Abstract
Cardiac arrest is the leading cause of death in the United States and other developed countries. Ventricular tachyarrhythmias are the most prominent cause of cardiac arrest, and patients with structural heart disease are at increased risk for these abnormal heart rhythms. Drug and device therapy have important limitations that make them inadequate to meet this challenge. We and others have proposed development of arrhythmia gene therapy as an alternative to current treatment methods. In this review, I discuss the basic mechanisms of ventricular arrhythmias and summarize the literature on the use of gene therapy for ventricular tachyarrhythmias.
Keywords: gene therapy, arrhythmia, ventricular tachycardia, ventricular fibrillation, myocardial infarction, heart failure
INTRODUCTION
Cardiac arrest is the leading cause of death in the United States and other developed countries. In 2008 (the most recent year with available statistics), cardiac arrest accounted for 14% of all deaths in the US.1 In cardiac arrest cases where a cause can be determined, the most common diagnosis is a ventricular tachyarrhythmia. Structural heart disease, most commonly ischemic heart disease or heart failure, is a frequent comorbidity that substantially increases risk of cardiac arrest. Baseline risk of cardiac arrest is 0.8 per 1000 patient-years. With history of prior myocardial infarction (MI) this risk increases to 13.7 per 1000 patient-years, and for patients with heart failure the risk is 21.9 per 1000 patient-years.2
Therapeutic options for reducing the risk of cardiac arrest are inadequate. Better heart failure therapies and more aggressive coronary artery disease risk factor modification have reduced cardiac arrest risk,1 but it remains the leading cause of death in spite of these advances. Antiarrhythmic drug therapy has been shown repeatedly to increase mortality,3-5 or at best to have a neutral effect on mortality (in studies of amiodarone or dofetilide).6, 7 Currently, the only safe and reliable therapy for ventricular arrhythmias is the implantable defibrillator, but defibrillators do not prevent arrhythmias; they treat the rhythm with rapid pacing or a painful shock after it occurs. In addition, frequent complications of defibrillator implantation procedures, including infection, cardiac perforation, and failure of either the lead or device, have further reduced enthusiasm for defibrillators as primary therapy.8, 9 These limitations have led to considerable speculation on the potential utility of gene therapy for ventricular arrhythmias. In this review, I will discuss basic concepts in cardiac arrhythmia mechanisms and the literature on ventricular arrhythmia gene therapy studies.
Cardiac Arrhythmia Mechanisms
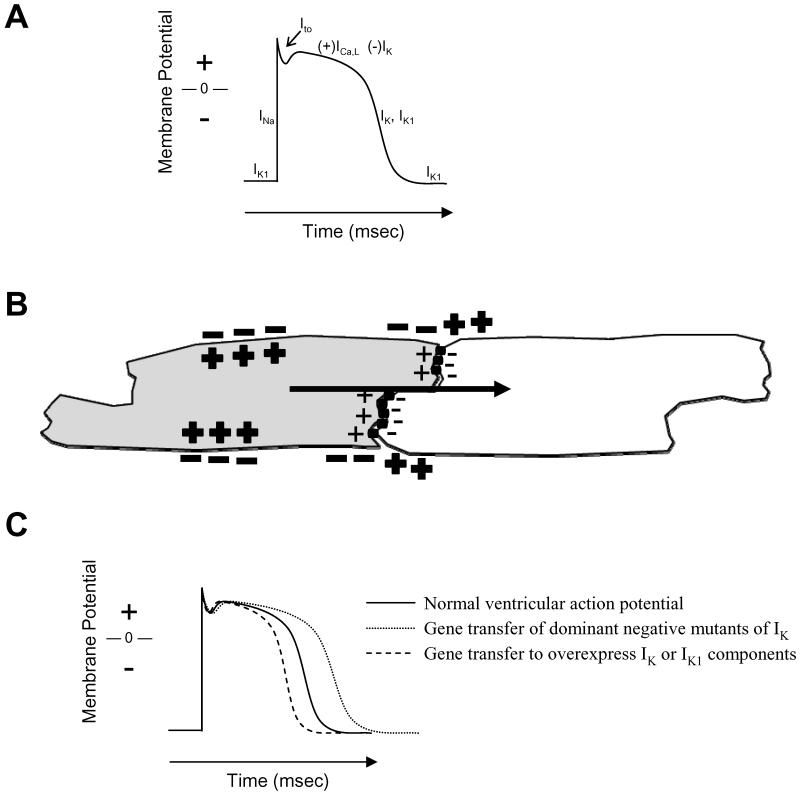
The cellular basis of cardiac electrical activity is the action potential (Figure 1a). The characteristic spike and dome morphology of the action potential is caused by time- and voltage-dependent activation of various sodium, calcium and potassium channels and pumps. Opening of the sodium and calcium channels generates a positive inward current that causes electrical activation of (depolarizes) the myocytes. Potassium currents are positive in the outward direction, reversing the electrical activation caused by the sodium and calcium channels and resetting (repolarizing) the myocyte. This electrical activity propagates from one myocyte to the next through low resistance intercellular connections provided by gap junctions and through changing electrochemical gradients in the extracellular space caused by the activated myocytes (Figure 1b). Normal cardiac electrical activation starts with activation of sinoatrial nodal myocytes that is transmitted initially to adjacent atrial myocytes and then to atrioventricular nodal myocytes and His-Purkinje myocytes that transmit the impulse to ventricular myocytes. Gene transfer-induced manipulation of any of these processes can (and to some extent has been shown to) alter cardiac electrical function (Figure 1c).
