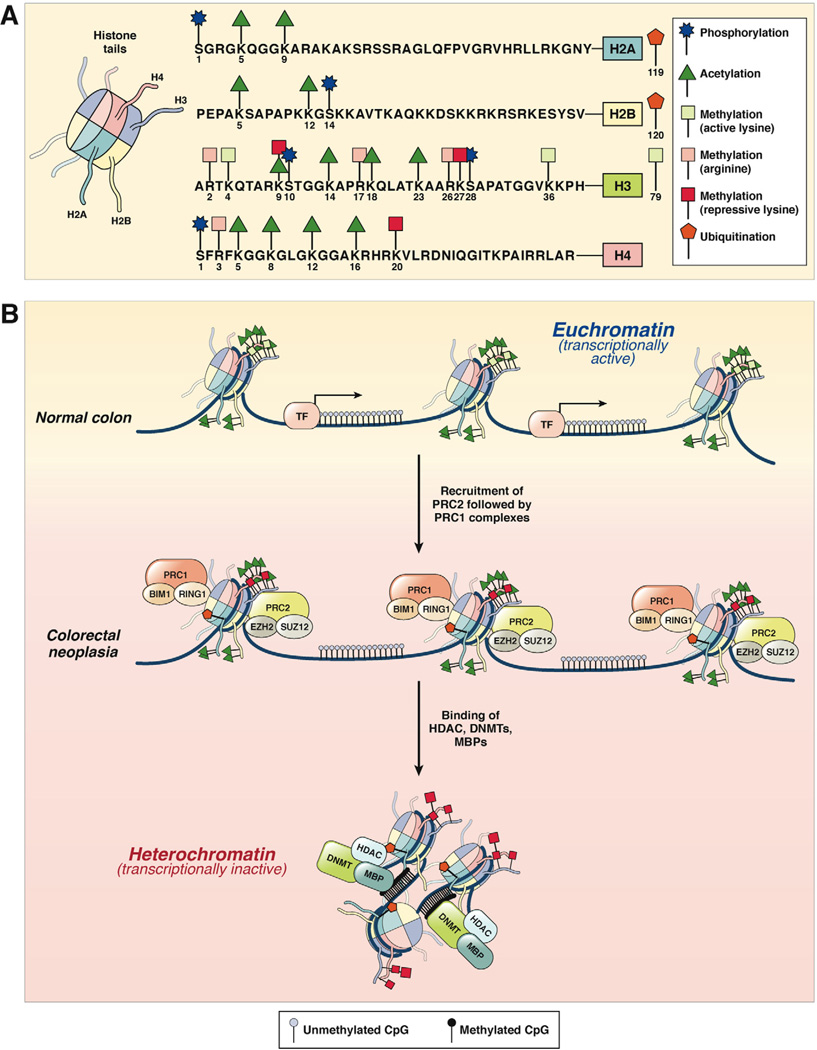
In addition to DNA methylation-induced transcriptional control of gene expression, posttranslational covalent modifications at histone tails constitute an epigenetic mechanism that regulates chromatin structure and gene expression in human cancers. However, in contrast to methylation-associated alterations, the understanding of histone modification patterns in human cancers is limited. Histone modifications typically occur at 4 histones (H2A, H2B, H3, and H4) that are organized in cylindrical structures and constitute the histone core. The nucleosome is a chromatin unit composed of 150 to 200 base pairs of DNA tightly wrapped around the cylindrical histone core. Histone N-terminal tails that protrude from this compact structure are frequent targets of posttranslational modifications, including phosphorylation, methylation, acetylation, ubiquitinylation, sumoylation, deamination, and ribosylation.57 The impact of histone modification is governed by 2 factors: the type of modification and the specific amino acid involved. Collectively, posttranslational modifications of histones establish a dynamic and potentially reversible “histone code” that permits active transcription in a euchromatin configuration but inactive transcription when chromatin is in a heterochromatin state (Figure 2).
Figure 2.
(A) Histone modification patterns in colorectal neoplasia. The 4 histone proteins (H2A, H2B, H3, and H4), each with 2 copies, associate in cylindrical structures that constitute the histone core, and each nucleosome is a chromatin unit composed of 150 to 200 base pairs of DNA tightly wrapped around the octameric histone core. Covalent modifications of each histone tail, including acetylation, methylation, phosphorylation, and ubiquitination, are also shown on the right. The amino acid sequences of the N-terminal tails and the key selected sites of modification of these 4 histones are shown. (B) In normal colon, histones are arranged in the open, euchromatin configuration, which represents transcriptionally active chromatin (upper panel). Histone tails may undergo multiple modifications, which include histone acetylation (green triangles) and lysine methylation (green squares). Gene promoters in euchromatin allow easy access for transcription factor (TF) binding, permitting active gene expression. In CRC cells, chromatin configuration is converted to the more compacted heterochromatin configuration, which is transcriptionally inactive and results in the hypermethylation-induced silencing of genes. This process is facilitated by the multimeric polycomb repressive protein complexes, PRC2 and PRC1, which sequentially bind to histones and initiate histone methylation (initiated by PRC2 complex containing EZH2 and SUZ12) followed by maintenance methylation and monoubiquitination (performed by PRC1 complex with BIM1 and RING1 proteins). This is followed by the recruitment of DNMTs, HDACs, and methyl binding proteins (MBPs) to complete the PRC-mediated transcriptional silencing of genes.
The consensus is that hypoacetylated loci typically silence gene expression whereas hyperacetylated histones are involved in gene activation. Acetylation of histone tail residues destabilizes the chromatin fiber, which permits enhanced mobility of nucleosomes along the chromosome and less impeded access of transcription factors to DNA.58 Although histone acetylation is mediated by histone acetyltransferases and histone deacetylases (HDACs), histone methylation is catalyzed by histone methyltransferases and histone demethylases.59
Polycomb Group Proteins and Histone Modifications in Cancer
The best understood histone modifications in CRC are acetylation/deacetylation and methylation/demethylation of lysine and arginine residues within histone tails. It has been shown that while dimethylation and trimethylation of histone H3 lysine (H3K4me2/me3) and acetylation of H3/H4 (H3K9Ac and H4K9Ac) amino acids constitute transcriptionally active marks, transcriptionally inactive gene promoters are frequently characterized by trimethylation of histone H3 lysine 9 and 27 (H3K9me3 and H3K27me3) residues.60 These bivalent histone modifications are mediated by transcriptional repressors, the polycomb group proteins, that are instrumental in silencing a specific group of tumor suppressor genes in human cancers.61 Two multimeric polycomb repressive complexes (PRCs), PRC1 and PRC2, can silence genes either independently or synergistically. The PRC2 complex is responsible for initiating methylation of histone H3 (H3K27me2/3) through its enzymatic subunits EZH1 and EZH2, whereas the PRC1 complex is involved in the maintenance of H3K27me2/3 silencing, as well as subsequent monoubiquitination of Lys 119 in histone H2A (H2AK119ub) via the ubiquitin ligases RING1A and RING1B.62 While the core PRC2 multimeric complex is composed of 4 components—EZH1/2, SUZ12, EED, and RbAp46/48 (or RBBP7/4)—the composition of PRC1 complexes is more variable with only 2 core common components: RING1A/B, together with BMI1, HPC/CBX, HPH, and YY162 (Figure 2).
PRC-mediated transcriptional silencing has been hypothesized to play a role in CRC,63 because many genes that are frequently hypermethylated in CRC are polycomb group targets. Both PRC1 and PRC2 proteins interact with DNA methyltransferases (DNMT1 and DNMT3b), establishing a potential key role for these proteins in catalyzing methylation-associated transcriptional silencing of target genes in cancer cells.64–66 Also, EZH2 is frequently overexpressed in CRCs67, 68 and predicts better recurrence-free survival in patients with CRC.67 Additionally, RNA interference–mediated intracellular depletion of EZH2 induces cell cycle arrest, inhibits CRC cell growth, and leads to reduced expression of several cancer-associated genes involved in proliferation or invasion, including Dag1, MageD1, SDC1, Timp2, and Tob1.69
Histone Modifications and DNA Methylation Cooperatively Induce Epigenetic Gene Silencing
In 1990, elevated c-Myc expression in 44% of CRCs was proposed as a consequence of a transregulatory mechanism that was controlled by an S-phase–specific histone H3 gene.70 A dozen years later, a relationship was noted between key histone code components and hypermethylation- induced silencing of the MLH1 gene in CRC cells.71 Deacetylation (H3K9) and simultaneous methylation of histone H3 (H3K9me2) in the MLH1 promoter was a key event mediating epigenetic inactivation of this gene, and treatment with the DNMT inhibitor 5-azacytidine led to complete reversal of MLH1 methylation and the corresponding histone code.71 These studies established that DNA methylation and histone modifications act in concert. This concept was consolidated in a study showing that elimination of DNA methylation from a p16INK4a gene altered the surrounding histones, and with additional passage of cells in the absence of the DNMT inhibitor, remethylation of p16INK4a occurred in conjunction with methylation of the histones in CRC cells.72 Furthermore, hypomethylation induced by DNMT inhibitors is insufficient to induce gene expression. Chromatin resetting, which includes nucleosome eviction, is also required for the activation of protein expression in SW48 CRC cells.73 These observations suggest that reversal of methylation alone may not be sufficient to reactivate methylationsilenced genes in human cancers, and this should be taken into consideration while designing epigenetic therapeutic regimens for cancer treatment in the future.
