Abstract
Objective
The purpose of this study is to assess possible diagnostic differences between general dentists (GPs) and oral and maxillofacial radiologists (RGs) in the identification of pathognomonic radiographic features of cemento-osseous dysplasia (COD) and its interpretation.
Methods
Using a systematic objective survey instrument, 3 RGs and 3 GPs reviewed 50 image sets of COD and similarly appearing entities (dense bone island, cementoblastoma, cemento-ossifying fibroma, fibrous dysplasia, complex odontoma and sclerosing osteitis). Participants were asked to identify the presence or absence of radiographic features and then to make an interpretation of the images.
Results
RGs identified a well-defined border (odds ratio (OR) 6.67, P < 0.05); radiolucent periphery (OR 8.28, P < 0.005); bilateral occurrence (OR 10.23, P < 0.01); mixed radiolucent/radiopaque internal structure (OR 10.53, P < 0.01); the absence of non-concentric bony expansion (OR 7.63, P < 0.05); and the association with anterior and posterior teeth (OR 4.43, P < 0.05) as key features of COD. Consequently, RGs were able to correctly interpret 79.3% of COD cases. In contrast, GPs identified the absence of root resorption (OR 4.52, P < 0.05) and the association with anterior and posterior teeth (OR 3.22, P = 0.005) as the only key features of COD and were able to correctly interpret 38.7% of COD cases.
Conclusions
There are statistically significant differences between RGs and GPs in the identification and interpretation of the radiographic features associated with COD (P < 0.001). We conclude that COD is radiographically discernable from other similarly appearing entities only if the characteristic radiographic features are correctly identified and then correctly interpreted.
Keywords: osseous dysplasia, jaws, radiography, interpretation
Introduction
Benign fibro-osseous lesion is a well-known, descriptive term that encompasses a wide range of conditions, the diagnoses of which may be challenging.1 In part, the challenge arises because the histopathological appearances of all fibro-osseous lesions are very similar, if not identical, making clinical diagnosis difficult based on microscopic features alone.2-4 Su et al5 cautioned that similar histopathological features could be seen in multiple fibro-osseous lesions and the presence of these features should not preclude the interpretation of one lesion over another. Waldron4 commented further that “in the absence of good clinical and radiologic information, a pathologist can only state that a given biopsy is consistent with a fibro-osseous lesion” and that “with adequate clinical and radiologic information, most lesions can be assigned with reasonable certainty into one of several categories”. One such fibro-osseous lesion is cemento-osseous dysplasia (COD), a group of non-neoplastic processes usually confined to the tooth-bearing areas of the jaws or edentulous alveolar processes.6 The diagnosis of COD, which can include several different but related entities, should consider tooth pulp vitality, the stage of lesion development and the possible co-existence of COD with other entities, namely simple bone cyst and osteomyelitis. Consequently, the diagnostic confusion often associated with COD has led to misinterpretation and mismanagement, often rendering these cases problematic.7-9 Therefore, the role of the oral and maxillofacial radiologist (RG) in the differential diagnosis is an important and essential one.
In 2005, the World Health Organization subdivided CODs into periapical, florid and other CODs.10 While the mandible is the preferred site for COD, a wide spectrum of radiographic appearances have been described which may further complicate interpretation. In the early immature osteolytic stage, COD lesions may be confused with periapical rarefying osteitis (i.e. radicular abscess, granuloma or cyst), particularly the small, solitary lesions. As COD lesions mature, the radiolucent defect(s) develop minute radiopacities which may ultimately coalesce and undergo substantial radiopacification.2,5,11 The shapes of the internal radiopacities have been described as being irregular, globular or ovoid, consisting of dense cementum-like structures which may be accompanied by a ground glass appearance in the bone. The radiographic appearances of these more mature florid COD lesions have been described by many authors to be similar to periapical COD; however, florid COD is thought to be associated with considerably more radiopacification.3,11,12 Finally, the presence of a radiolucent periphery surrounding the radiopacities is believed to be of diagnostic significance.5,11-13
Jaw expansion is a feature of COD that is not often reported. Kawai et al11 noted slight mandibular enlargement in 3 of 54 COD cases (periapical and florid) using occlusal radiographs. In contrast, Loh and Yeo14 and Tonioli and Schindler15 reported considerable expansion based on their observations of clinical examination alone. Melrose et al12 also reported jaw expansion with intact thinned cortices in their series of 34 florid COD, yet failed to report the frequency of this expansion.
