Abstract
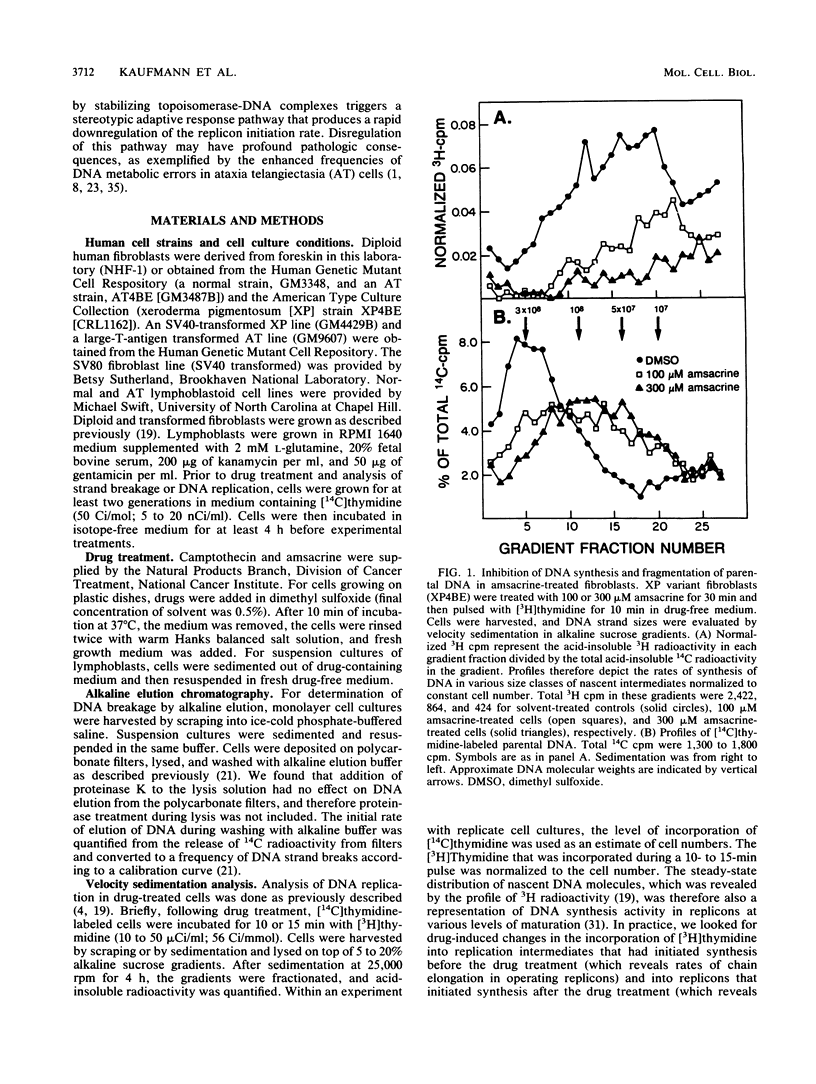
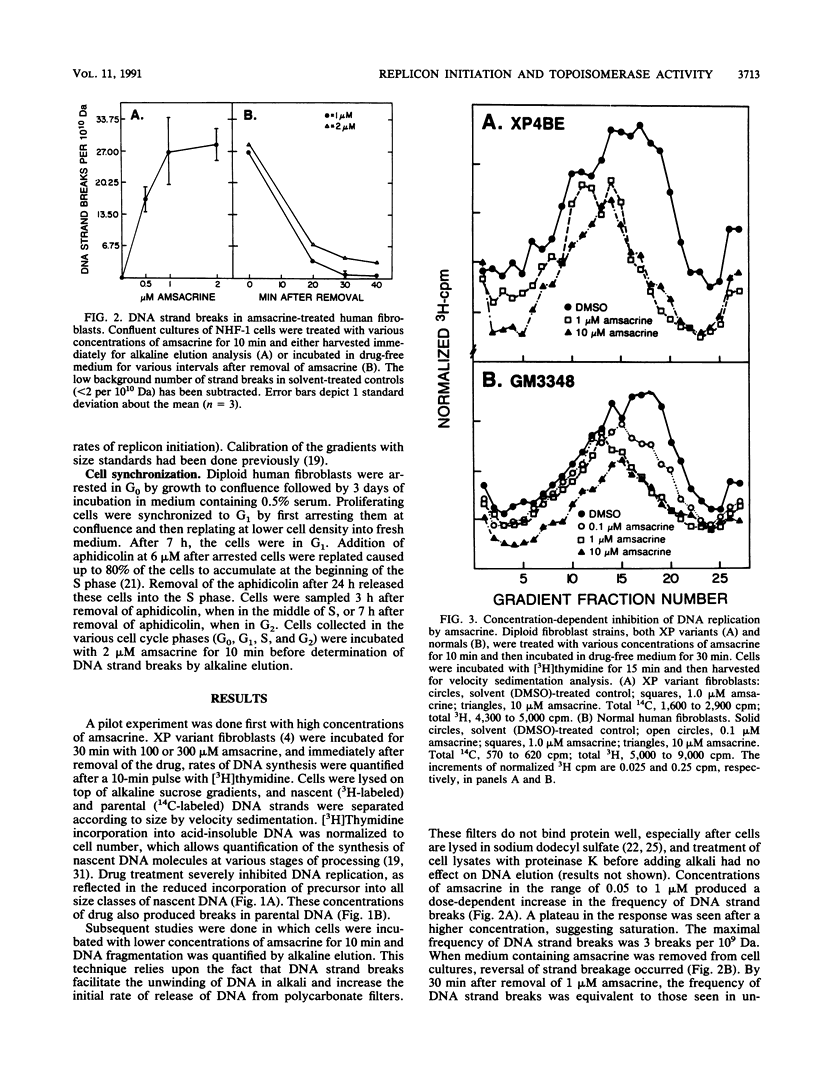
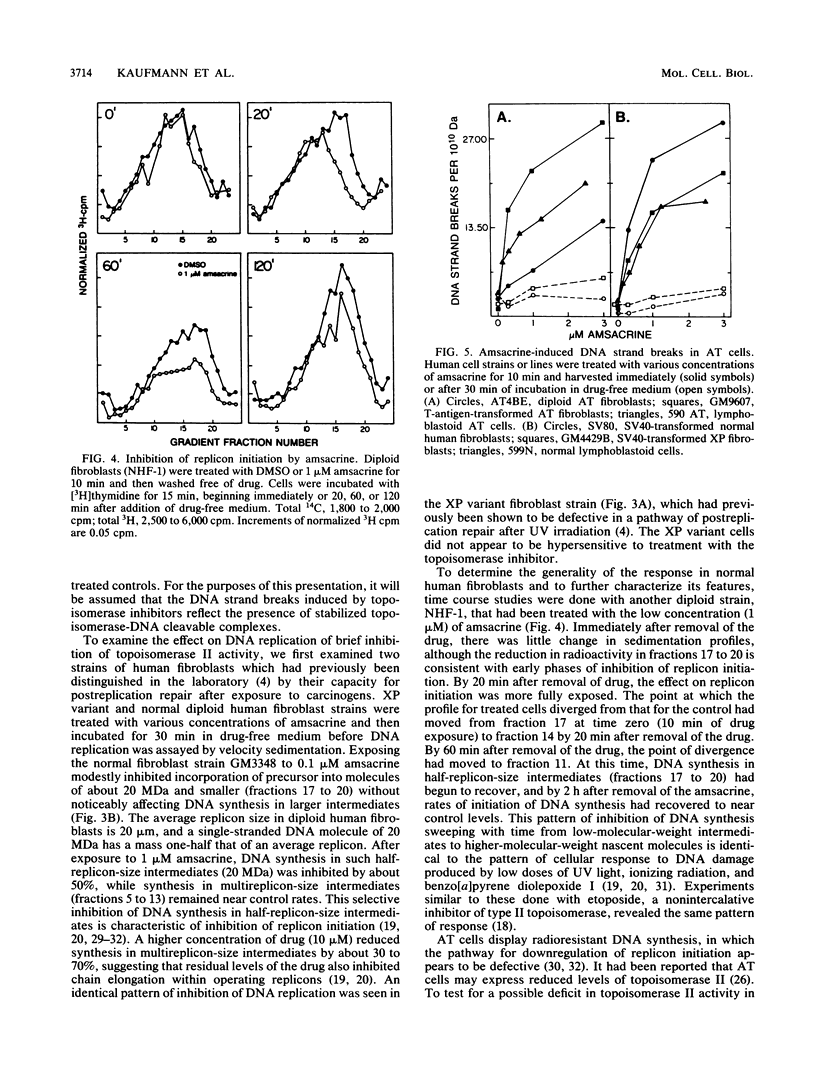
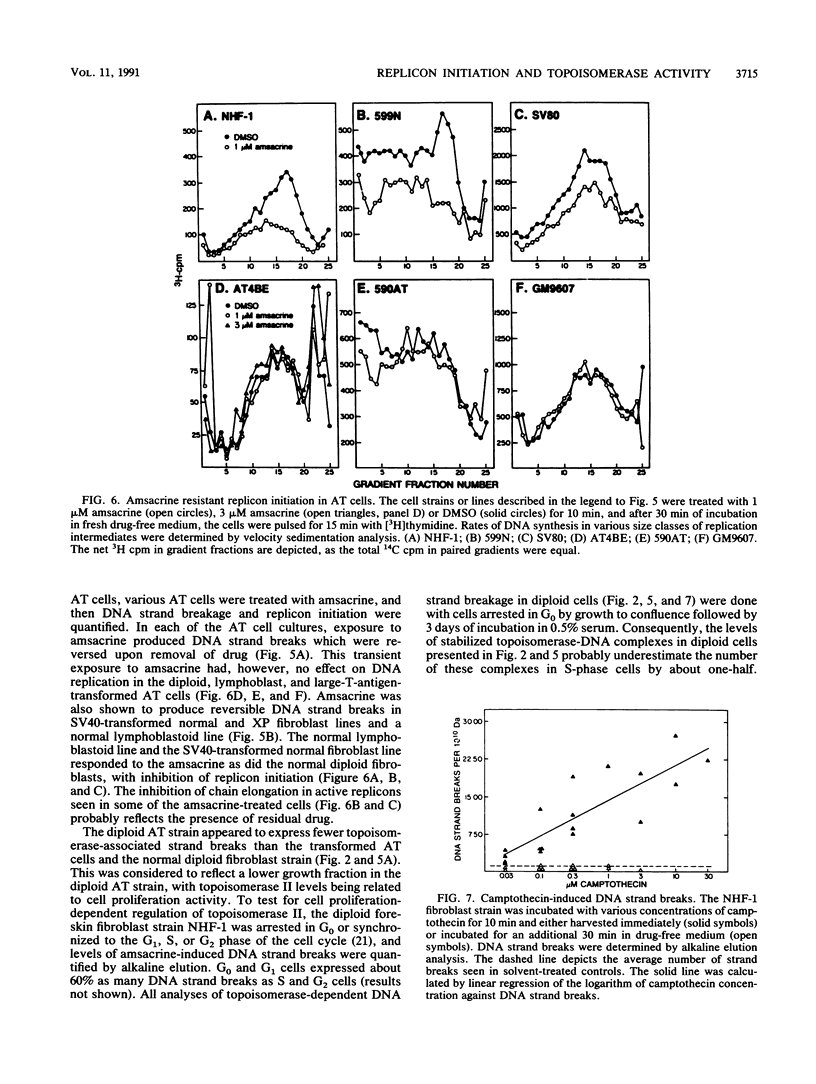
Diploid human fibroblast strains were treated for 10 min with inhibitors of type I and type II DNA topoisomerases, and after removal of the inhibitors, the rate of initiation of DNA synthesis at replicon origins was determined. By alkaline elution chromatography, 4'-(9-acridinylamino)methanesulfon-m-anisidide (amsacrine), an inhibitor of DNA topoisomerase II, was shown to produce DNA strand breaks. These strand breaks are thought to reflect drug-induced stabilization of topoisomerase-DNA cleavable complexes. Removal of the drug led to a rapid resealing of the strand breaks by dissociation of the complexes. Velocity sedimentation analysis was used to quantify the effects of amsacrine treatment on DNA replication. It was demonstrated that transient exposure to low concentrations of amsacrine inhibited replicon initiation but did not substantially affect DNA chainelongation within operating replicons. Maximal inhibition of replicon initiation occurred 20 to 30 min after drug treatment, and the initiation rate recovered 30 to 90 min later. Ataxia telangiectasia cells displayed normal levels of amsacrine-induced DNA strand breaks during stabilization of cleavable complexes but failed to downregulate replicon initiation after exposure to the topoisomerase inhibitor. Thus, inhibition of replicon initiation in response to DNA damage appears to be an active process which requires a gene product which is defective or missing in ataxia telangiectasia cells. In normal human fibroblasts, the inhibition of DNA topoisomerase I by camptothecin produced reversible DNA strand breaks. Transient exposure to this drug also inhibited replicon initiation. These results suggest that the cellular response pathway which downregulates replicon initiation following genotoxic damage may respond to perturbations of chromatin structure which accompany stabilization of topoisomerase-DNA cleavable complexes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurias A., Croquette M. F., Nuyts J. P., Griscelli C., Dutrillaux B. New data on clonal anomalies of chromosome 14 in ataxia telangiectasia: tct(14;14) and inv(14). Hum Genet. 