Abstract
Progenitor cells (PCs) are key components of vasculogenic remodeling and hematopoietic development. Decreases in the number and function of angiogenic progenitors have been observed in coronary artery disease, hypertension, and diabetic vasculopathy. Several recent studies have also demonstrated a close relationship between increased visceral fat and cardiovascular disease, implying an association between obesity and vascular dysfunction. However, very little is known about the role of PCs in obesity. We generated whole genome expression profiles of cultured PCs from 18 obese and 6 lean African-American women on Agilent microarrays and analyzed the data through bioinformatic pathway analysis using multiple databases and analytic tools. False-discovery rates (FDR) were calculated to assess statistical significance while controlling for multiple testing. We identified 1,545 upregulated and 2,257 downregulated genes associated with obesity (1.5-fold or greater absolute fold-change). Pathway analysis further identified a statistically significant downregulation of immune-response pathways in the obese subjects, including T-cell receptor signaling, natural killer cell signaling, and chemokine-signaling pathways (FDR <5%). Chemokine gene-expression patterns were consistent with an angiogenic–angiostatic imbalance and a downregulation of CXCR3 receptor-mediated signaling in the PCs from obese subjects. Overall, these findings reveal a novel transcriptional signature in cultured PCs from obese African-American women and further suggest that obesity-associated immune-compromise may originate much earlier in cellular development than currently appreciated. Clinically, this may translate into a lengthier period of immune dysregulation in obese subjects exposing them to greater risks of infection and other morbidities.
INTRODUCTION
Angiogenic progenitor cells (PCs) are a key component of vasculogenic remodeling in primary vascular networks. Reductions in the number of circulating PCs are associated with endothelial dysfunction and risk of future cardiovascular events (1). Additionally, impairments in the number and function of progenitors are also observed in coronary artery disease, hypertension, and diabetic vasculopathy (2).
The close inter-relationship between increased visceral fat, metabolic disturbances, low-grade inflammation, and cardiovascular diseases strongly suggests an association between obesity and vascular pathology. Obesity has been associated with vascular dysfunction and blunted endothelium-dependent vasodilation (3). Elevations in endothelin and reductions in nitric oxide availability have also been proposed as central mechanisms for the endothelial dysfunction associated with obesity (4). However, despite the strong association between obesity and vascular dysfunction and the known role of PCs in vascular remodeling, the precise contribution of these cells to obesity and obesity-associated dysfunctions has not been adequately addressed.
In the present study, we have investigated this relationship by conducting transcriptome analysis on cultured mononuclear PCs from obese and lean African-American women. PC cultures usually consist of a mixed population of hematopoietic and vasculogenic cells (5). We refer to these cells as cultured PCs while acknowledging their compositional heterogeneity (6). The heterogeneous nature of PCs, notably the presence of hematopoietic and angiogenic cell precursors, implies that PCs might play key roles in additional biological functions besides vasculogenesis. By providing a systems-based view of the transcriptome, whole-genome expression profiling allows us to interrogate such additional functions that might be altered in cultured PCs as a result of obesity.
We have previously identified obesity-associated transcriptomic changes in multiple signaling pathways in obese and lean subjects of Northern European ancestry (7–9). We have now adopted a similar approach to examine the gene-expression profiles in cultured PCs from obese and lean premenopausal African-American women, and have applied bioinformatics analysis to identify obesity-associated pathway alterations. Our results identify gene-expression patterns and biological pathways that provide additional insights into obesity-associated transcriptional remodeling and its possible pathophysiological consequences.
METHODS AND PROCEDURES
Study subjects
Healthy African-American women (18–45 years, self-reported race) without clinical evidence of cardiovascular disease were recruited. Exclusion criteria included the presence of any of the following at screening: coronary artery disease, ever smoking, diabetes mellitus (fasting plasma glucose >126 mg/dl or treatment of diabetes), hypercholesterolemia, hypertension (blood pressure >140/90 mm Hg or treatment for high blood pressure); rheumatoid arthritis/other chronic inflammatory diseases; pregnancy, lactation, use of vasoactive medications, sickle cell disease, renal insufficiency (estimated glomerular filtration rate of 60 ml/min/1.73 m2 or less) or hormonal therapy. None of the study participants were on statins or other lipid lowering medications. Volunteers enrolled into the study were divided into lean (BMI ≤25) and obese (BMI ≥30) classes. Subsets from a total of 39 obese and 9 lean subjects were drawn for genomic analysis and biochemical characterizations. Studies were conducted at the Clinical Research Center of the Morehouse School of Medicine. All protocols were approved by the institutional review board and informed consent was obtained from all volunteers. Volunteers fasted overnight and were instructed to refrain from drinking alcohol or caffeine 8 h prior to the Clinical Research Center visit.
Analyte measurements
Assays for insulin, glucose, and lipids were performed on contract (1777 Montreal circle; QUEST Labs, Tucker, GA) according to vendor-optimized assays.
PC culture
PCs were cultured in Endocult colony-forming unit-Hill liquid media system (Stem Cell Technologies, Vancouver, British Columbia, Canada) according to published procedures (10). Culture supplements were from different lots during the study, and the manufacturer’s specifications state that some variability is unavoidable. Peripheral blood mononuclear cells were obtained from buffy coats by subsequent purification over Ficoll gradients. After purification, 5 × 106 cells were plated per well of a 6-well fibronectin-coated plate. After 48 h, nonadherent cells were recovered, counted and subsequently replated on fibronectin-coated dishes at a density of 1 × 106 cells/well. Media was changed every 2 days. The attached cells were assessed immunologically by CD31 staining on day 9 after plating to confirm similarities to previously characterized human PC colonies. Total numbers of colony-forming unit-Hill colonies were quantified at day 5. Cells were cultured for a total of 14 days to provide enough RNA for microarray analysis.
RNA isolation and microarray hybridization
Procedures pertaining to RNA isolation, sample preparation, Agilent microarray hybridization, and raw data extraction are described in Supplementary Appendix S1.
Quantitative PCR analysis
Eighty seven genes were selected for quantitative PCR (qPCR) follow-up (based on differential expression or biological function of interest) in custom 96-well plates (Qiagen, CA). Quantitative SybrGreen PCR was performed in multi-well plates using the RT-PCR master mix (Qiagen) according to protocol. Details of qPCR procedure are described in Supplementary Appendix S2.
Differential gene-expression analysis
Details about microarray gene-expression data normalization, transformation, and outlier detection are described in Supplementary Appendix S3. Differential gene-expression analysis was conducted on 3,006 genes after excluding genes with an intragroup coefficient of variation >25% and an absolute fold-change <1.5-fold between obese and lean samples. Differentially expressed genes were identified via GenePattern (11) using standard t-test and asymptotic P values. The false-discovery rate (FDR) was controlled to correct for multiple testing (12).