FIGURE 1. Normal cardiac myocyte electrophysiology.
A. A schematic of the ventricular action potential shows the various currents active at different phases of activation and recovery. Normal ventricular myocytes have a stable resting membrane polarization maintained by the potassium current, IK1-. Externally applied activation of the cell causes opening of sodium channels in an abrupt depolarization step. The change in membrane voltage activates calcium (ICa,L) and potassium (IK) channels. The calcium current generates the plateau phase of the action potential where contraction occurs, and then potassium channel activity returns the membrane voltage to baseline. B. Propagation of cellular excitation between myocytes is shown in the schematic. The gray cell has been activated, so that the inside of the cell is positive relative to the outside due to inflow of sodium ions through the activated sodium channel. The gray cell is in open communication with the unexcited white cell through gap junctions (black cylinders) and contiguous extracellular space. The potential difference between cells causes current flow (black arrow) from the excited gray cell to the unexcited white cell. The current alters membrane voltage of the white cell, causing activation of sodium channels and generating an action potential. C. Examples of possible gene transfer induced changes in action potential morphology.
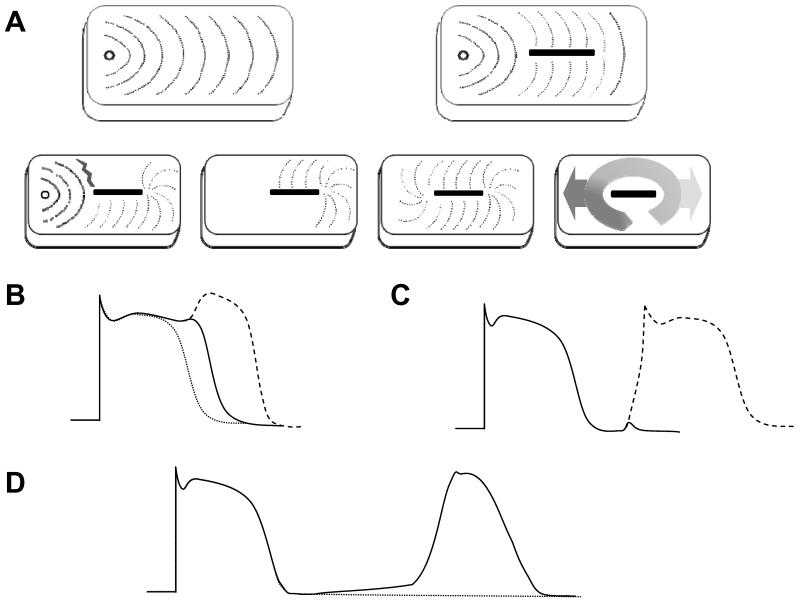
Arrhythmia mechanisms include reentry, abnormal automaticity and triggered activity (Figure 2). Reentry is a tissue-level phenomenon, requiring a critical mass of tissue for existence. Abnormal automaticity and triggered activity are cellular phenomena, capable of existing within individual cardiac myocytes. The arrhythmia occurs when the electrical impulse is propagated from this localized source of activation (tissue in the case of reentry, single cells or groups of synchronized cells in the case of triggered activity or abnormal automaticity) to the greater myocardium by the cell-to-cell transmission methods described above.
FIGURE 2. Basic mechanisms of arrhythmias.
