Abstract
We investigated the morphology, phylogeny of the 18S rDNA, and pH response of Oxytricha acidotolerans sp. nov. and Urosomoida sp. (Ciliophora, Hypotricha) isolated from two chemically similar acid mining lakes (pH ∼ 2.6) located at Langau, Austria, and in Lusatia, Germany. Oxytricha acidotolerans sp. nov. from Langau has 18 frontal-ventral-transverse cirri but a very indistinct kinety 3 fragmentation so that the assignment to Oxytricha is uncertain. The somewhat smaller species from Lusatia has a highly variable cirral pattern and the dorsal kineties arranged in the Urosomoida pattern and is, therefore, preliminary designated as Urosomoida sp. The pH response was measured as ciliate growth rates in laboratory experiments at pH ranging from 2.5 to 7.0. Our hypothesis was that the shape of the pH reaction norm would not differ between these closely related (3% difference in their SSU rDNA) species. Results revealed a broad pH niche for O. acidotolerans, with growth rates peaking at moderately acidic conditions (pH 5.2). Cyst formation was positively and linearly related to pH. Urosomoida sp. was more sensitive to pH and did not survive at circumneutral pH. Accordingly, we reject our hypothesis that similar habitats would harbour ciliate species with virtually identical pH reaction norm.
Keywords: Acid mining lakes, Growth rates, pH response, Oxytricha, SSU rDNA, Urosomoida
Introduction
Different freshwater ecosystems may vary widely in their pH (<2–12; Wetzel 2001), and this abiotic factor limits the occurrence of many species. The majority of species can potentially live in circumneutral water bodies, but many species are sensitive to low pH. The effect of reduced pH on the biota has been studied intensively in North America and Europe during the period of surface water acidification due to acid deposition from the 1970s to the early 1990s (e.g. Schindler 1988; Steinberg and Wright 1994). In spite of an early description on the effect of acid precipitation on the ciliate fauna in Florida lakes (Beaver and Crisman 1981), the response of ciliates to changing pH has been investigated in detail for only a few species (Weisse and Stadler 2006; Weisse et al. 2007; this study). This may reflect the fact that, relative to temperature and food, seasonal pH fluctuations of one unit, which are typical for many moderately productive, circumneutral lakes (Wetzel 2001), have only a minor effect on the population growth rate of ciliates (Weisse 2006a). However, the width of the pH niche determines the range of habitats that a given species may potentially colonise. This was demonstrated in laboratory experiments with three species of the common prostome ciliate genus Urotricha (Weisse and Stadler 2006): U. castalia tolerated pH changes of less than two units (6.5–8.2), U. furcata could survive over a wider pH range (5.4–9.2), and U. farcta showed positive growth over more than five pH units (4.1–9.5). Natural volcanic lakes and man-made acidic mining lakes (AML) and drainages are the most acidic freshwater habitats, some of them with extreme pH of <2 (for review, see Geller et al. 1998). Many of these extreme environments are characterized by pH ranging from 1.8–5.5 (Geller et al. 1998; Lackey 1938). At the lower end (pH < 3), the heterotrophic protist diversity is strongly reduced (Lackey 1938, 1939; McConathy and Stahl 1982; Packroff 2000; Packroff and Woelfl 2000; Wollmann et al. 2000). According to these reviews, several species of the genus Oxytricha are regularly encountered in such extreme habitats.
The original aim of the present investigation was to assess the pH reaction norm of two Oxytricha-like species that were isolated from two different AML of pH < 3. Since it turned out in the course of this investigation that at least one of the two isolates represents a novel species, we first describe both strains in detail. We performed laboratory experiments to test if both species show similar characteristics of either acidophilic (i.e. specialist) or acidotolerant (i.e. generalist) organisms. The former is typical for species that have specifically adapted to the extreme conditions prevailing in AML and grow better at low than at circumneutral pH; the latter are species that take advantage from reduced predation and/or competition under the unfavourable environmental conditions in AML (Moser and Weisse 2011a). The pH response of an acidophilic protist species has recently been reported for the mixotrophic alga Chlamydomonas acidophila (Gerloff-Elias et al. 2005; Moser and Weisse 2011a). The latter authors portrayed several species of the chrysomonad genus Ochromonas as acidotolerant protists.