Pathway analysis
Gene Set Enrichment analysis (GSEA)
GSEA was performed on a dataset of 35,543 Agilent features (multiple features often query the same gene, so the number of genes is less than the number of features). Statistically significant differences in predefined gene-sets (pathways) were identified by querying three separate pathway databases—Kyoto Encyclopedia of Genes and Genomes (KEGG), Biocarta, and a user-defined custom pathway database (Custom). To avoid spurious associations, pathways containing ≤10 genes or ≥200 genes were excluded from analysis, resulting in 180 (KEGG), 203 (Biocarta), and 152 (Custom) pathways for analysis. A weighted, Kolmogorov–Smirnov statistic (Normalized Enrichment Score) was calculated for each geneset, based on the over-representation of gene-set members toward the top or bottom of a ranked list of genes. Statistical significance of Normalized Enrichment Score was estimated by permutation. A FDR was calculated to control for multiple testing (12). The distribution of fold-changes for genes in selected pathways was visualized by MA-plots. MA plots characterize the dependencies between the log ratio of gene expression and the mean value of the gene expression between two compared groups (obese vs. lean, in this case).
Ingenuity Pathway analysis (IPA) and Database of Annotation, Visualization, and Integrated Discovery (DAVID)
IPA or DAVID-based pathway analysis was conducted with 1,145 obese-upregulated and 2,257 obese-downregulated Agilent features (absolute fold-change ≥1.5) by querying the respective knowledge repositories of the tools. Pathway enrichment was examined through Fisher’s exact test. Multiple testing corrections were calculated according to Benjamini and Hochberg (13).
Gene set similarity analysis
The degree of similarity among the significantly enriched gene-sets from KEGG, Biocarta, and Custom databases was quantified by (i) Venn diagram analysis and (ii) concordance analysis by the List of lists-annotated software program (14) (http://www.lola.gwu.edu/). Concordance between two gene lists was calculated as the number of genes in common divided by the number of genes in both. The variance (and 95% confidence intervals) of the concordance was calculated as A(B + C)/(A + B + C)3 where A is the number of common genes and B and C are the numbers of unique genes on the two lists. Significance of the concordance was calculated as
where c is the number of genes common to both lists, m1 and m2 are the sizes of the two lists, n1 and n2 are the sizes of the two arrays, and s is the total number of probes from which the two arrays were sampled.
RESULTS
Participants
Obese subjects differed significantly from the lean subjects for age, BMI, waist-to-hip ratio, and whole-body insulin resistance (homeostatic model assessment insulin resistance (HOMA2IR)) (Table 1). Blood chemistry results for all subjects were in the clinical normal ranges, not requiring medical treatment for hypertension, dyslipidemia, or hyperinsulinemia. Colony-forming unit-Hill colony count trended toward higher numbers in the lean group compared to the obese (statistically nonsignificant, P > 0.05) consistent with published findings for individuals with cardiovascular risk factors (15).
Table 1.
Demographic characteristics of the study subjects
| Obese | Lean | |||
|---|---|---|---|---|
| Variable | Normal range | Avg. (s.d.) | P value | |
| N | 39 | 9 | — | |
| Age (years) | 31.2 (6.4) | 25.6 (4.6) | <0.01 | |
| BMI (kg/m2) | 38.6 (5.17) | 22.5 (2.0) | <0.0001 | |
| Waist-to-hip ratio | 0.9 (0.1) | 0.7 (0.03) | <0.0001 | |
| BP, systolic (mm Hg) | ≤120 | 120.1 (15.1) | 109.0 (10.5) | 0.04 |
| BP, diastolic (mm Hg) | ≤80 | 82.2 (11.2) | 72.3 (7.3) | <0.01 |
| HOMAIR | ≤2 | 2.5 (1.9) | 1.6 (1.5) | 0.04 |
| Insulin (µIU/ml)a | <17 | 17.0 (29.1) | 7.3 (7.1) | 0.02 |
| Glucose (mg/dl)a | 65–99 | 89 (15.2) | 82.6 (7.1) | 0.16 |
| HDL-cholesterol (mg/dl) | >46 | 52.8 (11.4) | 74.6 (14.3) | <0.001 |
| LDL-cholesterol (mg/dl) | <130 | 96.3 (27.2) | 76.1 (29.2) | 0.08 |
| Cholesterol (mg/dl) | 125–200 | 166.0 (33.5) | 162.6 (33.6) | 0.66 |
| Triglycerides (mg/dl) | <150 | 84.2 (37.9) | 59.9 (19.1) | 0.02 |
| White blood cell count (WBC) | 4.1–10.9 × 103/µl | 5.99 (1.71) | 6.24 (2.17) | 0.35 |
| Lymphocytes (%) | 20–50% | 35.8 (7.5) | 32.3 (9.84) | 0.88 |
| Monocytes (%) | 2–12% | 6.51 (1.6) | 6.36 (1.28) | 0.60 |
| CFU-Hill colonies | 18.15 (16.09) | 37.5 (19.09) | 0.06 | |
Demographic and clinical parameters were measured in obese and lean subjects by standard techniques as described in the text. Due to the relatively small number of lean samples, a nonparametric P value (Wilcoxon rank sum test) is reported.
BP, blood pressure; HDL, high-density lipoprotein; HOMAIR, homeostatic model assessment insulin resistance; LDL, low-density lipoprotein.
Glucose measures were obtained from 8 lean and 33 obese subjects; insulin measures were obtained from 8 lean and 31 obese subjects.
Cell-type marker analysis
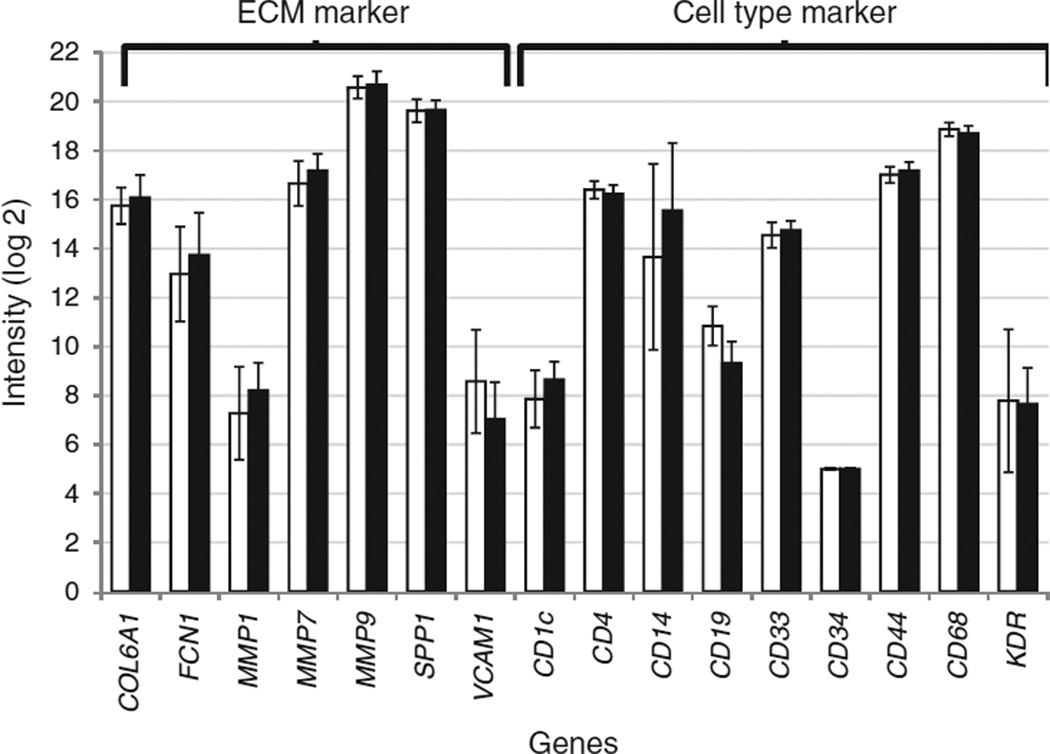
Microarray data was examined for expression of lymphoid, myeloid, and vascular lineage cell markers in the PC populations. A total of 32 cell markers were examined based on their reported selective expression on specific subsets of lymphoid- or myeloid-derived cells (16). Only 7/32 markers were reliably expressed in the dataset, demonstrating that the PCs were specific only to certain cell-types. These included CD4 (Thelper and Tregulatory cell marker), CD19 (B-cells), CD33 (myeloid progenitors), CD44 (eosinophils), CD14 (monocyte/macrophages), CD68 (macrophages), and CD1c (myeloid dendritic cells), confirming the heterogeneous composition of the cultured cell populations. With the exception of CD19 (threefold higher expression in lean, P < 0.003), none of the other genes were differentially expressed between the obese and lean samples. Three cell-surface markers (CD14, CD34, and KDR) were additionally examined by qPCR and no statistically significant differences were observed between the two groups. The expression levels of seven extracellular matrix remodeling vasculogenic-marker genes (COL6A1, FCN1, MMP1, MMP7, MMP9, SPP1, and VCAM1) were also examined (17). Again, none of the tested genes tested showed statistically significant differences in expression between the obese and lean samples. These findings suggest that despite possible compositional heterogeneity, the PCs from obese and lean subjects were largely similar to each other (Figure 1a).