Altered Expression of Histone-Modifying Enzymes in CRC
Multiple class I HDACs are up-regulated in a subset of CRCs, including HDAC1 in 36.4%, HDAC2 in 57.9%, and HDAC3 in 72.9% of specimens.74 Furthermore, the expression was enhanced in strongly proliferating and dedifferentiated tumors, and high HDAC expression levels were associated with reduced patient survival.74 Over-expression of HDAC2 was observed in 81.9% of colorectal adenomas, 62.1% of CRCs, and 53.1% of normal tissues.75 The overexpression in HDAC2 accompanied hypoacetylation at H4K12 and H3K18 histones during adenoma-to-carcinoma progression, suggesting that increased HDAC2 expression and subsequent loss of acetylation may accompany progression of CRC.75 Likewise, the HDAC1 and 2 histone acetyltransferase–related proteins CREB-binding protein and p300 are overexpressed in CRC tissues.76 Furthermore, up-regulation of p300 correlated with a poor prognosis, whereas high CREB-binding protein expression levels correlated with long-term survival.76 The class III HDAC, SIRT1, is an important epigenetic regulator of gene expression, and inhibition of SIRT1 can lead to reactivation of transcriptionally silenced genes, suggesting a possible therapeutic approach to reverse aberrantly silenced genes. In a study of 485 CRCs, it was observed that SIRT1 was overexpressed in ~40% of CRCs, which was associated with CIMP-high and MSI-H tumors, providing insights into the link between SIRT1 expression and gene silencing in CRC.77
MicroRNAs and CRC
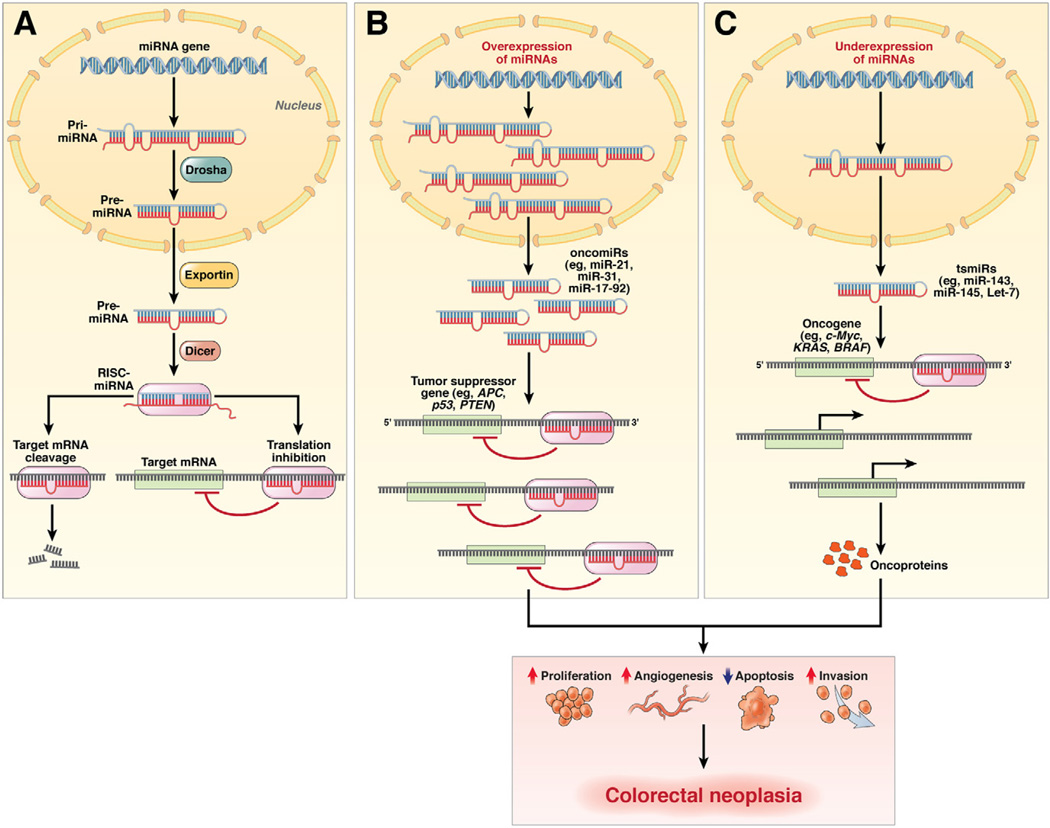
Noncoding RNAs, particularly microRNAs (miRNAs or miRs), are mechanistically involved in controlling the expression of various cancer-associated genes, and their expression may be altered in cancer. miRNAs are single-stranded, evolutionarily conserved, small RNA molecules (19–25 ribonucleotides) that mediate posttranscriptional gene repression. Typically primary miRNA genes are transcribed by RNA polymerase II in the nucleus, processed to precursor miRNAs by Drosha, and transported to the cytoplasm, where they undergo further processing by the ribonuclease III enzyme Dicer, resulting in mature miRNAs that are incorporated into the RNA-induced silencing complex. miRNAs act as endogenous suppressors of gene expression through imperfect binding of the RNA-induced silencing complex to 3′ untranslated regions of target mRNAs and induce either mRNA degradation or translational repression. Analogous to genes, miRNAs can act either as tumor suppressors (tsmiRs) by inhibiting the expression of oncogenes or as tumor promoters (oncomiRs) by suppressing the expression of target tumor suppressor genes78 (Figure 3).
Figure 3.
miRNA genesis in normal colon and their role as oncogenic and tumor suppressive genes in colorectal neoplasia. (A) In normal cells, primary miRNA transcripts are processed into precursor miRNAs by an enzymatic complex that includes the nuclear ribonuclease III enzyme Drosha. The resulting precursor miRNA is transported to the cytoplasm by exportin, where it is processed into ~22-nucleotide duplexes by the ribonuclease III enzyme Dicer. The strand corresponding to the mature miRNA is subsequently loaded onto the RNA-induced silencing complex (RISC). Mature miRNAs bind to the 3′ untranslated regions of target mRNAs and can suppress their expression through translational inhibition, degradation of the target miRNA, or both. (B) In cancer cells, miRNAs can act as oncogenes (or oncomiRs) and can be overexpressed during different stages of cancer development. Overexpression of specific oncomiRs in colorectal adenomas and cancers (eg, miR-21, −31, −17, −92) results in targeting and suppression of their target tumor suppressor genes (eg, APC, p53, PTEN). (C) Likewise, miRNAs may act as tumor suppressors (or tsmiRs), in which these miRNAs are down-regulated in CRC cells (eg, miR-143, -145, let-7) and are unable to block the expression of their target oncogenes completely (eg, c-Myc, KRAS, BRAF). The overall consequence of oncomiR and tsmiR regulation in cancer cells might involve increased proliferation, increased invasiveness or angiogenesis, and decreased apoptosis, all of which facilitate the development of colorectal neoplasia.
miRNAs were first discovered in worms, and at the time of this review, ~1950 human miRNAs have been described in release 18 of the Sanger Institute miRBase (www.mirbase.org).79 Cross-species comparisons have shown that miRNAs are evolutionarily conserved. Although the biology of miRNAs is still unclear, each miRNA can regulate the expression of several mRNA targets, making them master regulators of gene expression.
The Role of miRNAs in Cancer
Since the discovery of miRNAs in patients with chronic lymphocytic leukemia in 2002,80 evidence has implicated their role in multiple human diseases, prominently including cancer.81 Based on in silico predictions of miRNA/mRNA interactions, the expression of up to 5300 human genes (representing 30% of the human gene pool) may be controlled by various miRNAs.82 Cancer-associated alterations in miRNA expression occur in a tissuespecific manner, providing the basis for the development of highly robust molecular signatures to classify various human malignancies.83, 84 Relatively minor variations in miRNA expression can have important consequences because of the large number of targets modulated by each miRNA.78 Also, global miRNA expression patterns can distinguish different tissue and tumor types better than mRNA expression patterns, making them attractive targets for their development as cancer biomarkers.83
Altered miRNA Expression in CRC
Soon after the recognition of the involvement of miRNAs in human leukemia, 2 miRNAs, miR-143 and miR-145, were reported to be significantly down-regulated in colorectal adenomas and cancers compared with normal colon.85 Serial analysis of miRNA gene expression (miRAGE) led to the development of microRNAome signatures that identified 133 novel miRNAs in CRC and reconfirmed that miR-143 and miR-145 were significantly reduced in colorectal neoplasia.86 Subsequent functional studies have revealed that miR-143 and miR-145 act as tumor suppressor miRNAs in the colon.87–89
The introduction of high-throughput microarray-based miRNA profiling platforms revolutionized the field of miRNA expression pattern analysis. The list of aberrantly expressed miRNAs in colorectal adenomas and cancers is continuously growing, but a list of key miRNAs and their gene targets involved in colorectal carcinogenesis is shown in Table 1.