The present investigation is part of a larger project that investigates if the pelagial of AML is primarily colonised by pH specialists or generalists (Moser and Weisse 2011a, b; Weisse et al. 2011). Previous studies showed that the pH response, similar to the response to temperature and food, may vary within a ciliate genus and even within a given species (Weisse 2002; Weisse and Stadler 2006; Weisse et al. 2007). Accordingly, we expected to find some significant differences in the pH response of the two Oxytricha-like species that were isolated from two similar AML located at Langau in Lower Austria and in Lusatia, Germany. However, considering that we dealt with closely related species isolated from the same type of habitat, our hypothesis was that the shape of their pH reaction norm would not differ. Our results demonstrate that this is not the case; even closely related, potentially sympatric protist species may show different life strategies along the pH generalist to specialist continuum. These findings have important implications for our understanding of biodiversity of freshwater protists.
Material and Methods
Study organisms and their habitats of origin
Oxytricha acidotolerans sp. nov. was isolated from the pelagial of the smallest of three acidic mining lakes (AML), located about 2.3 km north-northeast of the village of Langau (48°51′ N, 15°44′ E; about 430 m above sea level) in Lower Austria during summer 2007. Surface area of this lake is ∼2 × 104 m2, maximum water depth 9.5 m. The pH ranges from 2.3 to 2.8 in the upper 5 m from which the ciliate originated. Hydro-chemical, limnological, and topographic characteristics of this AML have been reported by Moser and Weisse (2011c). The other species was isolated from AML Lake 111 (51°29′ N, 13°38′ E) located in Lusatia, East Germany, during 2002 and originally designated Oxytricha sp. strain 99X4 (Tittel et al. 2003) and here preliminary identified as Urosomoida sp. Surface area is ∼1 × 105 m2, maximum water depth close to 10 m; the pH ranges from 2.6 to 2.9 (Kamjunke et al. 2004; Wollmann et al. 2000). Both lakes originated from rising ground water of open-cast pits of lignite mines after the cessation of mining activities in the 1960s and were geogenically acidified. These man-made lakes are remarkably similar in their origin, age, and hydrochemical composition (Weithoff et al. 2010), but the German and the Austrian site are located approximately 325 km apart from each other. Both species were obtained as non-clonal cultures via enrichment cultures with the green alga Chlamydomonas acidophila Negoro as food and kept in Modified Woods Hole Medium (MWC; UKNCC 2001) at pH 2.8 and 17.5 °C in the dark.
Morphological methods, terminology
Living cells were studied using, inter alia, high-power oil immersion objective and differential interference contrast optics. Live measurements were made at magnifications of 125–1,250×. Although live values are more or less rough estimates, it is worth giving such data as specimens usually contract during fixation or shrink in preparations (for details see Foissner et al. 2002, p. 34). The infraciliature and nuclear apparatus were revealed with the protargol method according to protocol A in Foissner et al. (1999). Preparations of both species were made during the second half of 2009. Counts and measurements on prepared specimens were performed at a magnification of 1,250×. Illustrations of live specimens are based on freehand sketches and freezed images of video sequences of freely motile specimens, while those of prepared cells were made with a drawing device. For deposition of permanent preparations, see Results.
Focus stacking was done with the freeware CombineZP (www.hadleyweb.pwp.blueyonder.co.uk). The morphometric data shown in Table 1 are repeated in the text only if needed for clarity. Only specimens where (almost) all features were recognisable have been used for morphometric analysis. In the analysis of Oxytricha acidotolerans, we excluded such individuals which had, for example, a distinctly deviating cirral pattern (very likely often injured, regenerating, or malformed specimens) or an unusually small size (very likely often degenerating or just divided specimens). The inclusion of such specimens would artificially increase variability. The cirral and dorsal kinety pattern as well as the nuclear apparatus of the population from Lusatia was too variable for a decent description (for details, see below).