Figure 1.
Extracellular matrix and cell-type specific marker gene expression in cultured progenitor cells from obese and lean subjects. Gene-expression signals (log base 2) of extracellular matrix markers and cell-type markers in progenitor cells from 13 obese (black bars) and 6 lean (white bars) subjects. All gene-expression signals were obtained from Agilent microarrays with the exception of CD14, CD34, and KDR which were assessed by quantitative PCR.
Differential gene expression
Expression patterns of the top 50 differentially expressed genes (FDR<0.1) are shown in Supplementary Figure S1 online. A subset of 71 genes was further validated by qPCR. The magnitude of gene-expression fold-changes were significantly correlated between microarray and qPCR platforms (R2 = 0.68, P < 0.0001). 83% (59/71) of the genes were also concordant for the direction of fold-change between microarray and qPCR analyses (see Supplementary Figure S2 online).
Differentially regulated pathways
GSEA analysis
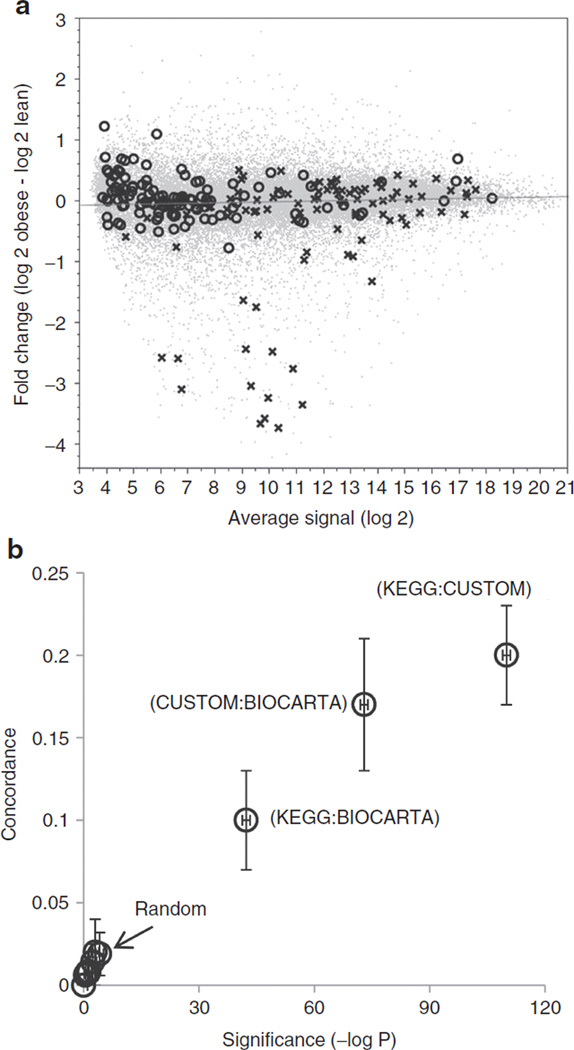
GSEA analysis was used to separately interrogate the KEGG, Biocarta, and Custom pathway databases for gene-set enrichment in obese or lean subjects. The enriched pathways were ranked by their Normalized Enrichment Score, nominal P value and FDR. Supplementary Table S1 online contains the results for the top 50 enriched pathways from each of the 3 databases. When sorted by significance level (P < 0.005 and FDR < 0.01), 12 KEGG pathways, 16 Biocarta pathways, and 12 Custom pathways were identified as downregulated in the obese subjects (Table 2). In contrast, none of the obese-upregulated pathways reached the same level of statistical significance in any of the databases queried (minimum observed FDR >0.29). The fold-change distribution for gene-members from the top obese-downregulated (T-cell receptor signaling) and obese-upregulated KEGG pathways (olfactory signal transduction) were compared via MA plots in Figure 2a. Genes unrelated to the selected pathways (gray dots) displayed a symmetric distribution of fold-changes around a log ratio of zero, indicating an overall parity in the number of up- and downregulated genes in the obese and lean subjects. Genes in the obese-upregulated, olfactory transduction pathway (circles) showed an enrichment for positive log ratios, but the effect-sizes were small (this explains the weaker results observed in GSEA). However, when considering the T-cell receptor signaling pathway (crosses), several genes with large negative log ratios were observed, contributing to a significant downregulation of this pathway in the obese subjects. To further interrogate how gene expression varied at the individual level, we plotted the subject-level expression patterns for the top 10 genes contributing to enrichment of one top pathway each from KEGG, Biocarta and Custom. Results are summarized as a heat map in Supplementary Figure S3 online.
Table 2.