A. Reentry. Under normal circumstances, activation spreads smoothly away from a point source (top left). In the top right, even when the myocardial tissue is disrupted by scar (depicted by the black box), activation progresses smoothly around the obstruction. The concept of reentry is depicted in the progression of activation shown across the bottom of the panel. If a stimulus is timed in a way that one pathway along the scar is refractory (gray jagged line) and the other pathway has recovered, the stimulus is prevented from propagating through the refractory pathway but it propagates normally along the recovered pathway (far left box). If the refractory tissue recovers before the stimulus wavefront reaches the end of the scar, the wavefront can now wrap around and activate the previously refractory tissue in the reverse direction (second box). The stimulus can complete the loop (third box). As long as each segment of tissue in the loop has a chance to recover before the stimulus wavefront reaches it, the stimulus will continue to revolve around the obstacle and escape along the forward and reverse directions at the beginning and end of the loop (far right box). B. Early Afterdepolarization. The dotted line shows a normal action potential. Prolongation of the action potential allows recovery of some inward sodium and calcium currents in the later stages of the prolonged action potential. Inward current from the sodium and/or calcium channels can generate a small perturbation in the action potential called an early afterdepolarization (solid line), or if the perturbation is sufficient to activate a majority of sodium and/or calcium channels it can generate a complete second action potential called a triggered beat (dashed line). C. Delayed Afterdepolarization. A small perturbation in membrane voltage after completion of the previous action potential is called a delayed afterdepolarization (solid line). If the perturbation is sufficient to activate a the inward sodium current, it can generate a triggered beat (dashed line). D. Abnormal Automaticity. Under normal circumstances, the resting membrane potential of ventricular myocytes is stable between action potentials elicited by external stimulation or propagation (dotted line). Leak or activation of depolarizing current can cause drift of the membrane potential away from baseline (solid line) that can generate secondary action potentials if the depolarizing current is sufficient to activate the cell’s sodium or calcium channels.
Reentry is the most common mechanism of arrhythmias in structural heart disease. Reentry is continual circulation of a cardiac impulse around an obstacle leading to repetitive stimulation of the surrounding cardiac tissue (Figure 2A). The obstacle can either be fixed and permanent (e.g. scar from prior MI or cardiac surgery) or functional (e.g. a mass of tissue that is excitable at slower heart rates or from a particular direction, but not at faster heart rates or from other directions). Two requirements must be met in order for reentry to occur: 1) the excitation wavefront must encounter unidirectional block and 2) either the speed of the wavefront needs to be slow enough, or the path of the reentrant circuit must be long enough in order for all cells along the circuit to regain excitability before the circulating wave returns. Conduction speed can be influenced by the inherent excitability of the tissue (mediated by sodium and/or calcium channels), or the degree of cell-to-cell coupling (mediated by gap junctions and fibrosis). Electrical recovery of the myocytes, or refractory period, is governed by factors that affect repolarization of the action potential, predominately sodium, potassium and calcium channels.
Triggered arrhythmias fall into two groups: 1) early afterdepolarizations (EADs, Figure 2B) and 2) delayed afterdepolarizations (DADs, Figure 2C). EADs occur when a secondary electrical activation occurs during the later phases of an existing action potential. These events are commonly associated with delays in repolarization of the action potential allowing recovery of the depolarizing currents (sodium and/or calcium channels) before completion of repolarization. This reactivation of the sodium or calcium currents at a point when the cell is in the process of recovering from the initial depolarization drives the cell membrane potential toward depolarization rather than allowing repolarization to continue. If this secondary depolarization has sufficient force, it can create an additional action potential. Any process that results in prolongation of the action potential duration can predispose to the development EADs.
DADs occur once the cell has fully repolarized. Increases in cytosolic calcium levels caused generally by leak from the sarcoplasmic reticulum calcium store, leads to activation of the sodium-calcium exchange pump on the cell membrane (INCX), thereby depolarizing the membrane and reaching threshold. EADs or DADs do not necessarily occur as individual, isolated incidents. Repetitive EADs or DADs occur, and the terminology for these events is ‘triggered arrhythmias.’
Abnormal automaticity arises from a more gradual leak of inward current or loss of outward current causing a loss of resting membrane potential over time after the previous action potential. When the membrane potential reaches the activation threshold for sodium or calcium channels, they open and the resulting inward current generates a new action potential. Mechanisms that cause a loss in the resting membrane potential are normally present and active in cells of the specialized conduction system (sinoatrial nodal, atrioventricular and His-Purkinje myocytes) that generate pacemaking activity for the heart, but not in normal atrial and ventricular myocytes that should ordinarily remain quiescent until activated by the specialized conduction system.
Ventricular arrhythmias during acute myocardial ischemia and post-ischemic reperfusion
Myocardial ischemia occurs when the demand for metabolites (oxygen, glucose, fatty acids, etc.) is greater than their supply, most often due to inadequate coronary blood flow. During acute ischemia, the inadequate supply of energy causes an attenuation of many physiological processes in an attempt to preserve cell survival. From an electrical standpoint, this reduces resting membrane potential, cellular excitability, and gap junctional communication; it also disrupts calcium handling and shortens repolarization time.10, 11 The end result of these changes is increased risk of reentrant arrhythmias from the conduction and repolarization changes, increased risk of DADs and triggered arrhythmias possibly due to myocardial stretch, and the potential for abnormal automaticity from the loss of resting membrane potential. In sum, ischemia can cause arrhythmias by all 3 of the above mentioned mechanisms.