One of the first comprehensive array-based analyses of 389 miRNAs in 84 CRC and matched normal colonic tissues identified 37 miRNAs differentially expressed in CRC, including the overexpression of miR-20a, miR-21, miR-106a, miR-181b, and miR-203 in tumor cells.90 In another report, miR-31, miR-183, miR-17-5, miR-18a, miR-20a, and miR-92 were expressed at significantly higher levels in CRC than normal tissues, while miR-143 and miR-145 were expressed at lower levels in tumors.91 It is beyond the scope of this article to elaborate on all studies that described variations in miRNA expression profiles in CRC cell lines or clinical specimens, but a high degree of concordance exists among these studies, regardless of whether candidate-based or array-based approaches were used.84, 91, 92
miRNAs and Distinct Phenotypic CRC Subgroups
CRCs are composed of various subtypes that can be categorized based on pathological features, familiality, location in the bowel, and so on. Although the functional details on the biological significance of miRNAs are still evolving, expression profiling studies have provided substantial evidence that miRNA expression patterns are unique and characteristic for some phenotypically different subtypes of CRCs. This is of clinical importance because CRCs with MSI and CIMP have significant differences in clinical outcome, including prognosis and therapeutic response to conventional chemotherapy.31 For instance, genome-wide miRNA profiling of 23 microsatellite stable (MSS) and 16 MSI CRCs showed that a subset of 8 miRNAs, which included various members of the oncogenic miR-17–92 family, reliably distinguished MSI from MSS CRCs.93 A gene signature of only 4 miRNAs (miR-142-3p, miR-212, miR-151, and miR-144) was able to successfully discriminate between MSI and MSS stage II CRC.88 A 6-gene panel was identified that was differentially expressed between mismatch repair (MMR)- deficient and MMR-proficient CRCs.94
A study from our group analyzed genome-wide miRNA signatures in 35 MSI CRCs (22 Lynch syndrome and 13 sporadic) and 19 MSS CRCs, as well as 20 normal colonic tissues.95 We identified patterns of miRNA expression that distinguished normal tissues from cancers and MSI from MSS tumors, but more importantly, we reported that expression of miR-622, miR-1238, and miR-192 could discriminate between Lynch syndrome MSI and sporadic MSI CRCs. In addition, the expression patterns of miRNAs were significantly similar between patients with Lynch syndrome who had identifiable mutations in DNA MMR genes and patients with Lynch syndrome in whom available mutation screening methods failed to identify the underlying mutation. This approach provided a diagnostic miRNA signature where the best available diagnostic tests failed, providing a possible alternative strategy to determine patient management.95
Epigenetic Mechanisms for miRNA Dysregulation in CRC
The mechanisms responsible for dysregulated expression of miRNAs in human cancers are still poorly understood. One mechanism that has received recent attention is aberrant methylation of the miRNA gene promoters when they are located in or near CpG islands. Aberrant hypermethylation of miR-34b, miR-34c, miR-9-1, miR-129-2, and miR-137, all of which are embedded in CpG islands, is associated with reduced expression in CRC cell lines and tumor tissues.96–98 By comparing miRNA expression and histone modifications (H3K4me3, H3K27me3, and H3K79me2) before and after DNA demethylation, 47 miRNAs, including miR-1-1, were found to be potential targets of epigenetic silencing in early and advanced CRCs.99 DNA demethylation at these miRNA promoters resulted in up-regulation of H3K4me3 and H3K27me3 at the promoters of these miRNAs, providing additional insight into the association between hypermethylation, chromatin modifications, and miRNA dysregulation in cancer.
Simultaneous methylation of EVL and miR-342, an intronic miR in the EVL gene, was seen in 86% of colorectal adenocarcinomas and in 67% of adenomas, indicating that aberrant methylation at this locus is an early event in colorectal carcinogenesis; furthermore, increased methylation is found in the normal mucosa adjacent to CRC and in some colons not associated with CRC, suggesting a “field effect.”100 Our group has reported that methylation of the miR-137 CpG island is a more cancer-specific event, observed in virtually all CRC cell lines, 82% of adenomas, and 82% of CRCs but in only 14% of normal mucosae from patients with CRC and 5% of healthy subjects.101 Using systematic microarray and bioinformatics approaches, we identified LSD1, a histone demethylase, to be a target of miR-137 in the colon.
Alterations in the expression of proteins involved in miRNA processing may provide another layer of miRNA regulation in cancer. Up-regulation of the mRNA levels of the nuclear ribonuclease Drosha and the cytoplasmic ribonucleases Dicer and Ago2 were found in CRC, and increased expression of Dicer is implicated in advanced CRCs.102 Overexpression of Dicer is an independent predictor of poor survival in CRC, regardless of age, sex, tumor site, stage, or differentiation.103, 104 These initial clues into the biology of miRNA provide a springboard for future studies.
Functional Association of miRNAs in Multistep Colorectal Carcinogenesis
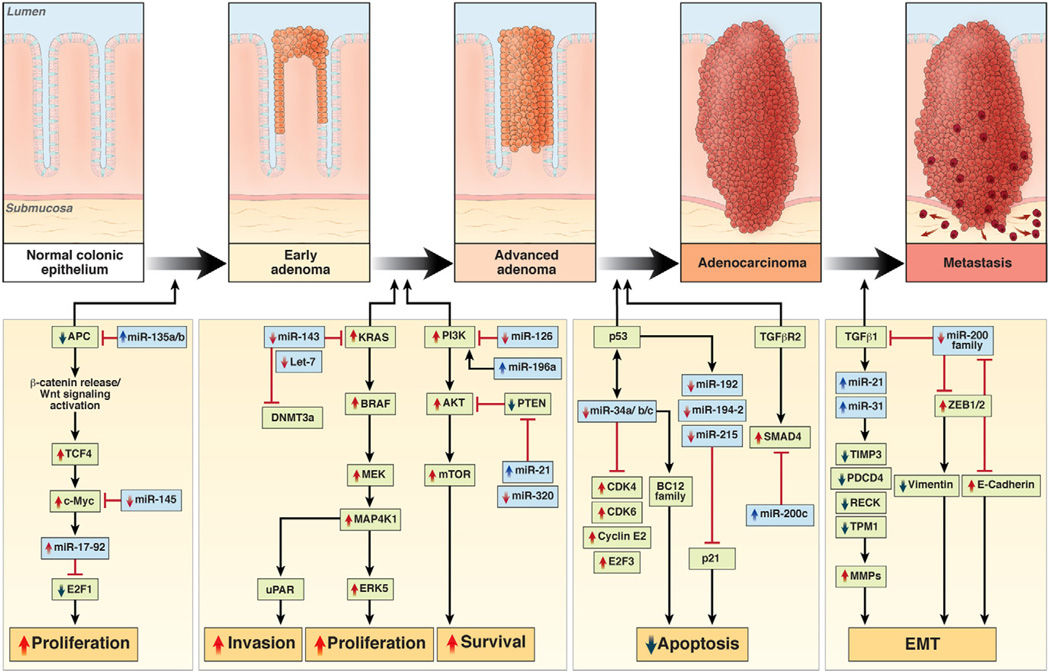
The classic multistep colorectal carcinogenesis model proposed by Fearon and Vogelstein in 1990 elegantly showed that most CRCs evolve through the sequential accumulation of “alterations” that manifest clinically in adenoma-to-carcinoma progression.105 Data gathered over the past several years on hypermethylation-induced silencing of genes provide new details on this process. It is beyond the scope of this article to summarize all relevant data in this regard, but there is evidence that miRNAs can regulate all the major pathways in colorectal neoplasia. In brief, this includes their impact on β-catenin/WNT signaling (miR-135a/b, miR-139, miR-145, miR-17-92),91, 94, 106–109 proliferation (let-7 family, miR-18a, miR-21, miR-126, miR-143, miR-200c),91, 107, 110 apoptosis (miR-34a, miR-133b, miR-195),107, 110 cell cycle control (miR-34a, miR-192, miR-215, miR-675),107, 110, 111 p53 signaling (miR-34b/c),98 differentiation (miR-141, miR-200c),104, 112 and migration and invasion (miR-126, miR-143, miR-196a, miR-200a/b/c, miR-373, miR-520c).104, 107, 110 It is likely that the altered expression of many miRNAs may target hundreds of growth regulatory genes and pathways that are critical in the multistep model of carcinogenesis (Figure 4 and Table 1).
Figure 4.
Involvement of miRNAs in multistep colorectal carcinogenesis. Various miRNAs have been identified that are dysregulated in colorectal neoplasia (in blue), along with validated gene targets (in green) in the multistep Vogelgram. Although the number of newly discovered miRNAs and gene targets is continuously growing, this figure provides evidence that miRNAs can regulate any of the major pathways in colorectal neoplasia, including their ability to regulate β-catenin/WNT signaling, MAPK and p53 signaling, proliferation, apoptosis, cell cycle control, migration, and invasion.
For instance, miR-135a and miR-135b are oncomiRs that directly target the 3′ untranslated region of the APC gene, suppress its expression, and activate WNT signaling106; likewise, the miR-17-92 family of genes directly suppresses APC function.91, 108 Because inactivation of APC is a gatekeeper event for the initiation of colorectal neoplasia, this suggests a miRNA-mediated mechanism for control of the APC gene and activation of the WNT signaling pathway. Moreover, higher expression of oncogenic miR-21 in adenomas and CRCs relative to normal tissues suggests that abnormal expression of this miRNA is an early event in the progression toward CRC.90, 113, 114 It has also been shown that miR-21 promotes cell migration and invasion by targeting the PDCD4 and PTEN tumor suppressor genes.115
Another important key signaling pathway in development of CRC involves activating the epidermal growth factor receptor, mediated by KRAS signaling that induces activation of various downstream effectors that mediate tumor growth, survival, and metastasis in many cancers. Down-regulated expression of the tsmiRs miR-143 and miR-145 has been shown to target multiple genes in the MAPK signaling pathway, including KRAS, ERK5, IRS-1, STAT1, and KLF4, all of which are instrumental during the transition of an early adenoma to advanced stages.88, 89, 116–122 Furthermore, the tumor suppressive role of miR-143 may be due to its ability to also target DNMT3A.123
Epithelial-to-mesenchymal transition is a cellular program involved in tumor cell invasion and metastasis. Activation of epithelial-to-mesenchymal transition allows tumor cells to detach, migrate, and disseminate through circulation and metastasize to distant organs. This process is initiated by transforming growth factor β1 produced by tumor cells, which triggers the expression of ZEB1 and ZEB2, which in turns causes transcriptional repression of E-cadherin and activation of vimentin.124 In addition, ZEB1 has been shown to induce transcription of the miR-200 family of miRNAs (miR-200a/b/c, miR-141, and miR-429), whose down-regulation is believed to an essential feature of epithelial-to-mesenchymal transition. Curiously, one of the putative targets of the miR-200 family is the ZEB1/2 genes themselves, because overexpression of miR-200c causes translational inhibition of ZEB1.125 It is likely that altered expresssion of miRs is involved in the full range of CRC development.