Comparative pathway enrichment results from Gene Set Enrichment Analysis (GSEA)
| KEGG | BIOCARTA | CUSTOM |
|---|---|---|
| T_CELL_RECEPTOR_SIGNALING_PATHWAY | TOB1_PATHWAY | LYMPHOCYTE_AFFYTECHNOTE |
| PRIMARY_IMMUNODEFICIENCY | CSK_PATHWAY | T_BCELL ACTIVATION |
| ALLOGRAFT_REJECTION | CTLA4_PATHWAY | XEF;VE CD4+ T CELLS |
| CELL_ADHESION_MOLECULES_CAMS | NKT_PATHWAY | IL12-MEDIATED SIGNALING EVENTS |
| INTESTINAL_IMMUNE_NETWORK_FOR_IGA_PRODUCTION | NKCELLS_PATHWAY | XEF; VE CD8+ T CELLS |
| ANTIGEN_PROCESSING_AND_PRESENTATION | IL12_PATHWAY | CALCINEURIN-REGULATED NFAT-DEPENDENT TRANSCRIPTION IN LYMPHOCYTES |
| NATURAL_KILLER_CELL_MEDIATED_CYTOTOXICITY | NO2IL12_PATHWAY | XEF; VE CD8+ T CELLS_1 |
| GRAFT_VERSUS_HOST_DISEASE | TH1TH2_PATHWAY | IMMUNOREGULATORY INTERACTIONS BETWEEN A LYMPHOID AND A NON-LYMPHOID CELL |
| HEMATOPOIETIC_CELL_LINEAGE | TCR_PATHWAY | CXCR4-MEDIATED SIGNALING EVENTS |
| ASTHMA | THELPER_PATHWAY | IL12 SIGNALING MEDIATED BY STAT4 |
| TYPE_I_DIABETES_MELLITUS | CTL_PATHWAY | PROINFLAMMATORYCYTOKINES |
| AUTOIMMUNE_THYROID_DISEASE | TCYTOTOXIC_PATHWAY | IL2-MEDIATED SIGNALING EVENTS |
| TCRA_PATHWAY | LYMPHOCYTE_AFFYTECHNOTE | |
| STATHMIN_PATHWAY | T_BCELL ACTIVATION | |
| TCAPOPTOSIS_PATHWAY | XEF;VE CD4+ T CELLS | |
| DC_PATHWAY | IL12-MEDIATED SIGNALING EVENTS |
The top scoring pathways (in descending order of FDR) identified from each of the three databases (KEGG, Biocarta, and Custom) are shown. The nominal P value is <0.005 and the q value (FDR) < 0.01 for all pathways on the list.
FDR, false-discovery rate.
Figure 2.
Pathway analyses by gene set enrichment (GSEA). (a) MA plots of log intensity averages (x axis; mean gene expression in obese and lean groups) vs. log ratios (y axis; mean differences between obese and lean log signals). Genes in gray are all genes from the Agilent microarray that were considered for analysis after filtering. Genes shown as crosses belong to the top obese-downregulated pathway (T-cell receptor signaling) and genes shown as open circles constitute the top obese-upregulated pathway (olfactory signal transduction). The regression line of best fit passes through zero on the y axis. (b) Concordance-significance plot quantifying the significance of the overlap among the GSEA selected pathways in comparison to randomly selected pathways. Negative of the log P values are plotted on the×axis and concordance measures, along with their confidence intervals, are plotted on the y axis. Pairwise concordance of gene lists from Kyoto Encyclopedia of Genes and Genomes (KEGG), Biocarta, and Custom are as indicated. Comparisons of selected gene lists with randomly selected gene lists are indicated by the “Random” identifier. Concordance values close to zero indicate no concordance among gene lists.
To quantify the similarities among the GSEA-derived top-scoring pathways from the different databases, we combined all genes from the top five pathways from each of KEGG, Biocarta, and Custom to create database-specific, nonredundant gene lists. This resulted in 277 unique genes from KEGG, 218 from Custom and 69 from Biocarta. The three gene lists were then compared among each other and against randomly selected gene lists. We first determined pathway similarity by Venn diagram analysis (see Supplementary Figure S4 online). Thirty genes overlapped between KEGG and Biocarta, 81 genes overlapped between KEGG and Custom, and 43 genes overlapped between Biocarta and Custom databases. 26 genes were found in common between all three databases. Nine of these twenty six genes were involved in T-cell receptor signaling and T-cell proliferation pathways (see Supplementary Table S2 online). We next determined the concordance and statistical significance of the gene list overlaps by use of the List of lists-annotated software. All comparisons among the three selected gene lists displayed concordance scores between 0.1–0.2 (95% confidence intervals not including zero) and were highly significant (−log P values between 42–110). In contrast, concordance estimates for these gene lists against three randomly selected gene lists of similar size (“small B-cell lymphomas,” 81 genes; “age-related changes in frontal cortex,” 142 genes; “lens gene expression,” 221 genes) demonstrated no evidence of concordance (concordance values ranged from 0 to 0.02; –logP between 0–4). These results are summarized in the concordance-significance plot in Figure 2b.
IPA analysis
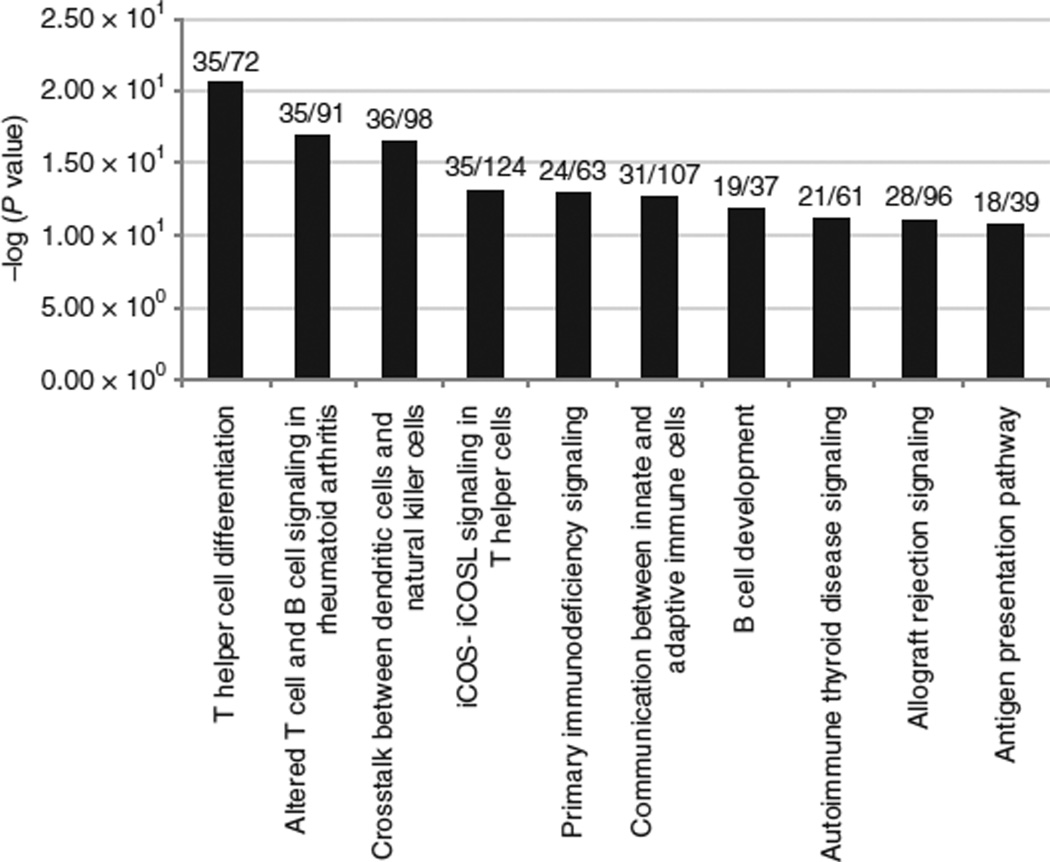
In IPA, we first examined over-representation of the “Diseases and Disorders” category in the obese-downregulated genelist. The top three obese-downregulated disease categories (P < 1 × 1035), were all related to immune/inflammatory responses. The primary sub-diseases contributing to the enrichment of “inflammatory response” (the top downregulated function) were related to immune response and activation of T-lymphocytes (see Supplementary Figure S5 online). A similar analysis to identify the top scoring “canonical pathways” that is enriched among the obesity-downregulated genes, demonstrated a similar enrichment of pathways belonging to T-cell mediated immune signaling (P < 1 × 1010) (Figure 3).