Table 1.
Morphometric data on Oxytricha acidotolerans sp. nov.
| Charactera | HT | Mean | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|---|
| Body, length | 83 | 76.5 | 79.0 | 8.3 | 1.8 | 10.9 | 55 | 88 | 21 |
| Body, width | 30 | 27.5 | 29.0 | 4.6 | 1.0 | 16.8 | 16 | 32 | 21 |
| Adoral zone of membranelles, length | 26 | 24.1 | 24.0 | 1.7 | 0.4 | 7.2 | 21 | 28 | 21 |
| AE to distal end of adoral zone, distance | 6 | 5.7 | 6.0 | 0.5 | 0.1 | 8.3 | 5 | 6 | 21 |
| Adoral membranelles, maximum width | 6 | 5.9 | 5.6 | 0.8 | 0.2 | 13.1 | 5 | 8 | 21 |
| AE to paroral, distance | 7 | 6.6 | 6.5 | 1.1 | 0.3 | 16.9 | 5 | 9 | 20 |
| AE to endoral, distance | 7 | 6.1 | 6.0 | 1.2 | 0.3 | 20.5 | 4 | 10 | 21 |
| Paroral, length | 10 | 10.5 | 10.0 | 1.0 | 0.2 | 9.6 | 9 | 13 | 20 |
| Endoral, length | 14 | 13.9 | 14.0 | 1.2 | 0.3 | 8.7 | 12 | 16 | 20 |
| AE to buccal cirrus, distance | 8 | 7.0 | 7.0 | 1.0 | 0.2 | 14.3 | 6 | 9 | 21 |
| AE to cirrus III/2, distance | 15 | 14.4 | 14.0 | 1.3 | 0.3 | 9.3 | 11 | 17 | 21 |
| AE to cirrus IV/3, distance | 18 | 17.5 | 18.0 | 1.4 | 0.3 | 8.2 | 14 | 20 | 21 |
| AE to cirrus VI/3, distance | 15 | 14.6 | 14.0 | 1.8 | 0.4 | 12.3 | 11 | 18 | 21 |
| AE to cirrus VI/4, distance | 12 | 11.1 | 10.0 | 1.5 | 0.3 | 13.7 | 10 | 14 | 21 |
| AE to frontmost postoral ventral cirrus, distance | 29 | 26.4 | 27.0 | 2.6 | 0.6 | 9.8 | 18 | 29 | 21 |
| AE to rearmost postoral ventral cirrus, distance | 37 | 33.9 | 34.0 | 2.7 | 0.6 | 7.8 | 37 | 37 | 18 |
| AE to left marginal row, distance | 22 | 21.6 | 22.0 | 1.5 | 0.3 | 7.1 | 18 | 24 | 21 |
| AE to right marginal row, distance | 19 | 15.9 | 15.0 | 2.2 | 0.5 | 13.6 | 13 | 19 | 21 |
| PE to anteriormost pretransverse ventral cirrus, distance | 10 | 11.9 | 12.0 | 2.0 | 0.4 | 17.2 | 7 | 16 | 21 |
| PE to rearmost transverse cirrus, distance | 3 | 3.1 | 3.0 | 0.7 | 0.2 | 23.5 | 1.5 | 4 | 21 |
| PE to anteriormost transverse cirrus, distance | 9 | 9.8 | 10.0 | 1.2 | 0.3 | 12.2 | 8 | 12 | 21 |
| Left caudal cirrus to middle caudal cirrus, distance | 3 | 2.6 | 2.5 | 0.4 | 0.1 | 17.1 | 1.6 | 3.2 | 20 |
| Middle caudal cirrus to right caudal cirrus, distance | 5 | 4.1 | 4.0 | 1.1 | 0.3 | 27.2 | 2 | 6 | 19 |
| AE to anterior macronuclear nodule, distance | 33 | 29.0 | 30.0 | 3.9 | 0.9 | 13.6 | 20 | 34 | 21 |
| Anterior macronuclear nodule, length | 10 | 10.6 | 10.0 | 1.4 | 0.3 | 13.2 | 9 | 14 | 21 |
| Anterior macronuclear nodule, width | 6 | 6.9 | 7.0 | 0.9 | 0.2 | 12.9 | 5 | 8 | 21 |
| Posterior macronuclear nodule, length | 10 | 9.8 | 10.0 | 1.2 | 0.3 | 11.9 | 8 | 12 | 21 |