Epigenetic Biomarkers and Their Applications
A large body of data supports the potential of DNA methylation and miRNAs to serve as biomarkers for the early detection, prognosis, and determination of predictive responses to chemotherapy in patients with colorectal neoplasia. Because epigenetic alterations occur early in cancer progression and can be present in premalignant adenomas, or even in normal mucosa of patients with colorectal mucosa due to a field effect, these markers are suitable for the diagnosis of early CRC. DNA methylation and miRNA expression changes can be easily measured in archival tissues, as well as in blood, feces, urine, and so on. In addition, unlike genetic mutations, epigenetic alterations can be measured quantitatively, which might be useful for monitoring disease progression and following patient responses to various treatments. Table 2 lists several epigenetic biomarkers with potential for clinical application.
Table 2.
DNA Methylation and miRNA-Based Biomarkers for Diagnosis, Prognosis, and Predictive Response to Chemotherapy in CRC
| Marker | Purpose | Specimen type |
Analysis method | Colorectal neoplasia |
Remarks | Reference |
|---|---|---|---|---|---|---|
| DNA methylation | ||||||
| MLH1 | Diagnosis | Serum | MSP | Hypermethylated | Diagnosis of sporadic MSI CRC | 127 |
| p16 | Diagnosis | Serum | MSP | Hypermethylated | Diagnosis of CRC | 128–130 |
| DAPK | Diagnosis | Serum | MSP | Hypermethylated | Diagnosis of CRC | 131 |
| RUNX3 | Diagnosis | Serum | MSP | Hypermethylated | Diagnosis of CRC | 132 |
| ALX4 | Diagnosis | Serum | MS-AP-PCR | Hypermethylated | Diagnosis of colorectal adenomas and cancers |
133 |
| TMEFF2, NGFR, SEPT9 | Diagnosis | Plasma | Microarray, qPCR | Hypermethylated | Diagnosis of CRC | 134 |
| Septin-9 | Diagnosis | Plasma | MSP | Hypermethylated | Diagnosis of colorectal adenomas and cancers | 135,136 |
| APC, MGMT, RASSF2A, WIF1 | Diagnosis | Plasma | MSP | Hypermethylated | Diagnosis of CRC | 192 |
| Vimentin | Diagnosis | Stool | MSP | Hypermethylated | Diagnosis of stage I and II CRCs | 137 |
| SFRP2, RASSF2 | Diagnosis | Stool | COBRA, Hi-SA | Hypermethylated | Diagnosis of colorectal adenomas and cancers, and distinction from gastric cancer |
138 |
| NDRG4 | Diagnosis | Stool | MSP | Hypermethylated | Diagnosis of CRC | 140 |
| GATA-5, GATA-5 | Diagnosis | Stool | MSP | Hypermethylated | Diagnosis of CRC | 139 |
| BMP3, NDRG4, TFPI2, Vimentin |
Diagnosis | Stool | QuARTS | Hypermethylated | Diagnosis of colorectal adenomas and cancers |
141 |
| Vimentin | Diagnosis | Stool, plasma |
Methyl-BEAMing | Hypermethylated | Diagnosis of colorectal adenomas and cancers |
193 |
| BMP3 | Diagnosis | Tissue | Bisulfite sequencing | Hypermethylated | Diagnosis of colorectal adenomas and cancers |
194 |
| HPP1, HLTF, MLH1 | Prognosis | Serum | MSP | Hypermethylated | Hypermethylation of HLTF/HPP1 genes associated with unfavorable prognosis |
195 |
| LINE-1 | Prognosis | Tissue | Pyrosequencing | Hypomethylation | Associated with worse OS | 142 |
| CIMP-high | Prognosis | Tissue | MSP | Hypermethylation | Associated with poor OS in advanced CRC | 28 |
| LINE-1 | Predictive | Tissue | Pyrosequencing | Hypomethylation | Improved survival in patients treated with 5-FU–based adjuvant chemotherapy |
142 |
| CIMP-high | Predictive | Tissue | Pyrosequencing | Hypermethylation | Lack of benefit from 5-FU–based adjuvant chemotherapy in MSI CRCs |
33 |
| TFAP2E | Predictive | Tissue | MethyLight | Hypermethylation | Lack of responsiveness in patients with CRC treated with 5-FU–based chemotherapy |
196 |
| miRNAs | ||||||
| miR-92, miR-17-3p | Diagnosis | Plasma | qRT-PCR | Up-regulated | Diagnosis of CRC and distinction from other gastrointestinal cancers and inflammatory bowel disease |
146 |
| miR-92a, miR-29a | Diagnosis | Plasma | qRT-PCR | Up-regulated | Diagnosis of colorectal adenomas and cancers | 147 |
| miR-221 | Diagnosis | Plasma | qRT-PCR | Up-regulated | Diagnosis of CRC and association with poor outcome |
148 |
| miR-21, miR-106a | Diagnosis | Stool | Mircoarray, qRT-PCR | Up-regulated | Diagnosis of colorectal adenomas and cancers | 145 |
| miR-135, miR-17-92 cluster | Diagnosis | Stool | qRT-PCR | Up-regulated | Diagnosis of CRC | 149 |
| miR-144 | Diagnosis | Stool | qRT-PCR | Up-regulated | Diagnosis of CRC | 150 |
| miR-92a, miR-21 | Diagnosis | Stool | qRT-PCR | Up-regulated | Diagnosis of colorectal adenomas and cancers | 197 |
| miR-21 | Prognosis | Tissue | Microarray, qRT-PCR | Up-regulated | Associated with poor survival | 90,152 |
| miR-155 | Prognosis | Tissue | qRT-PCR | Up-regulated | Associated with shorter DFS and OS | 152 |
| miR-320, miR-498 | Prognosis | Tissue | Microarray | Up-regulated | Correlated with shorter recurrence-free survival | 88 |
| miR-185 | Prognosis | Tissue | qRT-PCR | Up-regulated | Associated with shorter OS | 198 |
| miR-106a | Prognosis | Tissue | qRT-PCR | Down-regulated | Associated with shorter DFS and OS | 199 |
| miR-113b | Prognosis | Tissue | qRT-PCR | Down-regulated | Associated with shorter OS | 198 |
| miR-143 | Prognosis | Tissue | qRT-PCR | Down-regulated | Associated with shorter OS | 151 |
| miR-141 | Prognosis | Plasma | qRT-PCR | Up-regulated | Associated with poor survival | |
| Let-7, miR-181b | Predictive | Tissue | qRT-PCR | Down-regulated | Associated with improved response to capecitabine-based chemotherapy |
153 |
| miR-21 | Predictive | Tissue | Microarray, qRT-PCR | Up-regulated | Associated with poor response to 5-FU–based therapy |
90,152 |
| miR-125b, miR-137 | Predictive | Tissue | qRT-PCR | Associated with worse response to capecitabine-based chemoradiotherapy |
154 |
MSP, methylation-specific polymerase chain reaction; MS-AP-PCR, methylation-specific arbitrarily primer polymerase chain reaction; qPCR, quantitative polymerase chain reaction; QuARTS, quantitative allele-specific real-time target and signal amplification; OS, overall survival; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; DFS, disease-free survival.
Methylated DNA has been useful diagnostically in CRC. For instance, the methylation of MLH1 can be detected in CRC tumor samples126 or blood127 to help in the interpretation of MSI, because its presence helps exclude the diagnosis of Lynch syndrome. This conclusion can be further supported by the presence of a mutated BRAF gene.126 Although aberrant hypermethylation of the MLH1 gene promoter in serum provided only 33% sensitivity, this test had 100% specificity in the identification of patients with sporadic MSI.127 Taking cues from this initial evidence, a flurry of reports appeared in the literature that subsequently reported an additional list of aberrantly methylated genes that could be analyzed in blood. For example, p16 hypermethylation was frequently noted in sera or plasma of patients with CRC, and methylation was detectable even in the preneoplastic adenomas, highlighting the potential clinical usefulness of this approach.128–130 Hypermethylation of DAPK (death associated protein kinase),131 RUNX3,132 and ALX4 (aristaless-like homeobox-4)133 has also been shown to have potential for development as noninvasive diagnostic biomarkers for colorectal adenomas and cancers. The list of bloodbased methylation markers has evolved, and a study using a combinatorial microarray and quantitative polymerase chain reaction– based approach identified a panel of highly sensitive and specific biomarkers for methylated DNA in plasma.134 Using this strategy in 56 candidate genes, TMEFF2 (transmembrane protein with epidermal growth factor–like and 2 follistatin-like domains), NGFR (nerve growth factor receptor), and SEPT9 (septin-9) were found to be specific in discriminating healthy subjects from patients with colorectal neoplasia,134 a finding that has been validated in other studies.135, 136 In fact, an assay that detects hypermethylated SEPT9 is now being commercially offered in some parts of Europe to screen patients for CRC.