Figure 3.
Pathway enrichment by Ingenuity Pathway analysis (IPA). Identification of top 10 obese-downregulated canonical pathways identified by IPA. Pathway names are indicated on the×axis and the statistical significance of gene-set over-representation is indicated on the y axis (–log base 10 scale). The numbers at the top of each bar is a ratio of the number of genes in each pathway present in the obese downregulated dataset to the total number of genes representing that pathway in the IPA knowledge base.
IPA analysis of obese-upregulated genes identified “cardiovascular disease” as the second most enriched disease category (P < 6.6 × 1013), following “genetic disorders.” The primary disorders contributing to this enrichment included cardiovascular disorder, vascular disease, myocardial ischemia, coronary artery disease, and atherosclerosis. Among canonical pathways, “atherosclerotic signaling” was the second most enriched pathway (P < 2.5 × 102), followed by “differential regulation of cytokine production in macrophages and T-helper cells by interleukin (IL)-17A and IL-17F” (P < 1.03 × 101) (see Supplementary Figure S6 online). These data suggests an upregulation of genes linked to cardiovascular dysfunction and proinflammatory signaling in the obese. However, the strength of the evidence was weaker for the obese-upregulated pathways compared to the obese-downregulated pathways (based on a comparison of the relative significance levels).
DAVID analysis
Similar analyses were conducted in DAVID, by setting the EASE score to 0.01 to detect highly significant disease association with low FDRs. Again, the most significant disease-class association was observed for “immune” related disorders for the obese downregulated genes (P = 1.8 × 1015; FDR = 2.5 × 1012). Analysis of significantly enriched KEGG pathways with more than 30 gene members further identified the “chemokine-signaling pathway” as one of the top obese-downregulated pathways (P = 4.2 × 108; FDR = 1.5 × 104) (see Supplementary Figure S7). Analysis of the obese-upregulated genes pointed to an upregulation of cardiovascular disorders, similarly to what was observed in IPA, but again with weaker statistical significance.
Analysis of chemokines
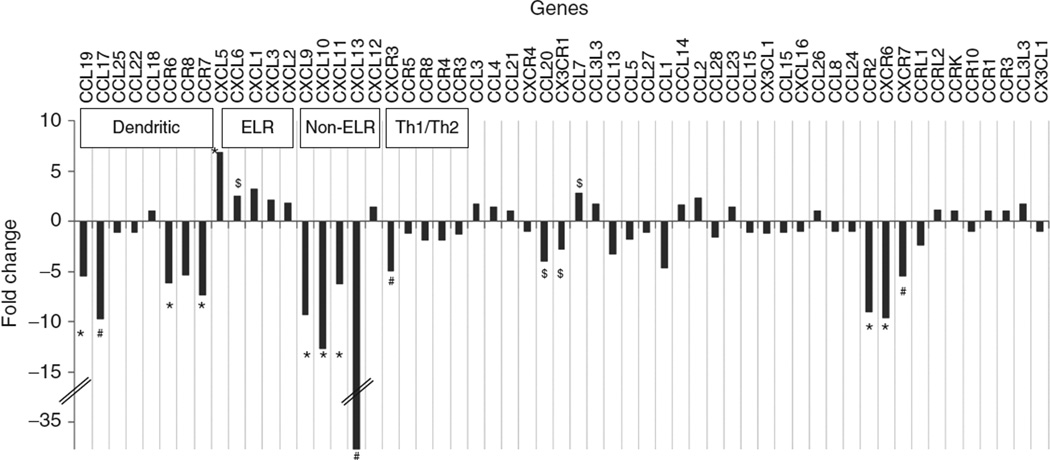
The identification of chemokine enriched clusters in DAVID prompted us to investigate the expression of chemokine genes in greater detail. A total of 57 chemokine ligands and receptors were found to be “expressed” in the samples and are plotted in Figure 4. The chemokines were further grouped into “dendritic cell,” “ELR,” “non-ELR,” and “Th1/Th2” types following the classification proposed by Zlotnik et al. (18). The majority of the “dendritic cell” type chemokines (CCR6, CCR7, CCR8, CCL17, CCL18, CCL19, CCL22, CCL25) were downregulated in the obese subjects (P < 0.01 for 4/8 genes). A similar strong downregulation was also noted for the majority of the “non-ELR” chemokines (CXCL9, CXCL10, CXCL11, CXCL12, CXCL13; P < 0.01 for 4/5 genes). In contrast, the ELR chemokines (CXCL1, CXCL2, CXCL3, CXCL5, and CXCL6) were upregulated in the obese subjects, although the statistical significances were lower. The “Th1/Th2” chemokines (CCR3, CCR4, CCR5, CCR8, and CXCR3) also demonstrated a coordinated downregulation in the obese (weaker significance levels). We further paired the chemokine ligands with their respective receptors (18) and compared expression patterns among ligand-receptor pairs. When we restricted the analysis to statistically significant (P < 0.01) expressions of both ligand-receptor pairs, the following were identified: CXCL9-CXCR3, CXCL10-CXCR3, CXCL11-CXCR3, CCL20-CCR6, CCL13-CCR2, CCL19-CCR7, CCL21-CCR7, and CCL17-CCR4. The first three pairs were all related to CXCR3 signaling and all three chemokines (CXCL9-11) belong to the non-ELR chemokine class. A subset of the chemokines (CCR2, CCL19, CXCL5, CXCL9, CXCL10, and CXCL13) and interferon-γ were further quantified by qPCR analysis and found to be in complete agreement with the microarray results (see Supplementary Figure S8).
Figure 4.
Chemokine gene expression in obese and lean subjects. Differential expression of chemokines is quantified by the fold-change in gene-expression signals in obese compared to lean subjects (from microarray). Chemokines are classified as described in the text. The symbols indicate the level of statistical significance for differential expression ($P < 0.05; #P < 0.01; *P < 0.005). The fold-change axis (y axis) is truncated to accommodate a large range of fold-change values.