Ventricular arrhythmias also occur during reperfusion after ischemia.12 The underlying cause of these arrhythmias remains incompletely understood, in part because investigation of these arrhythmias has been far less extensive than ischemic arrhythmia research. Intracellular alterations that potentially contribute to reperfusion arrhythmias include reactive oxygen species generation, intracellular calcium overload, inward current generation from reverse mode activity of the sodium-calcium exchanger, inositol-triphosphate generation from increased intracellular calcium, and heterogeneities in conduction and repolarization caused by variable recovery of individual myocytes from the ischemic insult.13-15 Postulated arrhythmia mechanisms in this complex environment include reentry and triggered arrhythmias. For more comprehensive discussion on the pathophysiology of arrhythmias during ischemia and reperfusion, please see reviews by Janse and Wit,10 and Carmeliet.11 The complex electrophysiological environment of ischemia and reperfusion makes it difficult to choose a single target for gene transfer to counteract the various element contributing to arrhythmias. Given this limitation, the strategies chosen by investigators active in this field have been to reduce either the underlying ischemic insult or the response to injury.
Yumoto et al. showed that gene transfer of hepatocyte growth factor (HGF) effectively reduced arrhythmias during acute myocardial ischemia.16 HGF is an angiogenic factor. They delivered the HGF gene by direct injection into the myocardial apex of rats using the hemagglutinating virus of Japan-coated liposomal vector system, and then they evaluated duration of ventricular fibrillation (VF) induced by alternating current during acute ischemia. The animals receiving HGF gene transfer had a shorter average duration of VF and higher electrical current requirement to induce VF than controls. The authors did not evaluate other electrophysiological parameters in the rats, but they noted increased collateral vessels on histology. They concluded that this increased collateralization reduce the ischemic insult and thereby reduced vulnerability to ischemia-related arrhythmias.
Yoshida et al. evaluated the ability of kallikrein to protect the myocardium during ischemia and reperfusion.17 Kallikrein is a component of the tissue kallikrein-kinin system upstream of bradykinin. Activation of the kallikrein-kinin system has numerous downstream effects including vasodilation, increased vascular permeability, and modulation of inflammation.18 Yoshida et al. evaluated the effects of AdKallikrein gene transfer in a rat model of ischemia-reperfusion. They delivered the virus via direct myocardial injection 1 week prior to a 30 minute ischemia-120 minute reperfusion cycle and found reductions in relative infarct size with concomitant reductions in the percentage of TUNEL-positive cells. Incidental to the primary observations on myocardial damage, Yoshida et al. also noted a reduction in the incidence of VF from a control level of 64% to 17%. They suggested that the antiarrhythmic effect was a component of the overall cardioprotective effect caused by kallikrein expression.
del Monte et al. assessed post-ischemic reperfusion arrhythmias after gene transfer of the 2A-isoform of the sarcoplasmic reticulum calcium ATPase pump (SERCA2a). SERCA2a is a pump that transports calcium out of the intracellular space and into the sarcoplasmic reticular space in cardiac myocytes. In an initial study, they delivered a recombinant adenovirus encoding SERCA2a (AdSERCA2a) to rat hearts using an aortic cross-clamping method previously described by the group.19, 20 After 2-6 days, the animals underwent coronary ligation for 30 minutes followed by 48 hours of reperfusion prior to sacrifice. During the ischemia and reperfusion phases of the experiment, the animals were monitored for arrhythmias. Relative to controls, the AdSERCA2a animals had better overall survival, and fewer episodes of ventricular tachycardia (VT) or VF. Although the authors do not explicitly state it, presumably these episodes were non-sustained because the data refer to the number of VT or VF episodes per hour. In a follow-up study, the investigators assessed AdSERCA2a in a porcine ischemia-reperfusion model.21 They delivered AdSERCA2a by intracoronary perfusion of the left anterior descending coronary artery during coronary venous occlusion. Seven days later, they occluded the LAD for 30 minutes with a balloon catheter and then continued electrocardiographic monitoring for 24 hours of reperfusion. An additional set of animals had permanent occlusion of the coronary artery using embolic coils. Comparing the AdSERCA2a animals to controls, the investigators saw no difference in frequency of VT or VF during active ischemia but a reduction in VT and VF episodes during the first 10 minutes of reperfusion. They acknowledged that the vast majority of observed arrhythmias were non-sustained, but they also observed fewer arrhythmias that they termed “life threatening” (3±1 in AdSERCA2a animals vs. 7±3 in controls). Of concern when looking at their individual data, the majority of risk reduction seems to be for either sustained or non-sustained VT and there was a trend toward increased number of VF episodes in the AdSERCA2a group. There were no differences seen in the animals with permanent occlusion.