A blood-based screening test seems attractive from a practical standpoint, but colonocytes are continuously exfoliated into the intestinal tract from tumors, so a stool-based test might be better for the detection of epigenetic signatures associated with CRC. Methylation of the vimentin promoter can be detected in stools of patients with asymptomatic CRC, and this has been added to the panel of diagnostic targets used in fecal DNA testing.137 Moreover, methylated RAFF2A and SFRP2 genes have been documented in the stools of patients with both CRC and gastric cancer.138 Recently it was shown that hypermethylation of the transcription factors GATA4 and GATA5 is frequent in CRC, and this epigenetic signature in fecal samples might serve as a noninvasive biomarker for detecting CRC.139 In this study, examination of GATA4/5 methylation in fecal DNA from 2 independent series of patients with CRC yielded a sensitivity of 71% and specificity of 84% for detection of CRC in the training set and a sensitivity of 51% and specificity of 93% in the validation set.139 The same group also reported that hypermethylation of the NDRG4 (N-Myc downstream-regulated gene 4) promoter in fecal DNA of patients with CRC yielded a sensitivity of 53% to 60% and a specificity of 93% to 100% in different patient cohorts.140 Another study analyzed the methylation status of BMP3, NDRG4, TFIP2, and vimentin genes in fecal DNA and reported that this test successfully identified 85% of patients with CRC and 54% of patients with large adenomas (≥1 cm in size) with 90% specificity, further highlighting the potential usefulness of fecal DNA–based tests for CRC screening.141 There is also evidence to suggest that CIMP status28, 33 and LINE-1 hypomethylation142, 143 might be useful prognostic or predictive molecular markers in CRC. It is very likely that the availability of more sophisticated sequencing technologies will permit the identification of additional methylated genes that are better prognostic and predictive biomarkers for CRC.
One of the most daunting aspects in molecular diagnostics of cancer is genetic heterogeneity. miRNAs are potentially useful in that there are only ~2000 of them, microarray platforms permit a comprehensive analysis of the miRNome, and there are expression patterns that might be useful diagnostically. Moreover, miRNAs are relatively stable and can be measured in body fluids144 and even feces.145 Accumulating evidence supports the existence of unique miRNA signatures that can facilitate earlier detection of colorectal adenomas and cancers. As listed in Table 2, expression levels of miR-92, miR-17, miR-29a, and miR-221 in plasma146–148 and the expression levels of several other miRNAs including miR-21, miR-106a, miR-135, and miR-144 in stool145, 149, 150 have diagnostic potential for patients with colorectal adenomas and cancers. Likewise, a growing list of miRNAs have either prognostic value (miR-21, miR-155, miR-320, miR-143)88, 90, 151, 152 or the ability to predict disease recurrence and therapeutic responses (miR-21, miR-181b, miR-137, Let-7)90, 153, 154 to chemotherapeutic regimens in patients with different stages of CRC. Some of these miRNA biomarkers may open new avenues for earlier diagnosis and personalization of therapies in patients with CRC.
Epigenetic Therapy
Unlike genetic changes, which are essentially fixed forever, epigenetic changes are intrinsically reversible, and altered gene expression can be turned on and off by the cell. This makes them attractive candidates for therapeutic intervention. Furthermore, there is growing evidence supporting the hypothesis that epigenetic alterations may be a driving force of drug resistance in human cancer, 155, 156 a phenomenon that has been reported in many solid tumors, including CRC cells.30 Consequently, 2 classes of chemical compounds that include DNMT and HDAC inhibitors have undergone major preclinical investigations and are currently being explored for efficacy in the treatment of various human cancers in several clinical trials. For instance, the DNA demethylating drugs 5-azacitidine and 5-aza-2′deoxycitidine (decitabine) are already in use clinically for various human malignancies, including myelodysplastic syndrome.157 These drugs act through their ability to be incorporated into DNA and act by preventing the resolution of a covalent reaction intermediate that traps and inactivates DNMT in the form of a covalent protein- DNA adduct, resulting in the rapid depletion of DNMT and concomitant demethylation with continued DNA replication.158 These drugs have potent activities in vitro and some responses are achieved clinically, but the demethylation activity is nonspecific and the toxicities are considerable.159
Seven classes of HDAC inhibitors have been developed thus far. Inhibition of these enzymes leads to the acetylation of histones, which is followed by a series of cellular processes that impact cell growth and promote tumorigenesis. The drug vorinostat has also been used clinically, but the HDAC family is large, and their therapeutic roles in cancer remain incompletely understood.160 There is evidence from in vitro and in vivo studies that vorinostat can down-regulate thymidylate synthase expression at the transcription level, which results in synergistic antitumor activity when combined with 5-FU in CRC cells.161, 162 Given the close collaboration between DNA methylation and histone modifications to inhibit transcription of tumor suppressor genes, another strategy is to combine DNMT and HDAC inhibitors, which may have a more synergistic effect in demethylating epigenetically silenced genes.163 Combination treatment with 5-azacitidine and valproic acid in a phase 1 clinical trial of patients with refractory solid tumors (including CRC) resulted in a significant decrease in global DNA methylation and induced histone deacetylation with stable disease lasting up to 12 months in a subset of patients.164
Although this is currently an emerging field, given the ubiquity of hypermethylation in a subset of CRCs, the opportunity to discover the appropriate use of these agents in this disease is apparent. The challenge will be to find epigenetic modifiers that are a proper “fit” with solid tumors, demethylate or deacetylate just the right loci, and improve the response to therapies. We have previously shown in vitro that the simultaneous use of a demethylating agent together with 5-FU in the CIMP CRC cell line SW48 overcomes the resistance to cell death.30 Because CRCs with CIMP have a poorer prognosis and diminished response to adjuvant chemotherapy, trials of epigenetic modifiers seem to be warranted. Also, the presence of the field effect of CIMP in the aging colon raises the possibility that these agents may be useful as chemopreventive agents. Also, there are several naturally occurring “botanical” agents such as curcumin and boswellic acid that are likely to be safe (because they have been used for centuries as food spices), and these may be useful as cancer-preventing agents or as adjuvants to conventional chemotherapy. 165–167 Clinical trials are currently exploring the safety and efficacy of various epigenetic drugs individually and in combination with chemotherapeutic drugs, which will reveal their true clinical potential. We speculate that epigenetic therapies in a variety of settings are about to enter into everyone’s armamentarium.
Conclusions
The study of epigenetic changes has greatly extended our understanding of the pathogenesis and pathophysiology of CRC and opens new horizons for diagnosis of asymptomatic tumors, characterization of various forms of CRC, and prediction of outcome and response to chemotherapy; it may also permit the development of novel preventive therapies or adjunctive therapeutic approaches. This promises to be an active area for investigation in the foreseeable future.
Supplementary Material
Acknowledgments
Funding
Supported by National Cancer Institute/National Institutes of Health grants R01 CA72851 (to C.R.B.) and R01 CA129286 (to A.G. and C.R.B.).
Abbreviations used in this paper
- CIMP
CpG island methylator phenotype
- CRC
colorectal cancer
- DNMT
DNA methyltransferase
- 5-FU
5-fluorouracil
- HDAC
histone deacetylase
- miRNA
microRNA
- MMR
mismatch repair
- MSI
microsatellite instability
- MSS
microsatellitestable
- PRC
polycomb repressive complex.