Confounder analysis
Since there was a statistically significant difference in age between the obese and lean subjects (P < 0.01, Table 1), we examined whether the observed variation in gene expression between the two groups could have been confounded by age. We used the nuclear factor-κB signaling pathway as a representative test case, since this pathway was one of the top differentially enriched pathways in both KEGG and BIOCARTA analyses (Table 2). We modeled the expression of 22 genes that contributed to core enrichment of the nuclear factor-κB signaling pathway using linear regression and used subject category (obese/lean) and age as independent and interacting variables. The results of the analysis (see Supplementary Table S3 online) demonstrated that the effect of age on gene-expression levels was statistically nonsignificant for all genes tested (P >0.05) whereas subject category was significantly related to individual gene expression for 13/22 genes (P <0.03). The interaction of subject category and age was significant for only 1/22 genes. These results suggest that the observed differences in gene expression between obese and lean subjects are primarily driven by the subject classes and not confounded by age.
DISCUSSION
In this study, we have combined whole-genome expression profiling with bioinformatic pathway analysis and identified a coordinated downregulation of several immune-related pathways in cultured mononuclear PCs from obese African-American women. We adopted a multifaceted pathway analysis strategy to maximize true discoveries and minimize false findings that often arise from differences in pathway enrichment algorithms and variability in the pathway database contents. Specifically, gene expression data was used to interrogate three pathway databases (KEGG, Biocarta and Custom) and analyzed via three different pathway enrichment tools (GSEA, IPA and DAVID) (19). The major finding from all pathway analysis pointed to a comprehensive downregulation of innate and adaptive immune-signaling pathways in the obese, suggestive of an immune deficiency state in their PCs. Quantitative assessment of similarities among the top scoring pathways from each database demonstrated considerable overlap and statistically significant concordances among the gene members of the different pathways. The T-cell receptor signaling and natural killer (NK) cell signaling pathways were identified as downregulated in multiple databases (KEGG and Biocarta) and by multiple methods (GSEA and IPA). These findings are consistent with previous reports of obesity-associated immunodeficiency. For example, reduced circulating CD4(+) T cells and impaired T-cell proliferation are observed in leptin-deficient obesity (20) and T-lymphopenia has been reported in normoleptinemic obese humans and Wistar fatty (fa/fa) rats (21). Obesity has also been associated with an impaired innate immune response and compromises in NK cell signaling (22). The consequence of a compromised immune status can result in greater frequency and severity of infections that has, indeed, been observed with viral infections in human and rodent obesity (23,24). However, while most of the reported studies have investigated dysfunction in differentiated immune cells, the present study has focused on a much earlier time-point representing the predifferentiated, progenitor state for many of these cells. Our findings suggest that impairments in immune signaling can be traced back to much earlier stages of PC differentiation, possibly representing a greater clinical concern than previously anticipated.
A comparison of the genes contributing to pathway enrichment pointed to chemokine signaling-related functions as being significantly downregulated in the obese samples (see Supplementary Figure S6 online). To ascertain the biological significance of this finding, we grouped the chemokines according to their functions (18) and analyzed the data for group expression trends. We observed statistically significant downregulation in 5 out of 8 “dendritic” chemokine ligands and receptors (CCL17, 18, 19,21,22,25 and CCR6, 7). These chemokines are expressed on dendritic cells and participate in the initiation of immune responses by T-cell recruitment. Thus, their downregulation in the obese PCs could ultimately result in impaired T-cell activation in response to environmental stimulation. We also observed an upregulation of the ELRCXC chemokines (CXCL 1–3, 5, 6) while the non-ELR-CXC chemokines (CXCL 9–13) were downregulated in the obese progenitors. ELR-CXC chemokines are potent angiogenic factors and stimulate endothelial cell and neutrophil chemotaxis, whereas non-ELR-CXC chemokines are strong angiostatic factors (25). Our results would imply a net proangiogenic program in the PCs from obese subjects that could possibly lead to an imbalance in the angiogenic/angiostatic ratio that, in turn, could contribute to a wide variety of disorders arising from aberrant angiogenesis (26). The angiogenic–angiostatic imbalance has also been shown to affect structural remodeling of the microvascular networks in obese subjects and contribute to peripheral vascular disease (27). Additionally, as has been observed with other inflammatory conditions such as chronic pancreatitis, such imbalances could also play a role in obesity-associated chronic inflammation.
We further interrogated the expression profiles of chemokine ligand-receptor gene pairs. Of all pairs tested, we identified a consistent downregulation of multiple ligands (CXCL9, 10, 11) that signal through the CXCR3 receptor. Since the CXCR3 ligands are known to be induced by IFN-γ, their observed downregulation could be a consequence of reductions in IFN-γ transcript levels. Indeed we observed a significant reduction in IFN-γ levels in the obese-derived PCs by both microarray and qPCR analysis (see Supplementary Figure S8 online). CXCR3 is expressed in several cells of hematopoietic lineage, including activated T-lymphocytes and natural killer cells, and marks the polarization of T-cells toward a Th1 phenotype (28). Knockout mice studies reveal a variety of disorders consequent to defective CXCR3 signaling, including impaired wound healing, interstitial lung fibrosis, adverse remodeling of myocardial infarcts, and increased susceptibility and mortality to viral infection (29). Thus, the coordinate downregulation of CXCR3 and its ligands in obese-derived PCs is striking and could have important clinical implications. In addition to CXCR3, we also observed statistically significant obesity-associated down-regulation of the chemokine receptors CCR2 (ninefold reduction, P < 0.001) and CXCR6 (9.6-fold reduction, P < 0.002), although their corresponding ligands (CCL2, CCL7 for CCR2 and CXCL16 for CXCR6) did not significantly differ in expression between the lean and obese subjects. The downregulation of CCR2 and CXCR6 could be important for several reasons. For example, CCR2 knockout mice display impaired monocyte and macrophage recruitment (30), insufficient dendritic cell activation and greater susceptibility to infections (31). On the other hand, CXCR6 is required for hepatic NK cell activation and NK-cell mediated antigen specific memory of haptens and viruses (32). Based on these observations, we hypothesize that the observed reduction in CCR2 and CXCR6 levels in the obese progenitors is perhaps most relevant in the context of impaired macrophage activation/recruitment, greater susceptibility to infection, and impaired NK cell activation, all again contributing to compromised innate and adaptive immunity states.