| Posterior macronuclear nodule, width | 8 | 7.5 | 8.0 | 0.8 | 0.2 | 10.7 | 5.0 | 8.0 | 21 |
| Macronuclear nodules, distance in between | 5 | 3.4 | 3.0 | 1.8 | 0.4 | 54.2 | 0 | 7 | 21 |
| Anterior micronucleus, diameter | 3 | 3.2 | 3.2 | 0.3 | 0.1 | 8.9 | 3 | 4 | 21 |
| Posterior micronucleus, diameter | 3 | 3.1 | 3.2 | 0.3 | 0.1 | 8.8 | 2.4 | 3.5 | 21 |
| Macronuclear nodules, number | 2 | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2 | 2 | 21 |
| Micronuclei, number | 2 | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2 | 2 | 21 |
| Adoral membranelles, number | 24 | 24.0 | 24.0 | 1.4 | 0.3 | 6.0 | 22 | 28 | 21 |
| Frontal cirri, number | 3 | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3 | 3 | 21 |
| Buccal cirri, number | 1 | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1 | 1 | 21 |
| Frontoventral cirri, numberb | 4 | 4.0 | 40.0 | 0.0 | 0.0 | 0.0 | 4 | 4 | 21 |
| Postoral ventral cirri, number | 3 | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3 | 3 | 18 |
| Pretransverse ventral cirri, number | 2 | 2.0 | 2.0 | 0.0 | 0.0 | 10.7 | 2 | 3 | 21 |
| Transverse cirri, number | 5 | 4.9 | 5.0 | 0.3 | 0.1 | 6.1 | 4 | 5 | 21 |
| Left marginal cirri, number | 20 | 20.6 | 21.0 | 1.5 | 0.3 | 7.4 | 17 | 23 | 21 |
| Right marginal cirri, number | 19 | 20.6 | 21.0 | 1.8 | 0.4 | 8.6 | 16 | 24 | 21 |
| Dorsal kineties (without dorsomarginal kineties), number | 4 | 4.0 | 4.0 | 0.2 | 0.0 | 5.4 | 4 | 5 | 21 |
| Dorsomarginal kineties, number | 1 | 1.6 | 2.0 | 0.5 | 0.1 | 30.7 | 1 | 2 | 21 |
| Dorsal kineties, total number | 5 | 5.7 | 6.0 | 0.6 | 0.1 | 10.2 | 5 | 7 | 21 |
| Dorsal kinety 1, number of bristles | 8 | 9.6 | 10.0 | 0.8 | 0.2 | 8.5 | 8 | 11 | 21 |
| Dorsal kinety 2, number of bristles | 10 | 11.2 | 11.0 | 1.6 | 0.4 | 14.6 | 8 | 14 | 21 |
| Dorsal kineties 3 and 4, number of bristles | 14 | 11.8 | 12.0 | 1.6 | 0.4 | 13.5 | 8 | 14 | 20 |
| Dorsal kinety 5, number of bristles | 6 | 6.7 | 7.0 | 1.0 | 0.2 | 15.2 | 4 | 8 | 21 |
| Dorsal kinety 6, number of bristlesc | 0 | 1.2 | 1.0 | 0.4 | 0.1 | 32.5 | 1 | 2 | 13 |
| Dorsal bristles, total number | 38 | 39.4 | 40.0 | 5.1 | 1.1 | 12.9 | 24 | 48 | 21 |
| Caudal cirri, number | 3 | 3.1 | 3.0 | 0.3 | 0.1 | 9.7 | 3 | 4 | 21 |
All data are based on protargol-impregnated specimens. Measurements in μm. AE, anterior end of cell; CV, coefficient of variation in %; HT, holotype specimen (also included in the sample n); M, median; Max, maximum; Mean, arithmetic mean; Min, minimum; n, number of specimens investigated; PE, posterior end of cell; SD, standard deviation; SE, standard error of arithmetic mean.