1986 Jan;72(1):22–24. doi: 10.1007/BF00278811. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Griggs H. G., Walker P. L. Mechanisms of chromosomal aberration production. I. Aberration induction by ultraviolet light. Mutat Res. 1973 Dec;20(3):387–402. doi: 10.1016/0027-5107(73)90060-2. [DOI] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. C., Kaufmann W. K., Brylawski B. P., Cordeiro-Stone M. Defective postreplication repair in xeroderma pigmentosum variant fibroblasts. Cancer Res. 1990 May 1;50(9):2593–2598. [PubMed] [Google Scholar]
- Brill S. J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. 1987 Mar 26-Apr 1Nature. 326(6111):414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima Y., Sasaki M. S. Enhanced expression of X-ray- and UV-induced chromosome aberrations by cytosine arabinoside in ataxia telangiectasia cells. Mutat Res. 1986 Jan-Feb;159(1-2):117–123. doi: 10.1016/0027-5107(86)90120-x. [DOI] [PubMed] [Google Scholar]
- Garg L. C., DiAngelo S., Jacob S. T. Role of DNA topoisomerase I in the transcription of supercoiled rRNA gene. Proc Natl Acad Sci U S A. 1987 May;84(10):3185–3188. doi: 10.1073/pnas.84.10.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Blazka M. E. Hypersensitivity of cultured ataxia-telangiectasia cells to etoposide. J Natl Cancer Inst. 1986 Jun;76(6):1007–1011. [PubMed] [Google Scholar]
- Horwitz S. B., Chang C. K., Grollman A. P. Studies on camptothecin. I. Effects of nucleic acid and protein synthesis. Mol Pharmacol. 1971 Nov;7(6):632–644. [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hsiang Y. H., Lihou M. G., Liu L. F. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989 Sep 15;49(18):5077–5082. [PubMed] [Google Scholar]