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2012.09.032.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 2.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graff JR, Herman JG, Myohanen S, et al. Mapping patterns of CpG island methylation in normal and neoplastic cells implicates both upstream and downstream regions in de novo methylation. J Biol Chem. 1997;272:22322–22329. doi: 10.1074/jbc.272.35.22322. [DOI] [PubMed] [Google Scholar]
- 4.Ooi SL, Henikoff S. Germline histone dynamics and epigenetics. Curr Opin Cell Biol. 2007;19:257–265. doi: 10.1016/j.ceb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Chadwick BP, Willard HF. Chromatin of the Barr body: histone and non-histone proteins associated with or excluded from the inactive X chromosome. Hum Mol Genet. 2003;12:2167–2178. doi: 10.1093/hmg/ddg229. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 7.Goelz SE, Vogelstein B, Hamilton SR, et al. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985;228:187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg AP, Gehrke CW, Kuo KC, et al. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988;48:1159–1161. [PubMed] [Google Scholar]
- 9.Baylin SB, Hoppener JW de BA, Steenbergh PH, et al. DNA methylation patterns of the calcitonin gene in human lung cancers and lymphomas. Cancer Res. 1986;46:2917–2922. [PubMed] [Google Scholar]
- 10.Issa JP, Ottaviano YL, Celano P, et al. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 11.Issa JP, Vertino PM, Boehm CD, et al. Switch from monoallelic to biallelic human IGF2 promoter methylation during aging and carcinogenesis. Proc Natl Acad Sci U S A. 1996;93:11757–11762. doi: 10.1073/pnas.93.21.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman JG, Civin CI, Issa JP, et al. Distinct patterns of inactivation of p15INK4B and p16INK4A characterize the major types of hematological malignancies. Cancer Res. 1997;57:837–841. [PubMed] [Google Scholar]
- 13.Ahuja N, Li Q, Mohan AL, et al. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–5494. [PubMed] [Google Scholar]
- 14.Toyota M, Ahuja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel A, Arnold CN, Tassone P, et al. Epigenetic inactivation of RUNX3 in microsatellite unstable sporadic colon cancers. Int J Cancer. 2004;112:754–759. doi: 10.1002/ijc.20472. [DOI] [PubMed] [Google Scholar]
- 16.Goel A, Arnold CN, Niedzwiecki D, et al. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004;64:3014–3021. doi: 10.1158/0008-5472.can-2401-2. [DOI] [PubMed] [Google Scholar]
- 17.Shin SK, Nagasaka T, Jung BH, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133:1849–1857. doi: 10.1053/j.gastro.2007.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashid A, Issa JP. CpG island methylation in gastroenterologic neoplasia: a maturing field. Gastroenterology. 2004;127:1578–1588. doi: 10.1053/j.gastro.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Worthley DL, Whitehall VL, Buttenshaw RL, et al. DNA methylation within the normal colorectal mucosa is associated with pathway-specific predisposition to cancer. Oncogene. 2010;29:1653–1662. doi: 10.1038/onc.2009.449. [DOI] [PubMed] [Google Scholar]
- 20.Ahuja N, Issa JP. Aging, methylation and cancer. Histol Histopathol. 2000;15:835–842. doi: 10.14670/HH-15.835. [DOI] [PubMed] [Google Scholar]
- 21.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 22.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Nagasaka T, Koi M, Kloor M, et al. Mutations in both KRAS and BRAF may contribute to the methylator phenotype in colon cancer. Gastroenterology. 2008;134:1950–1960. doi: 10.1053/j.gastro.2008.02.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 25.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 26.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 28.Shen L, Catalano PJ, Benson AB, III, et al. Association between DNA methylation and shortened survival in patients with advanced colorectal cancer treated with 5-fluorouracil based chemotherapy. Clin Cancer Res. 2007;13:6093–6098. doi: 10.1158/1078-0432.CCR-07-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003;106:66–73. doi: 10.1002/ijc.11176. [DOI] [PubMed] [Google Scholar]
- 31.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carethers JM, Smith EJ, Behling CA, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004;126:394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Jover R, Nguyen TP, Perez-Carbonell L, et al. 5-Fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology. 2011;140:1174–1181. doi: 10.1053/j.gastro.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel A, Arnold CN, Niedzwiecki D, et al. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003;63:1608–1614. [PubMed] [Google Scholar]
- 35.Goel A, Nagasaka T, Arnold CN, et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007;132:127–138. doi: 10.1053/j.gastro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Arnold CN, Goel A, Niedzwiecki D, et al. APC promoter hypermethylation contributes to the loss of APC expression in colorectal cancers with allelic loss on 5q. Cancer Biol Ther. 2004;3:960–964. doi: 10.4161/cbt.3.10.1113. [DOI] [PubMed] [Google Scholar]
- 37.Arnold CN, Goel A, Compton C, et al. Evaluation of microsatellite instability, hMLH1 expression and hMLH1 promoter hypermethylation in defining the MSI phenotype of colorectal cancer. Cancer Biol Ther. 2004;3:73–78. doi: 10.4161/cbt.3.1.590. [DOI] [PubMed] [Google Scholar]
- 38.Nagasaka T, Goel A, Notohara K, et al. Methylation pattern of the O6-methylguanine-DNA methyltransferase gene in colon during progressive colorectal tumorigenesis. Int J Cancer. 2008;122:2429–2436. doi: 10.1002/ijc.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 41.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 42.Chan TL, Yuen ST, Kong CK, et al. Heritable germline epimutation of MSH2 in a family with hereditary nonpolyposis colorectal cancer. Nat Genet. 2006;38:1178–1183. doi: 10.1038/ng1866. [DOI] [PubMed] [Google Scholar]
- 43.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet. 2009;41:112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 44.Lynch HT, Riegert-Johnson DL, Snyder C, et al. Lynch syndrome-associated extracolonic tumors are rare in two extended families with the same EPCAM deletion. Am J Gastroenterol. 2011;106:1829–1836. doi: 10.1038/ajg.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagasaka T, Rhees J, Kloor M, Gebert J, et al. Somatic hypermethylation of MSH2 is a frequent event in Lynch Syndrome colorectal cancers. Cancer Res. 2010;70:3098–3108. doi: 10.1158/0008-5472.CAN-09-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frazier ML, Xi L, Zong J, et al. Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res. 2003;63:4805–4808. [PubMed] [Google Scholar]
- 47.Gazzoli I, Loda M, Garber J, et al. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the MLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 2002;62:3925–3928. [PubMed] [Google Scholar]
- 48.Hitchins M, Williams R, Cheong K, et al. MLH1 germline epimutations as a factor in hereditary nonpolyposis colorectal cancer. Gastroenterology. 2005;129:1392–1399. doi: 10.1053/j.gastro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Hitchins MP, Wong JJ, Suthers G, et al. Inheritance of a cancer-associated MLH1 germ-line epimutation. N Engl J Med. 2007;356:697–705. doi: 10.1056/NEJMoa064522. [DOI] [PubMed] [Google Scholar]
- 50.Hitchins MP, Rapkins RW, Kwok CT, et al. Dominantly inherited constitutional epigenetic silencing of MLH1 in a cancer-affected family is linked to a single nucleotide variant within the 5=UTR. Cancer Cell. 2011;20:200–213. doi: 10.1016/j.ccr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Goel A, Nguyen TP, Leung HC, et al. De novo constitutional MLH1 epimutations confer early-onset colorectal cancer in two new sporadic Lynch syndrome cases, with derivation of the epimutation on the paternal allele in one. Int J Cancer. 2011;128:869–878. doi: 10.1002/ijc.25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogino S, Cantor M, Kawasaki T, et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55:1000–1006. doi: 10.1136/gut.2005.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrera LA, Prada D, Andonegui MA, et al. The epigenetic origin of aneuploidy. Curr Genomics. 2008;9:43–50. doi: 10.2174/138920208783884883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silver A, Sengupta N, Propper D, et al. A distinct DNA methylation profile associated with microsatellite and chromosomal stable sporadic colorectal cancers. Int J Cancer. 2012;130:1082–1092. doi: 10.1002/ijc.26104. [DOI] [PubMed] [Google Scholar]
- 56.Goel A, Xicola RM, Nguyen TP, et al. Aberrant DNA methylation in hereditary nonpolyposis colorectal cancer without mismatch repair deficiency. Gastroenterology. 2010;138:1854–1862. doi: 10.1053/j.gastro.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Mathews LA, Crea F, Farrar WL. Epigenetic gene regulation in stem cells and correlation to cancer. Differentiation. 2009;78:1–17. doi: 10.1016/j.diff.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 62.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 63.Ohm JE, McGarvey KM, Yu X, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vire E, Brenner C, Deplus R, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 65.Mohammad HP, Cai Y, McGarvey KM, et al. Polycomb CBX7 promotes initiation of heritable repression of genes frequently silenced with cancer-specific DNA hypermethylation. Cancer Res. 2009;69:6322–6330. doi: 10.1158/0008-5472.CAN-09-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin B, Yao B, Li JL, et al. DNMT1 and DNMT3B modulate distinct polycomb-mediated histone modifications in colon cancer. Cancer Res. 2009;69:7412–7421. doi: 10.1158/0008-5472.CAN-09-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fluge O, Gravdal K, Carlsen E, et al. Expression of EZH2 and Ki-67 in colorectal cancer and associations with treatment response and prognosis. Br J Cancer. 2009;101:1282–1289. doi: 10.1038/sj.bjc.6605333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang CG, Ye YJ, Yuan J, et al. EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis. World J Gastroenterol. 2010;16:2421–2427. doi: 10.3748/wjg.v16.i19.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fussbroich B, Wagener N, Macher-Goeppinger S, et al. EZH2 depletion blocks the proliferation of colon cancer cells. PLoS One. 2011;6:e21651. doi: 10.1371/journal.pone.0021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Viel A, Maestro R, Toffoli G, et al. c-myc overexpression is a tumor-specific phenomenon in a subset of human colorectal carcinomas. J Cancer Res Clin Oncol. 1990;116:288–294. doi: 10.1007/BF01612905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fahrner JA, Eguchi S, Herman JG, et al. Dependence of histone modifications and gene expression on DNA hypermethylation in cancer. Cancer Res. 2002;62:7213–7218. [PubMed] [Google Scholar]