Most COD cases are diagnosed on the basis of the radiographic features alone on intraoral radiographs.5 Regrettably, case reports or small case series of COD often have incomplete radiography and the subjective analyses of disease features may draw uncertainty to the diagnostic conclusions of the reports.
The purpose of this study was to focus on the radiographic features of COD, and to determine if an association could be made between the identification of one or more radiographic features of COD and a correct radiographic interpretation. This study used radiographic investigations of COD, and both general dentists (GPs) and specialist RGs, to determine the degree to which a comprehensive radiographic examination and clinical training were important in the correct interpretation COD. These questions remain unanswered in current literature for both COD and other disease entities arising in the jaws.
For the purposes of this study, we consider COD to represent a spectrum of conditions. We define periapical COD (synonymous with focal COD) as a condition related to one or more anterior or posterior teeth. We define florid COD as a condition related to extensive involvement in at least two quadrants.
Materials and methods
This study was approved by the University of Toronto Health Science Research Ethics Board (HREB).
The radiographic images of 37 cases of COD were obtained from the archives of the Special Procedures Clinic, Discipline of Oral and Maxillofacial Radiology, Faculty of Dentistry of the University of Toronto. 24 cases were periapical COD and 13 were florid COD. Ten COD cases were associated with secondary osteomyelitis and six were associated with simple bone cyst. In addition to these cases, 13 additional image sets consisting of similarly appearing entities (dense bone island, cementoblastoma, cemento-ossifying fibroma, fibrous dysplasia, complex odontoma and sclerosing osteitis) were also randomly included. The cases that were included were selected based on interpretations made by specialists in oral and maxillofacial radiology, and on the availability of a selection of plain radiographs that included one or more of panoramic, periapical and occlusal radiographs. Advanced imaging (CT and MRI) was available for 23 out of the 37 COD cases and these were archived in an eFilm database (eFilm, version 2.1, Merge Healthcare, Milwaukee, WI). While occlusal radiographs were not available for six COD cases, axial CT and/or MR images were available for four out of these six COD cases so that cortical expansion could be evaluated. The plain radiographic images of the total 50 cases were digitized using a digital flatbed scanner (Epson Expression 1680 version 1.01E, Epson® America, Long Beach, CA) and Adobe Photoshop CS2 Version 9.0 (Adobe® Systems, San Jose, CA). The images were then prepared in the form of a PowerPoint presentation (Microsoft® Corporation, Redmond, WA).
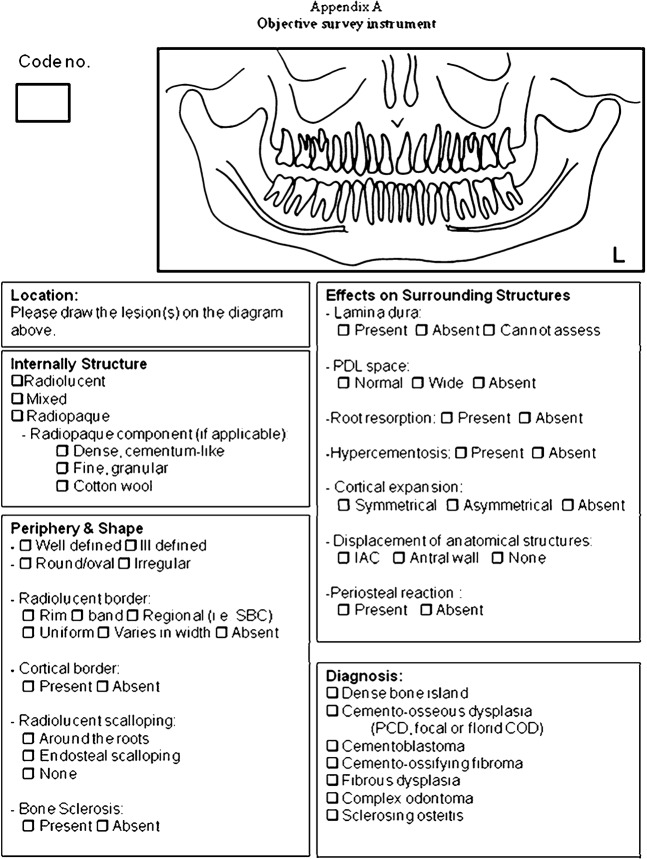
2 groups of reviewers, 3 GPs and 3 RGs independently examined the 50 radiographic cases and identified the presence or absence of particular radiographic features using an objective survey instrument (Figure 1). The examiners were calibrated on the basis of the radiographic features and their descriptors using example images of COD not included in the objective test. This was done prior to viewing the 50 case sets. For each of the 50 case sets, observers were also asked to provide a radiographic interpretation of the abnormality. A 3 month follow-up test was conducted with 1 GP and 1 RG using the same 50 case sets, randomly re-ordered, to test intraexaminer reliability. Two of the RGs who served as observers were recent graduates of a postgraduate programme in oral and maxillofacial radiology with less than 18 months of clinical experience as independent practitioners, while the third had approximately 10 years of experience. In contrast, the GPs who participated in this study were part-time instructors in oral and maxillofacial radiology in the faculty with a maximum of 18 months of undergraduate teaching experience.