A limitation of all currently available reports on gene therapy for ischemia or reperfusion arrhythmias is the absence of any cellular or tissue-level electrophysiology data to address mechanism for the benefit noted in gross assessment of arrhythmias.
Ventricular arrhythmias during the healing phase shortly after MI
Numerous remodeling events occur in the hours and days following an MI. Closure and migration of gap junctions away from cell borders causes slow and inhomogeneous conduction.22 Dysfunction of sodium, calcium and potassium channels reduces cellular excitability, alters repolarization and reduces the capacity of the cell to maintain resting membrane potential.23-26 The end results of these changes are possibilities for reentrant, triggered and automatic arrhythmias.
In an initial attempt to overcome the cellular electrophysiological dysfunction in infarct borderzone myocytes, Lau et al. tested the hypothesis that myocardial conduction velocity would improve and reentrant arrhythmias would be disrupted by introduction of a sodium channel with activity at the reduce membrane potential found in damaged myocytes.27 They noted that the skeletal muscle sodium channel isoform, SkM1, had a more positive voltage-dependence of inactivation than the cardiac variant, so they used adenovirus-mediated gene transfer and direct injection delivery to introduce SkM1 into myocytes of the infarct border zone. The sites targeted for AdSkM1 injections were those with wide-complex, bipolar epicardial electrograms. After gene transfer, the AdSkM1 group showed improved conduction and significant reduction in ventricular tachyarrhythmia inducibility during electrophysiology study.28, 29
The majority of gene transfer strategies aiming to modify electrophysiological function have overexpressed a specific gene of interest, with subsequent overexpression of the mRNA and protein. However, microRNAs have emerged as additional regulators of the translational of mRNA into protein. Yang and colleagues,30 noted an increase in microRNA, miR-1, after MI in humans and an experimental rat model. miR-1 inhibits expression of the gap junctional protein, connexin43, and the resting membrane potential-maintaining ion channel Kir2.1. In the rat MI model, they targeted miR-1 with antagomirs. Twelve hours after MI, animals treated with the antagomir to miR-1 showed suppression of arrhythmia vulnerability; in addition, significantly enhanced conduction velocity and decreased resting membrane potential were observed with antagomir pretreatment.
Ventricular arrhythmias after healing from MI
Approximately 4 weeks post-MI, the general healing process (i.e. inflammation, myocyte cell death and cell debris clearance, collagen deposition, etc.) has receded31. The MI scar border generally consists of surviving myocytes interdigitated with fibrosis. The disruption in cell-to-cell communication caused by the fibrosis most likely accompanied by some residual dysfunction of the surviving myocytes is the ideal substrate for reentrant arrhythmias. This substrate therefore represents the large majority of the post-MI patient population at risk of developing ventricular tachyarrhythmias.
Our lab has adopted a strategy of increasing either conduction velocity or cellular repolarization time to increase the probability of disrupting reentry by causing the electrical activation wavefront to meet refractory tissues. In initial proof-of-concept studies, we exploited a dominant-negative potassium channel mutation, KCNH2-G628S, that increased action potential duration by eliminating one of the key myocyte repolarization currents.32 We tested the hypothesis that localized in vivo gene transfer of this mutant channel to the VT site would prolong the refractory period, thereby extending the reentrant wavelength and eliminating VT circuit formation. In a model of reliable VT inducibility after healed MI,33 we delivered AdKCNH2-G628S to the anterior septum of pigs via the left anterior descending coronary artery and great cardiac vein.33, 34 Controls were either no gene transfer or adenovirus containing the beta-galactosidase gene. With this method, 50% of cells of the septal scar border show evidence of transgene expression. One week after gene transfer, the animals receiving AdKCNH2-G628S no longer had any ventricular arrhythmias, but all control animals continued to have inducible sustained monomorphic VT.32
An important consideration brought out by our data is the difference between antiarrhythmic drugs and genetic interventions. We observed improved efficacy and reduced toxicity in this model with KCNH2-G628S gene transfer compared to dofetilide (a pharmacological blocker of the channel produced by KCNH2). Animals no longer had any ventricular arrhythmias after gene transfer, but animals remained inducible after dofetilide. One of the dofetilide animals also had spontaneous Torsades des Pointes, a known proarrhythmic toxicity of the drug. Since both drug and gene operate by the same mechanism (i.e. block of the same ion channel), we concluded that the pharmacokinetic and pharmacodynamic properties of the drug likely played a role in the reduced efficacy of the drug. These limitations to drug therapy suggest that we cannot make assumptions on the behavior of a genetic manipulation based on the actions of similar pharmacotherapy.