- 72.Bachman KE, Park BH, Rhee I, et al. Histone modifications and silencing prior to DNA methylation of a tumor suppressor gene. Cancer Cell. 2003;3:89–95. doi: 10.1016/s1535-6108(02)00234-9. [DOI] [PubMed] [Google Scholar]
- 73.Si J, Boumber YA, Shu J, et al. Chromatin remodeling is required for gene reactivation after decitabine-mediated DNA hypomethylation. Cancer Res. 2010;70:6968–6977. doi: 10.1158/0008-5472.CAN-09-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weichert W, Roske A, Niesporek S, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14:1669–1677. doi: 10.1158/1078-0432.CCR-07-0990. [DOI] [PubMed] [Google Scholar]
- 75.Ashktorab H, Belgrave K, Hosseinkhah F, et al. Global histone H4 acetylation and HDAC2 expression in colon adenoma and carcinoma. Dig Dis Sci. 2009;54:2109–2117. doi: 10.1007/s10620-008-0601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ishihama K, Yamakawa M, Semba S, et al. Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol. 2007;60:1205–1210. doi: 10.1136/jcp.2005.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nosho K, Shima K, Irahara N, et al. SIRT1 histone deacetylase expression is associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Mod Pathol. 2009;22:922–932. doi: 10.1038/modpathol.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slaby O, Svoboda M, Michalek J, et al. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer. 2009;8:102. doi: 10.1186/1476-4598-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Griffiths-Jones S, Saini HK, van Dongen S, et al. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 82.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 83.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 84.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michael MZ, O’Connor SM, van Holst Pellekaan NG, et al. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- 86.Cummins JM, He Y, Leary RJ, et al. The colorectal microRNAome. Proc Natl Acad Sci U S A. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bandres E, Cubedo E, Agirre X, et al. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schepeler T, Reinert JT, Ostenfeld MS, et al. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 89.Wang CJ, Zhou ZG, Wang L, et al. Clinicopathological significance of microRNA-31, -143 and -145 expression in colorectal cancer. Dis Markers. 2009;26:27–34. doi: 10.3233/DMA-2009-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Motoyama K, Inoue H, Takatsuno Y, et al. Over- and under-expressed microRNAs in human colorectal cancer. Int J Oncol. 2009;34:1069–1075. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- 92.Slaby O, Svoboda M, Fabian P, et al. Altered expression of miR-21, miR-31, miR-43 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 93.Lanza G, Ferracin M, Gafa R, et al. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Mol Cancer. 2007;6:54. doi: 10.1186/1476-4598-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sarver AL, French AJ, Borralho PM, et al. Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC Cancer. 2009;9:401. doi: 10.1186/1471-2407-9-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balaguer F, Moreira L, Lozano JJ, et al. Colorectal cancers with microsatellite instability display unique miRNA profiles. Clin Cancer Res. 2011;17:6239–6249. doi: 10.1158/1078-0432.CCR-11-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bandres E, Agirre X, Bitarte N, et al. Epigenetic regulation of microRNA expression in colorectal cancer. Int J Cancer. 2009;125:2737–2743. doi: 10.1002/ijc.24638. [DOI] [PubMed] [Google Scholar]
- 97.Toyota M, Suzuki H, Sasaki Y, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68:4123–4132. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 98.Vogt M, Munding J, Gruner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki H, Takatsuka S, Akashi H, et al. Genome-wide profiling of chromatin signatures reveals epigenetic regulation of MicroRNA genes in colorectal cancer. Cancer Res. 2011;71:5646–5658. doi: 10.1158/0008-5472.CAN-11-1076. [DOI] [PubMed] [Google Scholar]
- 100.Grady WM, Parkin RK, Mitchell PS, et al. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene. 2008;27:3880–3888. doi: 10.1038/onc.2008.10. [DOI] [PubMed] [Google Scholar]
- 101.Balaguer F, Link A, Lozano JJ, et al. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010;70:6609–6618. doi: 10.1158/0008-5472.CAN-10-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papachristou DJ, Korpetinou A, Giannopoulou E, et al. Expression of the ribonucleases Drosha, Dicer, and Ago2 in colorectal carcinomas. Virchows Arch. 2011;459:431–440. doi: 10.1007/s00428-011-1119-5. [DOI] [PubMed] [Google Scholar]
- 103.Faber C, Horst D, Hlubek F, et al. Overexpression of Dicer predicts poor survival in colorectal cancer. Eur J Cancer. 2011;47:1414–1419. doi: 10.1016/j.ejca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 104.Stratmann J, Wang CJ, Gnosa S, et al. Dicer and miRNA in relation to clinicopathological variables in colorectal cancer patients. BMC Cancer. 2011;11:345. doi: 10.1186/1471-2407-11-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 106.Nagel R, le Sage C, Diosdado B, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68:5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 107.Arndt GM, Dossey L, Cullen LM, et al. Characterization of global microRNA expression reveals oncogenic potential of miR-145 in metastatic colorectal cancer. BMC Cancer. 2009;9:374. doi: 10.1186/1471-2407-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monzo M, Navarro A, Bandres E, et al. Overlapping expression of microRNAs in human embryonic colon and colorectal cancer. Cell Res. 2008;18:823–833. doi: 10.1038/cr.2008.81. [DOI] [PubMed] [Google Scholar]
- 109.Oberg AL, French AJ, Sarver AL, et al. miRNA expression in colon polyps provides evidence for a multihit model of colon cancer. PLoS One. 2011;6:e20465. doi: 10.1371/journal.pone.0020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Earle JS, Luthra R, Romans A, et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. J Mol Diagn. 2010;12:433–440. doi: 10.2353/jmoldx.2010.090154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsang WP, Ng EK, Ng SS, et al. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 112.Cheng H, Zhang L, Cogdell DE, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6:e17745. doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmitz KJ, Hey S, Schinwald A, et al. Differential expression of microRNA 181b and microRNA 21 in hyperplastic polyps and sessile serrated adenomas of the colon. Virchows Arch. 2009;455:49–54. doi: 10.1007/s00428-009-0804-0. [DOI] [PubMed] [Google Scholar]
- 114.Yamamichi N, Shimomura R, Inada K, et al. Locked nucleic acid in situ hybridization analysis of miR-21 expression during colorectal cancer development. Clin Cancer Res. 2009;15:4009–4016. doi: 10.1158/1078-0432.CCR-08-3257. [DOI] [PubMed] [Google Scholar]
- 115.Kumar MS, Lu J, Mercer KL, et al. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 116.La RG, Badin M, Shi B, et al. Mechanism of growth inhibition by MicroRNA 145: the role of the IGF-I receptor signaling pathway. J Cell Physiol. 2009;220:485–491. doi: 10.1002/jcp.21796. [DOI] [PubMed] [Google Scholar]
- 117.Suzuki HI, Yamagata K, Sugimoto K, et al. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 118.Akao Y, Nakagawa Y, Naoe T. MicroRNA-143 and-145 in colon cancer. DNA Cell Biol. 2007;26:311–320. doi: 10.1089/dna.2006.0550. [DOI] [PubMed] [Google Scholar]
- 119.Sachdeva M, Zhu S, Wu F, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gregersen LH, Jacobsen AB, Frankel LB, et al. MicroRNA-145 targets YES and STAT1 in colon cancer cells. PLoS One. 2010;5:e8836. doi: 10.1371/journal.pone.0008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu N, Papagiannakopoulos T, Pan G, et al. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 122.Chen X, Guo X, Zhang H, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 123.Ng EK, Tsang WP, Ng SS, et al. MicroRNA-143 targets DNA methyltransferases 3A in colorectal cancer. Br J Cancer. 2009;101:699–706. doi: 10.1038/sj.bjc.6605195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang H, Li Y, Lai M. The microRNA network and tumor metastasis. Oncogene. 2010;29:937–948. doi: 10.1038/onc.2009.406. [DOI] [PubMed] [Google Scholar]
- 125.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 126.Wang L, Cunningham JM, Winters JL, et al. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res. 2003;63:5209–5212. [PubMed] [Google Scholar]
- 127.Grady WM, Rajput A, Lutterbaugh JD, et al. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61:900–902. [PubMed] [Google Scholar]
- 128.Zou H, Yu B, Zhao R, et al. Detection of aberrant p16 methylation in the serum of colorectal cancer patients. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36:499–501. [PubMed] [Google Scholar]
- 129.Nakayama H, Hibi K, Takase T, et al. Molecular detection of p16 promoter methylation in the serum of recurrent colorectal cancer patients. Int J Cancer. 2003;105:491–493. doi: 10.1002/ijc.11117. [DOI] [PubMed] [Google Scholar]
- 130.Nakayama G, Hibi K, Nakayama H, et al. A highly sensitive method for the detection of p16 methylation in the serum of colorectal cancer patients. Anticancer Res. 2007;27:1459–1463. [PubMed] [Google Scholar]
- 131.Yamaguchi S, Asao T, Nakamura J, et al. High frequency of DAP-kinase gene promoter methylation in colorectal cancer specimens and its identification in serum. Cancer Lett. 2003;194:99–105. doi: 10.1016/s0304-3835(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 132.Tan SH, Ida H, Lau QC, et al. Detection of promoter hypermethylation in serum samples of cancer patients by methylation-specific polymerase chain reaction for tumour suppressor genes including RUNX3. Oncol Rep. 2007;18:1225–1230. [PubMed] [Google Scholar]
- 133.Ebert MP, Model F, Mooney S, et al. Aristaless-like homeobox-4 gene methylation is a potential marker for colorectal adenocarcinomas. Gastroenterology. 2006;131:1418–1430. doi: 10.1053/j.gastro.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 134.Lofton-Day C, Model F, deVos T, et al. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414–423. doi: 10.1373/clinchem.2007.095992. [DOI] [PubMed] [Google Scholar]
- 135.deVos T, Tetzner R, Model F, et al. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337–1346. doi: 10.1373/clinchem.2008.115808. [DOI] [PubMed] [Google Scholar]
- 136.Grutzmann R, Molnar B, Pilarsky C, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen WD, Han ZJ, Skoletsky J, et al. Detection in fecal DNA of colon cancer-specific methylation of the non-expressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–1132. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