Figure 1.
Objective survey instrument
So that all participants made their observations and interpretations under the same conditions, images were viewed in a quiet, dimly-lit room on the same 19 inch LCD computer monitors (Dell UltraSharp1907FPc, Dell Inc., Round Rock, TX). The viewing conditions used are a standard of practice for faculty and resident RGs at our institution. Furthermore, the same amount of time was allotted to each participant to view the images.
Univariate analysis of the data was performed using descriptive statistics (frequencies, proportions) for qualitative variables. Inter and intraexaminer agreements were assessed using Cohen's Kappa test to analyse the reliability of the examiners. To assess the contribution of examiner type (i.e. GP vs RG) on the identification of a particular radiographic feature and on the final interpretation, bivariate analysis was performed using χ2 testing. To determine which radiographic feature(s) were associated with a correct interpretation of COD, a stepwise logistic regression model was developed for both examiner groups to determine the effect(s) of radiographic feature(s) on the probability of the outcome (i.e. the final interpretation).
Results
The RGs correctly interpreted 79.3% of the cases as COD whereas the GPs were able to correctly interpret only 38.7%. These differences were significant to P < 0.001. Using Cohen's Kappa test, the GPs showed an overall “slight to moderate” interexaminer agreement in their final interpretation (Kappa score 0.13-0.58) whereas the RGs showed an overall “substantial to almost perfect” agreement (Kappa score 0.80–0.84).
RGs and GPs generally agreed on the radiographic locations of the COD lesions, and many of the radiographic features related to the effects of COD on surrounding structures (Table 1). RGs identified the presence of a radiolucent band or rim (P < 0.05), a mixed radiolucent/radiopaque internal structure (P < 0.001), dense cementum-like radiopacities (P < 0.05), endosteal or root scalloping (P < 0.005), the absence of lamina dura (P < 0.001) and normal periodontal ligament space (P < 0.005) more frequently than GPs.
Table 1. Frequency of reported radiographic features of cemento-osseous dysplasia.
| Examiner group |
Pa | ||
| GP | RG | ||
| Location | |||
| Mandible only | 78.4 % | 77.5 % | ns |
| Anterior and posterior teeth | 50.5 % | 53.2 % | ns |
| Multiple and bilateral | 52.3 % | 52.3 % | ns |
| Periphery | |||
| Well defined | 55.9 % | 61.3 % | ns |
| Radiolucent band/rim | 64.0 % | 78.4 % | 0.03 |
| No cortical border | 61.3 % | 68.5 % | ns |
| Irregular shape | 66.6 % | 67.6 % | ns |
| Peripheral sclerosis | 47.7 % | 61.3 % | ns |
| Internal structure | |||
| Mixed radiolucent/radiopaque | 27.0 % | 55.9 % | <0.001 |
| Dense, cementum-like radiopacities | 53.1 % | 71.1 % | 0.02 |
| Effects on surrounding structures | |||
| Endosteal or root scalloping | 36.9 % | 54.1 % | 0.002 |
| No lamina dura | 52.9 % | 74.8 % | <0.001 |
| Normal PDL space | 54.1 % | 71.2 % | 0.003 |
| No root resorption | 83.8 % | 88.3 % | ns |
| No hypercementosis | 68.5 % | 66.7 % | ns |
| Non-concentric cortical expansion | 54.1 % | 55.9 % | ns |
| No displacement of anatomic structures | 87.4 % | 89.2 % | ns |
| No periosteal reaction | 89.2 % | 94.6 % | ns |
GP, general dentist; RG, oral and maxillofacial radiologist; ns, non-significant; PDL, periodontal ligament. a χ2 test
A stepwise logistic regression model was developed for both examiner groups to determine which radiographic feature(s) was most strongly associated with a correct interpretation of COD. Adjusted odds ratio (OR) and 95% confidence intervals (CI) were also reported. For GPs (Table 2), the two radiographic features that, when recognized, were most strongly associated with a correct interpretation of COD were the absence of root resorption (OR 4.52; CI 1.18–17.30; P < 0.05) and localization of lesions to the anterior and posterior teeth (OR 3.22; CI 1.42–7.52; P < 0.05). In comparison, for RGs (Table 3), the radiographic features that were most strongly associated with a correct interpretation of COD were the presence of a well-defined periphery (OR 6.67; CI 1.50–28.57; P < 0.05); the bilateral occurrence of lesions (OR 10.23; CI 2.00–52.56; P < 0.05); an internal mixed radiolucent/radiopaque appearance (OR 10.53; CI 2.06–52.63; P < 0.05); the absence of non-concentric bony expansion (OR 7.63; CI 1.46–40.00; P < 0.05); involvement of both the anterior and posterior teeth (OR 4.34; CI 1.11–17.54; P < 0.05); and the presence of a radiolucent rim or band (OR 8.28; CI 2.14–32.56; P < 0.001).