Lyon et al. assessed SERCA2a in a rat model of post-infarction cardiomyopathy.35 They used both AdSERCA2a and an adeno-associated virus serotype 9 (AAV9) vector encoding SERCA2a. They infarcted rats by ligation of the LAD. Sixteen weeks later, they delivered either AdSERCA2a by intramyocardial injection or AAV9SERCA2a via tail vein infusion. The Ad animals were followed for 1 week, and the AAV animals were followed for 4-6 weeks prior to sacrifice and analysis. SERCA2a-overexpressing animals had fewer spontaneous premature ventricular contractions, and fewer spontaneous or induced non-sustained VT episodes. Spontaneous sustained VT or VF was not sufficiently common in the model to assess differences. SERCA2a-overexpressing animals also had fewer sustained VT or VF episodes after isoproterenol administration. Cellular analysis showed a reduction in spontaneous SR calcium release events, total SR calcium leak, and triggered arrhythmias.
Ventricular arrhythmias in cardiomyopathic hearts
The presence of beat-beat alternation of the cardiac action potential duration is linked to the onset of ventricular arrhythmias and sudden death in cardiomyopathies.36 The cellular mechanisms underlying these events have been proposed to be either alterations in ionic currents or intracellular calcium cycling, manifested by a steep slope of restitution. Cutler et al.,37 assessed mechanisms and potential therapies for alternans in normal guinea pig hearts, but the basic findings of their study should be relevant to cardiomyopathic hearts where calcium handling is impaired. They used adenovirus-mediated overexpression of SERCA2a (via the aortic cross-clamp method noted above) to improve the beat-beat removal of diastolic calcium. 72 hours after guinea pig hearts had received AdSERCA2a, myocytes were isolated. AdSERCA2a treated cells demonstrated faster calcium reuptake kinetics and calcium transient amplitude compared to non-infected cells. In addition, AdSERCA2a myocytes exhibited a lack of APD and calcium transient alternans during pacing at 200 and 240 bpm when compared to control. Furthermore, in vivo measurements of the threshold (heart rate) for APD alternans was significantly increased in the AdSERCA2a treated group. The overall arrhythmogenic efficacy of rapid pacing was significantly reduced in the AdSERCA2a group.
Inherited ventricular arrhythmia syndromes
Brunner et al. investigated the possibility of using gene transfer to correct the QT interval in a mouse model of the long QT syndrome. The long QT syndrome is a disorder of cardiac repolarization. In general, an imbalance between depolarizing and repolarizing currents lengthens the action potential, which is manifest on the 12-lead electrocardiogram as a prolongation of the QT interval. A variety of mutations in sodium, calcium and potassium channels and associated proteins have been described to cause the Long QT Syndrome (for a more extensive review on mechanisms of inherited arrhythmia syndromes, see a review by Kaufman).38 The transgenic model expresses an N-terminal tag of Kv1.1 that exhibits dominant negative suppression of repolarizing currents by trapping Kv1 subfamily channels in the endoplasmic reticulum.39 The animals have QT prolongation in ECG and spontaneous ventricular arrhythmias. In an initial gene transfer study, they injected adenoviruses encoding Kv1.5 into transgenic mice bearing a dominant negative Kv1.1 mutation and evaluated effects 7 days after gene transfer.40 In a follow-up study the same group used AAV vectors and looked 6 months after injection.41 In both studies, the investigators found shortening of action potential duration and QT interval after gene transfer. All animals had a negligible incidence of arrhythmias, so the study could not realistically test the effect of therapy on arrhythmia prevention. Overall, these studies demonstrated the important concept that a dominant negative mutation could be rescued by transfer of a gene that replaced the function but that was not susceptible to the dominant negative interaction. In essence, they corrected the phenotype without correcting the genotype.
More recently, Denegri et al. evaluated gene transfer in mice with Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT).42 The general mechanism for this inherited arrhythmia syndrome is inappropriate calcium handling in the sarcoplasmic reticulum (SR) associated with mutations either of the SR calcium release channel (ryanodine receptor) or an intra-SR calcium buffering protein (calsequestrin). In this syndrome, diastolic release of calcium from the sarcoplasmic reticulum causing DADs and triggered arrhythmias, predominately in the presence of adrenergic stimulation. Denegri et al. used a calsequestrin knock-out transgenic mouse line and evaluated gene transfer with an AAV vector encoding wild-type calsequestrin. Neonatal animals were transduced by intraperitoneal injection, and animals were studied at 20 weeks of age. They found that calsequestrin replacement occurred in 50-60% of ventricular myocytes. Cells expressing the transgene had reduced susceptibility to DADs in the presence of isoproterenol, and the calsequestrin gene transfer-animals had a significant reduction in VT after epinephrine infusion.