- Hsiang Y. H., Wu H. Y., Liu L. F. Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res. 1988 Jun 1;48(11):3230–3235. [PubMed] [Google Scholar]
- Kalwinsky D. K., Look A. T., Ducore J., Fridland A. Effects of the epipodophyllotoxin VP-16-213 on cell cycle traverse, DNA synthesis, and DNA strand size in cultures of human leukemic lymphoblasts. Cancer Res. 1983 Apr;43(4):1592–1597. [PubMed] [Google Scholar]
- Kao-Shan C. S., Micetich K., Zwelling L. A., Whang-Peng J. Cytogenetic effects of amsacrine on human lymphocytes in vivo and in vitro. Cancer Treat Rep. 1984 Jul-Aug;68(7-8):989–997. [PubMed] [Google Scholar]
- Kaufmann W. K., Boyer J. C., Smith B. A., Cordeiro-Stone M. DNA repair and replication in human fibroblasts treated with (+/-)-r-7,t-8-dihydroxy-t-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene . Biochim Biophys Acta. 1985 Feb 20;824(2):146–151. doi: 10.1016/0167-4781(85)90091-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Cleaver J. E. Mechanisms of inhibition of DNA replication by ultraviolet light in normal human and xeroderma pigmentosum fibroblasts. J Mol Biol. 1981 Jun 25;149(2):171–187. doi: 10.1016/0022-2836(81)90297-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K. Pathways of human cell post-replication repair. Carcinogenesis. 1989 Jan;10(1):1–11. doi: 10.1093/carcin/10.1.1. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Wilson S. J. DNA repair endonuclease activity during synchronous growth of diploid human fibroblasts. Mutat Res. 1990 Jul;236(1):107–117. doi: 10.1016/0921-8777(90)90038-7. [DOI] [PubMed] [Google Scholar]
- Little J. B., Nagasawa H. Effect of confluent holding on potentially lethal damage repair, cell cycle progression, and chromosomal aberrations in human normal and ataxia-telangiectasia fibroblasts. Radiat Res. 1985 Jan;101(1):81–93. [PubMed] [Google Scholar]
- Lock R. B., Ross W. E. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in Chinese hamster ovary cells. Cancer Res. 1990 Jun 15;50(12):3761–3766. [PubMed] [Google Scholar]