Table 2. Multiple logistic regression analysis for general dentists.
| Radiographic feature | Adjusted OR | 95.0% CI for EXP (β) |
P | |
| Lower | Upper | |||
| No root resorption | 4.52 | 1.18 | 17.30 | 0.03 |
| Anterior and posterior teeth | 3.22 | 1.42 | 7.52 | 0.01 |
| Constant | 3.45 | 0.05 | ||
Goodness of fit: R2 = 0.11 (Cox & Snell R2), –2 log likelihood = 135.112. P = 0.54 (Hosmer-Lemeshow). OR, odds ratio; CI, confidence interval
Table 3. Multiple logistic regression analysis for oral and maxillofacial radiologists.
| Radiographic feature | Adjusted OR | 95.0% CI for EXP (β) |
P | |
| Lower | Upper | |||
| Well-defined periphery | 6.67 | 1.50 | 28.57 | 0.01 |
| Bilateral | 10.23 | 2.00 | 52.56 | 0.01 |
| Mixed radiolucent/radiopaque | 10.53 | 2.06 | 52.63 | 0.01 |
| No cortical expansion | 7.63 | 1.46 | 40.00 | 0.02 |
| Anterior and posterior teeth | 4.34 | 1.11 | 17.54 | 0.04 |
| Radiolucent rim/band | 8.28 | 2.14 | 32.56 | <0.001 |
| Constant | 14.81 | 0.01 | ||
Goodness of fit: R2 = 0.33 (Cox & Snell R2); –2 log likelihood = 68.461. P = 0.66 (Hosmer-Lemeshow). OR, odds ratio; CI, confidence interval
One RG and one GP completed a 3 month post test. The RG's Kappa score showed “almost perfect” intraobserver agreement (Kappa score 0.88) and the GP's intraobserver agreement was “substantial” (Kappa score 0.65).
Discussion
COD is easily confused with other entities that may arise in the jaws. Indeed, oral and maxillofacial radiology may be the most valuable diagnostic test for differentiating COD from other lesions that may also be categorized as being “fibro-osseous” in nature.
Comparing the abilities of GPs and specialists in oral and maxillofacial radiology in feature identification and image interpretation of COD has not been studied before. Indeed, few studies have examined the interpretive skills of non-oral and maxillofacial radiologists and RGs. Raitz et al16 and Makris et al17 have examined the differences between non-oral and maxillofacial radiologists and RGs using analogue and digital images, but found no differences.
As a group, the RGs showed higher interexaminer agreement as evidenced by higher Kappa scores than the GPs. This may be partly related to the uniformity of the graduate training programme in oral and maxillofacial radiology and the increased familiarity that RGs have with the identification of radiographic features. As a group, the RGs were able to correctly interpret COD cases compared with GPs at a rate of almost 2:1.
The 50 image sets that were reviewed consisted mainly of plain radiographs. While it is true that GPs may not be familiar with advanced imaging modalities (CT and MRI), their inclusion was always in addition to the “traditional dental images” that are regularly made and interpreted by general practitioners. Furthermore, advanced images were selective, with only representative image slices included to augment conventional radiographs; advanced images were not presented separately.