CONCLUSIONS
Literature reports of gene therapy for ventricular arrhythmias shows early signs of success. Strategies have focuses on intervening either to eliminate the underlying disease process (in the case of ischemic arrhythmias) or to prevent the basic elements necessary to perpetuate the arrhythmias in cases where the underlying disease is not readily reversible (in cases of myocardial scar or heart failure). All of the available literature in this area consists of early phase proof-of-concept studies. Study size has been small; some of the ideas have only been tested in rodents, and none have comprehensively evaluated the functional manifestations of the proposed therapy. Potential limitations to arrhythmia gene therapy that have not yet been assessed include the possibility of conduction or repolarization gradients from heterogeneous gene transfer (assumed to be a problem based on theory but not observed to be a problem in the above discussed examples), of adaptation or maladaptation affecting efficacy or toxicity after chronic transgene expression (not yet assessed in any arrhythmia study), or the possibility of interactions between disease process and therapeutic intervention as the underlying cardiac disease progresses. With further study to more fully define therapeutic mechanism, to investigate potential side effects of therapy and to test efficacy in models relevant to the human disease, gene therapy has the potential to revolutionize our treatment of these life-threatening syndromes.
ACKNOWLEDGEMENTS
This work was funded by the grants from National Institutes of Health (HL67148, HL93486, EB2846).
Footnotes
CONFLICT OF INTEREST. The author declares no conflicts.
REFERENCES
- (1).Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Rea TD, Pearce RM, Raghunathan TE, Lemaitre RN, Sotoodehnia N, Jouven X, et al. Incidence of out-of-hospital cardiac arrest. Am J Cardiol. 2004;93:1455–1460. doi: 10.1016/j.amjcard.2004.03.002. [DOI] [PubMed] [Google Scholar]
- (3).Echt D, Liebson P, Mitchell L, Peters R, Obias-Manno D, Barker A, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- (4).Waldo A, Camm A, deRuyter H, Friedman P, MacNeil D, Pauls J, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- (5).Kuck K, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- (6).Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- (7).Kober L, Bloch Thomsen PE, Moller M, Torp-Pedersen C, Carlsen J, Sandoe E, et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–2058. doi: 10.1016/s0140-6736(00)03402-4. [DOI] [PubMed] [Google Scholar]
- (8).Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28:926–932. doi: 10.1111/j.1540-8159.2005.00195.x. [DOI] [PubMed] [Google Scholar]
- (9).Gould P, Krahn A, Canadian Heart Rhythm Society Working Group on Device Advisories Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295:1907–1911. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- (10).Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- (11).Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- (12).Goldberg S, Greenspon AJ, Urban PL, Muza B, Berger B, Walinsky P, et al. Reperfusion arrhythmia: a marker of restoration of antegrade flow during intracoronary thrombolysis for acute myocardial infarction. Am Heart J. 1983;105:26–32. doi: 10.1016/0002-8703(83)90274-0. [DOI] [PubMed] [Google Scholar]
- (13).Woodcock EA, Arthur JF, Matkovich SJ. Inositol 1,4,5-trisphosphate and reperfusion arrhythmias. Clin Exp Pharmacol Physiol. 2000;27:734–737. doi: 10.1046/j.1440-1681.2000.03328.x. [DOI] [PubMed] [Google Scholar]
- (14).Brooks WW, Conrad CH, Morgan JP. Reperfusion induced arrhythmias following ischaemia in intact rat heart: role of intracellular calcium. Cardiovasc Res. 1995;29:536–542. [PubMed] [Google Scholar]
- (15).Valverde CA, Kornyeyev D, Ferreiro M, Petrosky AD, Mattiazzi A, Escobar AL. Transient Ca2+ depletion of the sarcoplasmic reticulum at the onset of reperfusion. Cardiovasc Res. 2010;85:671–680. doi: 10.1093/cvr/cvp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yumoto A, Fukushima KK, Nakamura K, Hashimoto K, Aoki M, Morishita R, et al. Hepatocyte growth factor gene therapy reduces ventricular arrhythmia in animal models of myocardial ischemia. Acta Med Okayama. 2005;59:73–78. doi: 10.18926/AMO/31982. [DOI] [PubMed] [Google Scholar]
- (17).Yoshida H, Zhang JJ, Chao L, Chao J. Kallikrein gene delivery attenuates myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Hypertension. 2000;35:25–31. doi: 10.1161/01.hyp.35.1.25. [DOI] [PubMed] [Google Scholar]