- 138.Nagasaka T, Tanaka N, Cullings HM, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst. 2009;101:1244–1258. doi: 10.1093/jnci/djp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hellebrekers DM, Lentjes MH, van den Bosch SM, et al. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res. 2009;15:3990–3997. doi: 10.1158/1078-0432.CCR-09-0055. [DOI] [PubMed] [Google Scholar]
- 140.Melotte V, Lentjes MH, van den Bosch SM, et al. N-Myc downstream- regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst. 2009;101:916–927. doi: 10.1093/jnci/djp131. [DOI] [PubMed] [Google Scholar]
- 141.Ahlquist DA, Zou H, Domanico M, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142:248–256. doi: 10.1053/j.gastro.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ogino S, Nosho K, Kirkner GJ, et al. A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer. J Natl Cancer Inst. 2008;100:1734–1738. doi: 10.1093/jnci/djn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kawakami K, Matsunoki A, Kaneko M, et al. Long interspersed nuclear element-1 hypomethylation is a potential biomarker for the prediction of response to oral fluoropyrimidines in microsatellite stable and CpG island methylator phenotype-negative colorectal cancer. Cancer Sci. 2011;102:166–174. doi: 10.1111/j.1349-7006.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- 144.Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 145.Link A, Balaguer F, Shen Y, et al. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomarkers Prev. 2010;19:1766–1774. doi: 10.1158/1055-9965.EPI-10-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 147.Huang Z, Huang D, Ni S, et al. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 148.Pu XX, Huang GL, Guo HQ, et al. Circulating miR-221 directly amplified from plasma is a potential diagnostic and prognostic marker of colorectal cancer and is correlated with p53 expression. J Gastroenterol Hepatol. 2010;25:1674–1680. doi: 10.1111/j.1440-1746.2010.06417.x. [DOI] [PubMed] [Google Scholar]
- 149.Koga Y, Yasunaga M, Takahashi A, et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res (Phila) 2010;3:1435–1442. doi: 10.1158/1940-6207.CAPR-10-0036. [DOI] [PubMed] [Google Scholar]
- 150.Kalimutho M, Del Vecchio BG, Di CS, et al. Differential expression of miR-144* as a novel fecal-based diagnostic marker for colorectal cancer. J Gastroenterol. 2011;46:1391–1402. doi: 10.1007/s00535-011-0456-0. [DOI] [PubMed] [Google Scholar]
- 151.Kulda V, Pesta M, Topolcan O, et al. Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet. 2010;200:154–160. doi: 10.1016/j.cancergencyto.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 152.Shibuya H, Iinuma H, Shimada R, et al. Clinicopathological and prognostic value of MicroRNA-21 and MicroRNA-155 in colorectal cancer. Oncology. 2010;79:313–320. doi: 10.1159/000323283. [DOI] [PubMed] [Google Scholar]
- 153.Nakajima G, Hayashi K, Xi Y, et al. Non-coding microRNAs hsalet-7g and hsa-miR-181b are associated with chemoresponse to S-1 in colon cancer. Cancer Genomics Proteomics. 2006;3:317–324. [PMC free article] [PubMed] [Google Scholar]
- 154.Svoboda M, Izakovicova HL, Sefr R, et al. Micro-RNAs miR125b and miR137 are frequently upregulated in response to capecitabine chemoradiotherapy of rectal cancer. Int J Oncol. 2008;33:541–547. [PubMed] [Google Scholar]
- 155.Baylin SB. Resistance, epigenetics and the cancer ecosystem. Nat Med. 2011;17:288–289. doi: 10.1038/nm0311-288. [DOI] [PubMed] [Google Scholar]
- 156.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higherrisk myelodysplastic syndromes. Blood. doi: 10.1182/blood-2012-06-434639. 2012 Aug 22 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lyko F, Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst. 2005;97:1498–1506. doi: 10.1093/jnci/dji311. [DOI] [PubMed] [Google Scholar]
- 159.Garcia-Manero G. Demethylating agents in myeloid malignancies. Curr Opin Oncol. 2008;20:705–710. doi: 10.1097/CCO.0b013e328313699c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 161.Ocker M, Alajati A, Ganslmayer M, et al. The histone-deacetylase inhibitor SAHA potentiates proapoptotic effects of 5-fluorouracil and irinotecan in hepatoma cells. J Cancer Res Clin Oncol. 2005;131:385–394. doi: 10.1007/s00432-004-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim JC, Shin ES, Kim CW, et al. In vitro evaluation of histone deacetylase inhibitors as combination agents for colorectal cancer. Anticancer Res. 2009;29:3027–3034. [PubMed] [Google Scholar]
- 163.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 164.Braiteh F, Soriano AO, Garcia-Manero G, et al. Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res. 2008;14:6296–6301. doi: 10.1158/1078-0432.CCR-08-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 166.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 167.Shen Y, Takahashi M, Byun HM, et al. Boswellic acid induces epigenetic alterations by modulating DNA methylation in colorectal cancer cells. Cancer Biol Ther. 2012;13:542–552. doi: 10.4161/cbt.19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsang WP, Kwok TT. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30:953–959. doi: 10.1093/carcin/bgp094. [DOI] [PubMed] [Google Scholar]
- 169.Gabriely G, Wurdinger T, Kesari S, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhu S, Si ML, Wu H, et al. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 171.Sayed D, Rane S, Lypowy J, et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell. 2008;19:3272–3282. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yu XF, Zou J, Bao ZJ, et al. miR-93 suppresses proliferation and colony formation of human colon cancer stem cells. World J Gastroenterol. 2011;17:4711–4717. doi: 10.3748/wjg.v17.i42.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jalava SE, Urbanucci A, Latonen L, et al. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene. doi: 10.1038/onc.2011.624. 2012 Jan 23 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 175.Wang X, Lam EK, Zhang J, et al. MicroRNA-122a functions as a novel tumor suppressor downstream of adenomatous polyposis coli in gastrointestinal cancers. Biochem Biophys Res Commun. 2009;387:376–380. doi: 10.1016/j.bbrc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 176.Valeri N, Gasparini P, Fabbri M, et al. Modulation of mismatch repair and genomic stability by miR-155. Proc Natl Acad Sci U S A. 2010;107:6982–6987. doi: 10.1073/pnas.1002472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schaefer JS, Montufar-Solis D, Vigneswaran N, et al. Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10-/- mice precedes expression in the colon. J Immunol. 2011;187:5834–5841. doi: 10.4049/jimmunol.1100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bandres E, Bitarte N, Arias F, et al. MicroRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 179.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let=7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 180.Raver-Shapira N, Marciano E, Meiri E, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 181.Strillacci A, Griffoni C, Sansone P, et al. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp Cell Res. 2009;315:1439–1447. doi: 10.1016/j.yexcr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 182.Schepeler T, Holm A, Halvey P, et al. Attenuation of the betacatenin/TCF4 complex in colorectal cancer cells induces several growth-suppressive microRNAs that target cancer promoting genes. Oncogene. 2012;31:2750–2760. doi: 10.1038/onc.2011.453. [DOI] [PubMed] [Google Scholar]
- 183.Ma Y, Zhang P, Yang J, et al. Candidate microRNA biomarkers in human colorectal cancer: systematic review profiling studies and experimental validation. Int J Cancer. 2012;130:2077–2087. doi: 10.1002/ijc.26232. [DOI] [PubMed] [Google Scholar]
- 184.Hu M, Xia M, Chen X, et al. MicroRNA-141 regulates Smad interacting protein 1 (SIP1) and inhibits migration and invasion of colorectal cancer cells. Dig Dis Sci. 2010;55:2365–2372. doi: 10.1007/s10620-009-1008-9. [DOI] [PubMed] [Google Scholar]
- 185.Braun CJ, Zhang X, Savelyeva I, et al. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68:10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Boni V, Bitarte N, Cristobal I, et al. miR-192/miR-215 influence 5-fluorouracil resistance through cell cycle-mediated mechanisms complementary to its post-transcriptional thymidilate synthase regulation. Mol Cancer Ther. 2010;9:2265–2275. doi: 10.1158/1535-7163.MCT-10-0061. [DOI] [PubMed] [Google Scholar]
- 187.Liu L, Chen L, Xu Y, et al. MicroRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun. 2010;400:236–240. doi: 10.1016/j.bbrc.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 188.Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial- mesenchymal transition. Cancer Biol Ther. 2010;10:219–222. doi: 10.4161/cbt.10.6312548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bronisz A, Godlewski J, Wallace JA, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. 2012;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang H, Wu J, Meng X, et al. MicroRNA-342 inhibits colorectal cancer cell proliferation and invasion by directly targeting DNA methyltransferase 1. Carcinogenesis. 2011;32:1033–1042. doi: 10.1093/carcin/bgr081. [DOI] [PubMed] [Google Scholar]
- 191.Nakano H, Miyazawa T, Kinoshita K, et al. Functional screening identifies a microRNA, miR-491 that induces apoptosis by targeting Bcl-X(L) in colorectal cancer cells. Int J Cancer. 2010;127:1072–1080. doi: 10.1002/ijc.25143. [DOI] [PubMed] [Google Scholar]
- 192.Lee BB, Lee EJ, Jung EH, et al. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res. 2009;15:6185–6191. doi: 10.1158/1078-0432.CCR-09-0111. [DOI] [PubMed] [Google Scholar]
- 193.Li M, Chen WD, Papadopoulos N, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol. 2009;27:858–863. doi: 10.1038/nbt.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zou H, Harrington JJ, Shire AM, et al. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemiol Biomarkers Prev. 2007;16:2686–2696. doi: 10.1158/1055-9965.EPI-07-0518. [DOI] [PubMed] [Google Scholar]
- 195.Wallner M, Herbst A, Behrens A, et al. Methylation of serum DNA is an independent prognostic marker in colorectal cancer. Clin Cancer Res. 2006;12:7347–7352. doi: 10.1158/1078-0432.CCR-06-1264. [DOI] [PubMed] [Google Scholar]
- 196.Ebert MP, Tanzer M, Balluff B, et al. TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med. 2012;366:44–53. doi: 10.1056/NEJMoa1009473. [DOI] [PubMed] [Google Scholar]
- 197.Wu CW, Ng SS, Dong YJ, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739–745. doi: 10.1136/gut.2011.239236. [DOI] [PubMed] [Google Scholar]
- 198.Akcakaya P, Ekelund S, Kolosenko I, et al. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol. 2011;39:311–318. doi: 10.3892/ijo.2011.1043. [DOI] [PubMed] [Google Scholar]
- 199.Diaz R, Silva J, Garcia JM, et al. Deregulated expression of miR-106a predicts survival in human colon cancer patients. Genes Chromosomes Cancer. 2008;47:794–802. doi: 10.1002/gcc.20580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.