With regard to the reporting of particular radiographic features of COD, GPs sometimes either under-or over-interpreted a radiographic feature compared with RGs. RGs identified the presence of mixed radiolucent/radiopaque internal structure more frequently than GPs (55.9% vs 27%). In contrast, GPs interpreted a lesion as having a completely radiolucent internal structure when a lesion had subtle internal radiopacities, and a completely radiopaque appearance as being more associated with COD. These differences were significant to P < 0.001. The presence of a radiolucent band or rim in COD was more frequently identified by RGs than GPs (78.4% vs 64%), as was the presence of dense cementum-like radiopacities (71.1% vs 53.1%), the presence of endosteal or root scalloping (54.1% vs 36%) and the absence of lamina dura (74.8% vs 52.9%) and periodontal ligament space (71.2% vs 54.1%). These differences were all statistically significant.
In terms of effects on surrounding structures, the RGs reported that 54.1% of COD cases were associated with endosteal and/or inter-radicular scalloping compared with 36.9% of GPs. Also, RGs reported that COD lesions resulted in loss of the lamina dura in 74.8% of the cases while the GPs considered the majority of COD (52.9%) to have a normal lamina dura. Some radiographic features may be subtle and visible only to the trained eye, making fine radiographic distinctions difficult. RGs may have a greater appreciation for normal variation whereas GPs may lack this knowledge or expertise and, therefore, might interpret a variation of normal to be abnormal or vice versa. Recently, Raitz et al18 compared the interpretive skills of different examiner groups: undergraduate dental students; GPs; oral surgeons; oral pathologists; oral radiologists; and stomatologists. Similar to our study, Raitz et al found that the interpretive skills of specialists were superior to those of the GPs and concluded that the GPs' knowledge of the different presentations of lesions examined was low.
To determine which radiographic feature(s) analysed was more frequently associated with a correct interpretation of COD, a multivariate logistic regression model associating a given radiographic feature with examiner type (GP or RG) and a correct interpretation of COD was developed. Using this model for the GPs' data, there were only two radiographic features of COD that, when identified, were most strongly associated with a correct interpretation of COD. In the absence of root resorption, GPs were 4.52 times more likely to correctly interpret a case as being COD (95% CI = 1.18–17.30). If COD was related to anterior and posterior teeth together, the odds of the GPs in making the correct interpretation were 3.22 (CI = 1.42–7.52). In contrast, RGs identified six radiographic features as being more strongly associated with a correct interpretation of COD. If the COD lesion had a well-defined periphery, the odds of a correct interpretation were 6.67 times higher (CI = 1.50–28.57). In the presence of a radiolucent border, the odds of a correct interpretation were 8.28 times higher (CI = 2.14–32.56). If the internal structure of the COD lesion was mixed radiolucent/radiopaque, RGs were 10.52 times more likely to correctly interpret COD (CI = 2.06–52.63). When COD was bilaterally distributed, RGs were 10.23 times more likely to correctly interpret COD than if the lesions were unilateral (CI = 2.00–52.56). When COD was associated with anterior and posterior teeth, the odds of a correct interpretation were 4.34 (CI = 1.11–17.54). Although oral radiologists reported that 55.9% of COD caused non-concentric expansion (Table 1), this radiographic feature was not associated with correct interpretation of COD. Rather, in the absence of non-concentric cortical expansion, RGs were 7.63 times more likely to correctly differentiate COD from the other similarly appearing entities (CI = 1.46–40.00).
The systematic evaluation of radiographic features (location, periphery, internal structure and effects on the surrounding structures) of 37 cases of COD presented to 6 clinicians represents the largest objective radiology-based study of COD to date. We found significant differences in the abilities of RGs and GPs to correctly interpret COD, and to distinguish it from other similarly appearing entities. These results suggest that most COD can be differentiated from other similarly appearing entities, but only if the characteristic radiographic features are correctly identified and then correctly interpreted. The former relies on adequate training and expertise and the latter relies on knowledge and education.
There are, however, several shortcomings of the current study. Firstly, the sample of cases used may be more difficult and challenging for the GPs as these patient referrals were initiated by both GPs and dental specialists to our oral and maxillofacial radiology referral clinic. Secondly, as with all diagnostic tests, diagnostic radiology has its limitations. Radiographic distinction of COD from small cemento-ossifying fibromas, for example, may be difficult. Under these circumstances, “periodic monitoring” may be recommended to evaluate changes so that a neoplastic process may be differentiated from one that is dysplastic. In the absence of clinical signs or symptoms related to COD lesions, follow-up radiography is favoured, together with a conservative management approach.