- Mattern M. R., Mong S. M., Bartus H. F., Mirabelli C. K., Crooke S. T., Johnson R. K. Relationship between the intracellular effects of camptothecin and the inhibition of DNA topoisomerase I in cultured L1210 cells. Cancer Res. 1987 Apr 1;47(7):1793–1798. [PubMed] [Google Scholar]
- Mohamed R., Pal Singh S., Kumar S., Lavin M. F. A defect in DNA topoisomerase II activity in ataxia-telangiectasia cells. Biochem Biophys Res Commun. 1987 Nov 30;149(1):233–238. doi: 10.1016/0006-291x(87)91629-9. [DOI] [PubMed] [Google Scholar]
- Olivieri G., Micheli A. Mitotic delay and repair in human lymphocytes. Mutat Res. 1983 Oct;122(1):65–72. doi: 10.1016/0165-7992(83)90144-6. [DOI] [PubMed] [Google Scholar]
- Painter R. B. Effect of caffeine on DNA synthesis in irradiated and unirradiated mammalian cells. J Mol Biol. 1980 Nov 5;143(3):289–301. doi: 10.1016/0022-2836(80)90191-6. [DOI] [PubMed] [Google Scholar]
- Painter R. B. Inhibition and recovery of DNA synthesis in human cells after exposure to ultraviolet light. Mutat Res. 1985 Jan-Mar;145(1-2):63–69. doi: 10.1016/0167-8817(85)90041-0. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Formation of nascent DNA molecules during inhibition of replicon initiation in mammalian cells. Biochim Biophys Acta. 1976 Jan 19;418(2):146–153. doi: 10.1016/0005-2787(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7315–7317. doi: 10.1073/pnas.77.12.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Mattern M. R., Schwartz R. E., Zwelling L. A. Absence of swiveling at sites of intercalator-induced protein-associated deoxyribonucleic acid strand breaks in mammalian cell nucleoids. Biochemistry. 1984 Jun 19;23(13):2922–2927. doi: 10.1021/bi00308a011. [DOI] [PubMed] [Google Scholar]
- Rose K. M. DNA topoisomerases as targets for chemotherapy. FASEB J. 1988 Jun;2(9):2474–2478. doi: 10.1096/fasebj.2.9.2836254. [DOI] [PubMed] [Google Scholar]