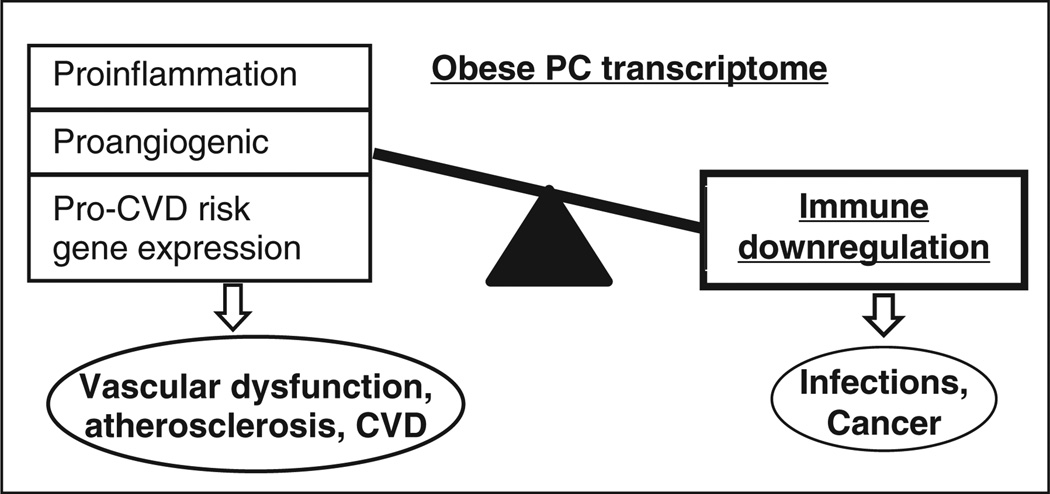
Although the statistical evidence is strongest for an obesity-associated immune compromise, transcriptome analysis also implicated (with weaker evidence of statistical significance) the presence of proinflammatory and cardiovascular risk signatures in the obese-derived PCs. Thus we observed an upregulation of a series of proinflammatory chemokines including CXCL1, CXCL5, CXCL6, and CCL7. Notably, all four chemokines are linked to proinflammatory nuclear factor-κb signaling while CXCL5 is also involved in neutrophil activation. In addition, there was upregulation of CCL2 (monocyte chemoattractant protein 1) which facilitates mononuclear cell infiltration in several disorders including atherosclerosis. As noted previously, the differential regulation of ELR and non-ELR chemokines is also suggestive of an angiogenic–angiostatic imbalance resulting in a net proangiogenic signaling in the obese-derived PCs. Additionally, we observed a strong upregulation of the angiogenesis-associated sushi-repeat containing protein, X-linked 2 (SRPX2, 5.8-fold upregulated in obese). Together, these results may provide an explanation for some of the sources of increased angiogenesis that is observed in obesity. Finally, enrichment analysis in IPA and DAVID implicated that multiple genes related to several subtypes of cardiovascular disease (myocardial ischemia, coronary artery disease, atherosclerosis etc.) were also upregulated in the obese-derived PCs. We speculate that such upregulation may, in fact, be an explanatory mechanism for the increased cardiovascular risk, including atherosclerosis and stroke that is associated with obesity. We have summarized all of these findings into a schematic model of the obese-derived PC transcriptome that links immune dysfunction, angiogenesis, and cardiovascular disease (Figure 5).
Figure 5.
Schematic of the obese-derived progenitor cell transcriptome. A summary of the results observed in the present study indicates a net downregulation of the immune response pathways and upregulation of selected proinflammatory cytokines and genes related to a spectrum of cardiovascular disorders in the obese-derived progenitor cells (PCs). Additionally, chemokine gene expression suggests an angiostatic–angiogenic imbalance resulting in a net proangiogenic milieu in the cells. Immune compromise may be responsible for increased infections and cancers whereas the proinflammatory, net angiogenic and higher cardiovascular disk (CVD) risk gene expression may lead to cardiovascular dysfunction in the obese. In the figure, the thickness of the rectangles is qualitatively proportional to the strength of the statistical significance of the findings.
We now discuss some aspects of the cell population that was used in the present study. There is ongoing debate about the in vitro phenotype of the PCs. Although circulating cells expressing CD34, VEGFR-2 (or KDR), and CD133 (CD34+/CD133+/KDR+ triple positives) have been traditionally considered to represent endothelial PCs (33), some recent references suggest that these cells may actually represent primitive hematopoietic cells. There is also accumulating evidence that even undifferentiated hematopoietic cells have angiogenic potential. Conversely, peripheral blood endothelial PCs often express monocyte/macrophage markers (CD14, Mac-1, and CD11c) and are largely derived from the monocyte–macrophage containing CD34-mononuclear cell population (34). Therefore, cultured PCs may not represent a unique cell type but consist of a highly adaptive and functionally heterogeneous population of cells that can respond to different microenvironments as needed. In fact, intrinsic cell population heterogeneity is considered inevitable even when cells are cultured in consistent in vitro conditions (35). In this context, some degree of heterogeneity and a mix of vasculogenic and hematopoietic precursors are to be expected in the cells used in this study. We examined this heterogeneity by analyzing microarray gene expression levels of cell-surface markers of the major lymphoid and myeloid lineage cell types from obese and lean subjects. We observed no significant differences in marker gene expression between obese and lean samples (with the exception of the B-cell marker CD19). Additionally, qPCR analysis demonstrated high CD14 expression, low KDR expression, and very low CD34 expression in the cells from obese and lean subjects with no statistically significant differences between the groups. The low level of CD34 expression is suggestive of a monocyte-enriched cell population. In addition to cell-surface marker analysis, we interrogated the expression levels of 7 ECM component genes that have been previously associated with endothelial phenotype in late-phase (day 14) cultured PCs (17). The patterns of expression observed in our study, also from cells at day 14 of culture, closely mimic previous reports with lower expression of vascular cell adhesion molecule 1 (VCAM1) and high expression of the remaining genes. Based on these results, our overall interpretation is that the peripheral blood-derived cells used in the study are composed of mononuclear myeloid endothelial cells and endothelial PCs with a primarily monocytic phenotype and without activation of VCAM1 expression. Specifically, although VCAM1 expression levels were low, we did not observe any statistically significant differences between VCAM1 expressions in obese or lean-derived PCs. Increased circulating VCAM1 is observed in obesity and reportedly contributes to the development of endothelial dysfunction and atherosclerosis. Protein and mRNA levels of VCAM1 are also increased in visceral adipose tissue of obese subjects (36). However, the absence of increased VCAM1 expression in obese-derived PCs most likely suggest that obesity-associated elevation of VCAM1 expression is a function of differentiated cells (endothelial and adipose) that is not reflected in the progenitors.
Comparison of the results from the present study with similar gene expression studies conducted either in whole blood or isolated peripheral blood mononuclear cells from obese and lean subjects show close similarities and some differences. For example, a whole-blood transcriptome analysis performed in obese and lean white subjects also reported a general downregulation in immune-function related genes in the obese cohort (8). This study also reported significant upregulation of pathways related to “oxidative phosphorylation,” “apoptosis,” and “ribosome” in the obese subjects, that was not observed in the present study (most likely reflecting differences in cell populations and levels of differentiation). On the other hand, a study of the mRNA levels of selected cytokines in peripheral blood mononuclear cells from obese and lean subjects also reported a decrease in transcript levels for IL-6, IL-1β, and tumor necrosis factor-α in the obese (37).
We now discuss some limitations of the present study. As with many human studies involving ethnic minorities, the number of samples available for lean subjects was low due to recruitment challenges and typical validation strategies (e.g., splitting the cohorts into discovery and replication groups) were not practical. Also, while the results indicate a suppressed immune gene expression signature in obese-derived PCs, the present study does not distinguish if such suppression originates from reduced expression of immunity-related genes and/or from differences in the proportions of cells expressing these genes between obese and lean subjects. Although our analysis of lymphocyte and monocyte proportions (Table 1) as well as gene expression analysis of lymphoid, myeloid, and endothelial cell-surface markers argues against a gross imbalance in cellular composition between the groups, one cannot fully exclude the possibility of differential enrichment of specific, unexamined cell subpopulations between them. Another potential limitation of the present work is the lack of functional analysis to evaluate the findings from the microarray study; this will be addressed in future studies. Finally, as this study was restricted only to African-American women, further studies are warranted to determine whether the findings are generalizable to males and to other race/ethnicities.