In conclusion, COD is radiographically discernable from other similarly appearing entities and RGs are better able to correctly interpret the radiographic features of COD than GPs. Logistic regression modelling of our data demonstrated that the radiographic features most strongly associated with a correct interpretation of COD by RGs were their bilateral occurrence; involvement of anterior and posterior teeth together; the presence of a well-defined border with an associated radiolucent band/rim; and a mixed radiolucent/radiopaque internal structure.
We suggest that a greater emphasis should be placed on the identification of radiographic features and their biological meaning in COD and radiographically similar diseases. As the characteristic radiographic features of COD become more widely recognized and appreciated, fewer cases should be mismanaged.
References
- 1.Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol 2001;8:126–143 [DOI] [PubMed] [Google Scholar]
- 2.Summerlin DJ, Tomich CE. Focal cemento-osseous dysplasia: a clinicopathologic study of 221 cases. Oral Surg Oral Med Oral Pathol 1994;78:611–620 [DOI] [PubMed] [Google Scholar]
- 3.MacDonald-Jankowski DS. Florid cemento-osseous dysplasia: a systematic review. Dentomaxillofac Radiol 2003;32:141–149 [DOI] [PubMed] [Google Scholar]
- 4.Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg 1993;51:828–835 [DOI] [PubMed] [Google Scholar]
- 5.Su L, Weathers DR, Waldron CA. Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifying fibromas. II. A clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:540–549 [DOI] [PubMed] [Google Scholar]
- 6.Mahomed F, Altini M, Meer S, Coleman H. Cemento-osseous dysplasia with associated simple bone cysts. J Oral Maxillofac Surg 2005;63:1549–1554 [DOI] [PubMed] [Google Scholar]
- 7.Galgano C, Samson J, Kuffer R, Lombardi T. Focal cemento-osseous dysplasia involving a mandibular lateral incisor. Int Endod J 2003;36:907–911 [DOI] [PubMed] [Google Scholar]
- 8.Smith S, Patel K, Hoskinson AE. Periapical cemental dysplasia: a case of misinterpretation. Br Dent J 1998;185:122–123 [DOI] [PubMed] [Google Scholar]
- 9.Islam MN, Cohen DM, Kanter KG, Stewart CM, Katz J, Bhattacharyya I. Florid cemento-osseous dysplasia mimicking multiple periapical pathology—an endodontic dilemma. Gen Dent 2008;56:559–562 [PubMed] [Google Scholar]
- 10.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press, 2005 [Google Scholar]
- 11.Kawai T, Hiranuma H, Kishino M, Jikko A, Sakuda M. Cemento-osseous dysplasia of the jaws in 54 Japanese patients: A radiographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:107–114 [DOI] [PubMed] [Google Scholar]
- 12.Melrose RJ, Abrams AM, Mills BG. Florid osseous dysplasia. A clinical-pathologic study of thirty-four cases. Oral Surg Oral Med Oral Pathol 1976;41:62–82 [DOI] [PubMed] [Google Scholar]
- 13.Baden E, Saroff SA. Periapical cemental dysplasia and periodontal disease. A case report with review of the literature. J Periodontol 1987;58:187–191 [DOI] [PubMed] [Google Scholar]
- 14.Loh FC, Yeo JF. Florid osseous dysplasia in Orientals. Oral Surg Oral Med Oral Pathol 1989;68:748–753 [DOI] [PubMed] [Google Scholar]
- 15.Tonioli MB, Schindler WG. Treatment of a maxillary molar in a patient presenting with florid cemento-osseous dysplasia: A case report. J Endod 2004;30:665–667 [DOI] [PubMed] [Google Scholar]
- 16.Raitz R, Correa L, Curi M, Dib L, Fenyo-Pereira M. Conventional and indirect digital radiographic interpretation of oral unilocular radiolucent lesions. Dentomaxillofac Radiol 2006;35:165–169 [DOI] [PubMed] [Google Scholar]
- 17.Makris N, Tsiklakis K, Alexiou KE, Vierrou AM, Stefaniotis T. The subjective image quality of conventional and digital panoramic radiography among 6 to 10 year old children. J Clin Pediatr Dent 2006;31:109–112 [DOI] [PubMed] [Google Scholar]
- 18.Raitz R, Assunção Júnior JN, Correa L, Fenyo-Pereira M. Parameters in panoramic radiography for differentiation of radiolucent lesions. J Appl Oral Sci 2009;17:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]