- Russo G., Isobe M., Pegoraro L., Finan J., Nowell P. C., Croce C. M. Molecular analysis of a t(7;14)(q35;q32) chromosome translocation in a T cell leukemia of a patient with ataxia telangiectasia. Cell. 1988 Apr 8;53(1):137–144. doi: 10.1016/0092-8674(88)90495-3. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Anderson C. O., Watson J. V. Predominant role for DNA damage in etoposide-induced cytotoxicity and cell cycle perturbation in human SV40-transformed fibroblasts. Cancer Res. 1986 Nov;46(11):5641–5645. [PubMed] [Google Scholar]
- Snapka R. M. Topoisomerase inhibitors can selectively interfere with different stages of simian virus 40 DNA replication. Mol Cell Biol. 1986 Dec;6(12):4221–4227. doi: 10.1128/mcb.6.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmach L. J., Jones R. W., Busse P. M. The action of caffeine on X-irradiated HeLa cells. I. Delayed inhibition of DNA synthesis. Radiat Res. 1977 Sep;71(3):653–665. [PubMed] [Google Scholar]
- Wang J. C., Caron P. R., Kim R. A. The role of DNA topoisomerases in recombination and genome stability: a double-edged sword? Cell. 1990 Aug 10;62(3):403–406. doi: 10.1016/0092-8674(90)90002-v. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetti-Bosseler F., Scott D. Cell death, chromosome damage and mitotic delay in normal human, ataxia telangiectasia and retinoblastoma fibroblasts after x-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 May;39(5):547–558. doi: 10.1080/09553008114550651. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Estey E., Silberman L., Doyle S., Hittelman W. Effect of cell proliferation and chromatin conformation on intercalator-induced, protein-associated DNA cleavage in human brain tumor cells and human fibroblasts. Cancer Res. 1987 Jan 1;47(1):251–257. [PubMed] [Google Scholar]
- Zwelling L. A., Mattern M. R. DNA-repair deficiencies do not affect intercalator-induced cytotoxicity or DNA scission in human cells. Mutat Res. 1982 May-Jun;104(4-5):295–304. doi: 10.1016/0165-7992(82)90159-2. [DOI] [PubMed] [Google Scholar]
- van Maanen J. M., Retèl J., de Vries J., Pinedo H. M. Mechanism of action of antitumor drug etoposide: a review. J Natl Cancer Inst. 1988 Dec 7;80(19):1526–1533. doi: 10.1093/jnci/80.19.1526. [DOI] [PubMed] [Google Scholar]