The results from the present study point to a coordinate immune-dysfunction and possible angiogenic–angiostatic imbalance in cultured mononuclear cells in the obese. Although the association of immune defects to obesity is not new, our study places obesity-associated immune dysfunction as a very early pathophysiology, observable in predifferentiated PCs. Further studies elucidating the functional properties and compositional heterogeneity of obesity-associated PCs will provide a novel window to study the intersection of metabolic, immune and vascular dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Natalia Silvestrov for biochemical assays; Michelle Leander Griffith for RNA processing; Jerry Manlove–Simmons for cell culture; Patricia Jackson and Eddie Stanley for subject recruitment and Jacqueline Ali for administrative support. This work has been supported by NCRR 2R25RR017694-06A1, 5U54RR022814, and 5P20RR11104 (P.P. and B.E.G.); NHLBI 5R25HL003676 and NCRR G12-RR03034 (L.A.); NCRR U54 RR026137, UO1HL084891 and UL1 RR025008 (E.O.O.); NHLBI 5R25HL059868-10, NCMHD P20MD000175-09, NIDDK 1R21DK088319-01, and American Heart Association grant AHA10SDG4230068 (S.G.).
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Schmidt-Lucke C, Rössig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 2.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Zeng L, Ding S, Xu K. Adult endothelial progenitor cells retain hematopoiesis potential. Transplant Proc. 2010;42:3745–3749. doi: 10.1016/j.transproceed.2010.07.094. [DOI] [PubMed] [Google Scholar]
- 6.Urbich C, Heeschen C, Aicher A, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 7.Gerrits MF, Ghosh S, Kavaslar N, et al. Distinct skeletal muscle fiber characteristics and gene expression in diet-sensitive versus diet-resistant obesity. J Lipid Res. 2010;51:2394–2404. doi: 10.1194/jlr.P005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S, Dent R, Harper ME, et al. Gene expression profiling in whole blood identifies distinct biological pathways associated with obesity. BMC Med Genomics. 2010;3:56. doi: 10.1186/1755-8794-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh S, Dent R, Harper ME, Stuart J, McPherson R. Blood gene expression reveal pathway differences between diet-sensitive and resistant obese subjects prior to caloric restriction. Obesity (Silver Spring) 2011;19:457–463. doi: 10.1038/oby.2010.209. [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 11.Reich M, Liefeld T, Gould J, et al. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 12.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- 14.Cahan P, Ahmad AM, Burke H, et al. List of lists-annotated (LOLA): a database for annotation and comparison of published microarray gene lists. Gene. 2005;360:78–82. doi: 10.1016/j.gene.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Hill JM, Zalos G, Halcox JPJ, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New England J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 16.Golden-Mason L, Curry MP, Nolan N, et al. Differential expression of lymphoid and myeloid markers on differentiating hematopoietic stem cells in normal and tumor-bearing adult human liver. Hepatology. 2000;31:1251–1256. doi: 10.1053/jhep.2000.7713. [DOI] [PubMed] [Google Scholar]
- 17.Furuhata S, Ando K, Oki M, et al. Gene expression profiles of endothelial progenitor cells by oligonucleotide microarray analysis. Mol Cell Biochem. 2007;298:125–138. doi: 10.1007/s11010-006-9359-4. [DOI] [PubMed] [Google Scholar]
- 18.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, Dent R, Harper ME, Stuart J, McPherson R. Blood gene expression reveal pathway differences between diet-sensitive and resistant obese subjects prior to caloric restriction. Obesity (Silver Spring) 2011;19:453–467. doi: 10.1038/oby.2010.209. [DOI] [PubMed] [Google Scholar]
- 20.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka S, Isoda F, Kiuchi Y, et al. T lymphopenia in genetically obese-diabetic Wistar fatty rats: effects of body weight reduction on T cells. Metab Clin Exp. 2000;49:1261–1266. doi: 10.1053/meta.2000.9516. [DOI] [PubMed] [Google Scholar]
- 22.O’Shea D, Cawood TJ, O’Farrelly C, Lynch L. Natural killer cells in obesity: impaired function and increased susceptibility to the effects of cigarette smoke. PLoS ONE. 2010;5:e8660. doi: 10.1371/journal.pone.0008660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huttunen R, Syrjänen J. Obesity and the outcome of infection. Lancet Infect Dis. 2010;10:442–443. doi: 10.1016/S1473-3099(10)70103-1. [DOI] [PubMed] [Google Scholar]
- 24.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 25.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 26.Cui A, Anhenn O, Theegarten D, et al. Angiogenic and angiostatic chemokines in idiopathic pulmonary fibrosis and granulomatous lung disease. Respiration. 2010;80:372–378. doi: 10.1159/000245332. [DOI] [PubMed] [Google Scholar]
- 27.Frisbee JC. Obesity, insulin resistance, and microvessel density. Microcirculation. 2007;14:289–298. doi: 10.1080/10739680701282945. [DOI] [PubMed] [Google Scholar]
- 28.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wuest TR, Carr DJ. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J Immunol. 2008;181:7985–7993. doi: 10.4049/jimmunol.181.11.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Lu P, Ishida Y, et al. Attenuated liver tumor formation in the absence of CCR2 with a concomitant reduction in the accumulation of hepatic stellate cells, macrophages and neovascularization. Int J Cancer. 2006;118:335–345. doi: 10.1002/ijc.21371. [DOI] [PubMed] [Google Scholar]
- 31.Peters W, Dupuis M, Charo IF. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J Immunol. 2000;165:7072–7077. doi: 10.4049/jimmunol.165.12.7072. [DOI] [PubMed] [Google Scholar]
- 32.Germanov E, Veinotte L, Cullen R, et al. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J Immunol. 2008;181:81–91. doi: 10.4049/jimmunol.181.1.81. [DOI] [PubMed] [Google Scholar]
- 33.Steurer M, Kern J, Zitt M, et al. Quantification of circulating endothelial and progenitor cells: comparison of quantitative PCR and four-channel flow cytometry. BMC Res Notes. 2008;1:71. doi: 10.1186/1756-0500-1-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 35.Luni C, Doyle FJ, Elvassore N. Cell population modelling describes intrinsic heterogeneity: a case study for hematopoietic stem cells. IET Syst Biol. 2011;5:164–173. doi: 10.1049/iet-syb.2009.0059. [DOI] [PubMed] [Google Scholar]
- 36.Bosanská L, Michalský D, Lacinová Z, et al. The influence of obesity and different fat depots on adipose tissue gene expression and protein levels of cell adhesion molecules. Physiol Res. 2010;59:79–88. doi: 10.33549/physiolres.931705. [DOI] [PubMed] [Google Scholar]
- 37.O’Rourke RW, Kay T, Lyle EA, et al. Alterations in peripheral blood lymphocyte cytokine expression in obesity. Clin Exp Immunol. 2006;146:39–46. doi: 10.1111/j.1365-2249.2006.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 40.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.