- (18).Sharma JN. Cardiovascular activities of the bradykinin system. ScientificWorldJournal. 2008;8:384–393. doi: 10.1100/tsw.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).del MF, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hajjar R, Schmidt U, Matsui T, Guerrero L, Lee K, Gwathmey J, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118:614–624. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- (22).Peters N, Coromilas J, Severs N, Wit A. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- (23).Dun W, Boyden P. Diverse phenotypes of outward currents in cells that have survived in the 5-day-infarcted heart. Am J Physiol. 2005;289:H667–H673. doi: 10.1152/ajpheart.00180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Jiang M, Cabo C, Yao J, Boyden P, Tseng G. Delayed rectifier K currents have reduced amplitudes and altered kinetics in myocytes from infarcted canine ventricle. Cardiovasc Res. 2000;48:34–43. doi: 10.1016/s0008-6363(00)00159-0. [DOI] [PubMed] [Google Scholar]
- (25).Lue W, Boyden P. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Circulation. 1992;85:1175–1188. doi: 10.1161/01.cir.85.3.1175. [DOI] [PubMed] [Google Scholar]
- (26).Pu J, Boyden P. Alterations of Na+ currents in myocytes from epicardial border zone of the infarcted heart. A possible ionic mechanism for reduced excitability and postrepolarization refractoriness. Circ Res. 1997;81:110–119. doi: 10.1161/01.res.81.1.110. [DOI] [PubMed] [Google Scholar]
- (27).Lau DH, Clausen C, Sosunov EA, Shlapakova IN, Anyukhovsky EP, Danilo P, Jr., et al. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: an in silico, in vivo, in vitro study. Circulation. 2009;119:19–27. doi: 10.1161/CIRCULATIONAHA.108.809301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lau DH, Clausen C, Sosunov EA, Shlapakova IN, Anyukhovsky EP, Danilo P, Jr., et al. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: an in silico, in vivo, in vitro study. Circulation. 2009;119:19–27. doi: 10.1161/CIRCULATIONAHA.108.809301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Coronel R, Lau DH, Sosunov EA, Janse MJ, Danilo P, Jr., Anyukhovsky EP, et al. Cardiac expression of skeletal muscle sodium channels increases longitudinal conduction velocity in the canine 1-week myocardial infarction. Heart Rhythm. 2010;7:1104–1110. doi: 10.1016/j.hrthm.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- (31).Fishbein MC, Maclean D, Maroko PR. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978;90:57–70. [PMC free article] [PubMed] [Google Scholar]
- (32).Sasano T, McDonald AD, Kikuchi K, Donahue JK. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat Med. 2006;12:1256–1258. doi: 10.1038/nm1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sasano T, Kelemen K, Greener ID, Donahue JK. Ventricular tachycardia from the healed myocardial infarction scar: validation of an animal model and utility of gene therapy. Heart Rhythm. 2009;6:S91–S97. doi: 10.1016/j.hrthm.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sasano T, Kikuchi K, Feng N, McDonald A, Lai S, Donahue J. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42:954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- (37).Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2:686–694. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kaufman ES. Mechanisms and clinical management of inherited channelopathies: long QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia, and short QT syndrome. Heart Rhythm. 2009;6:S51–S55. doi: 10.1016/j.hrthm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- (39).London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, et al. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci U S A. 1998;95:2926–2931. doi: 10.1073/pnas.95.6.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Brunner M, Kodirov S, Mitchell G, Buckett P, Shibata K, Folco E, et al. In vivo gene transfer of Kv1.5 normalizes action potential duration and shortens QT interval in mice with long QT phenotype. Am J Physiol. 2003;285:H194–H203. doi: 10.1152/ajpheart.00971.2002. [DOI] [PubMed] [Google Scholar]
- (41).Kodirov S, Brunner M, Busconi L, Koren G. Lonng-term restitution of 4-aminopyridine-sensitive currents in Kv1DN ventricular myocytes using adeno-associated virus-mediated delivery of Kv1.5. FEBS Lett. 2003;550:74–78. doi: 10.1016/s0014-5793(03)00822-6. [DOI] [PubMed] [Google Scholar]
- (42).Denegri M, Avelino-Cruz JE, Boncompagni S, De Simone SA, Auricchio A, Villani L, et al. Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias. Circ Res. 2012;110:663–668. doi: 10.1161/CIRCRESAHA.111.263939. [DOI] [PubMed] [Google Scholar]