This group is composed of the frontoterminal cirri (VI/3, VI/4), the parabuccal cirrus (III/2), and cirrus IV/3 (e.g. Fig. 1f).
Specimens with lacking dorsal kinety 6 (e.g. holotype) not included.
General terminology is mainly according to Lynn (2008); for explanation of terms specific for hypotrichs (e.g. DE-value), see Berger and Foissner (1997), Berger (1999, 2006, 2008, 2011), and Foissner and Al-Rasheid (2006). For the individual designation of the frontal-ventral-transverse cirri and their anlagen, the numbering system by Wallengren (1900) was used (details see Berger 1999, p. 16). The term 18-cirri hypotrich means a hypotrich with 18 frontal-ventral-transverse cirri (e.g. Berger 2008, p. 27). For authorities of scientific names of hypotrichs, see Berger (1999, 2001) and Shao et al. (2012).
DNA extraction, amplification, sequencing, and phylogenetic analyses
DNA was extracted from ethanol fixed cells using the NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany). The nuclear SSU rDNA was amplified using universal Eukarya-specific primers (F01 and R01; Mattern and Schlegel 2001). PCR conditions were as follows: 3 min initial denaturation at 94 °C, followed by 40 cycles of 1 min at 94 °C, 1 min at 48 °C, and 2 min at 72 °C, and a final elongation of 7 min at 72 °C (Wylezich et al. 2002). The reaction mix contained 10 pl of genomic DNA, 10 pmol of each primer, 1 U Taq-Polymerase (SIGMA, Taufkirchen, Germany), 1× PCR buffer, 2 mM MgCl2 and 200 pM dNTPs in a total volume of 25 pl. PCR products were purified with the NucleoSpin Extract II Kit (Macherey-Nagel) and directly sequenced on an ABI 3100 automatic sequencer. Sequencing reactions were performed in both directions using the same universal Eukarya-specific primers and different internal primers (Mattern and Schlegel 2001; Wylezich et al. 2002). Sequences were assembled using the program BioEdit 7.0.5.3 (Hall 1999) and have been submitted to GenBank (accession numbers are FN429123 for O. acidotolerans sp. nov. designated in GenBank as “Oxytricha sp. Oc_L1, isolate Langau” and FN429124 for Urosomoida sp. from Lusatia designated in GenBank as “Oxytricha sp. Ox_L1, isolate ML111”). The analysed data set for phylogenetic reconstructions contained 35 SSU rDNA sequences of spirotrichous ciliates including the two new sequences. Two sequences from oligotrichous representatives served as outgroup in all analyses. The final alignment thus comprised 37 taxa and was manually trimmed to the lengths of the shortest sequences in the dataset resulting in an overall length of 1763 bp including gaps. For GenBank accession numbers see Fig. 5. Alignments were estimated with the program ClustalW2 (Larkin et al. 2007). The program jModelTest 0.1.1 (Posada 2008) was used to select the best-fit model of nucleotide substitution. The GTR+I+G proved to be the best model of sequence evolution for the data set. Neighbor-joining (NJ), Maximum-likelihood (ML), and Bayesian analyses were performed with Geneious pro 5.0.3 (Drummond et al. 2010) using PhyML (Guindon and Gascuel 2003) and MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) implemented in Geneious. Empirical NJ and ML calculations were performed using the GTR+I+G model with 1,000 bootstrap replications; we did not apply any filters. For the ML and Bayesian analyses, the jModeltest output parameter for invariable sites (I = 0.6960) and the gamma shape parameter (G = 0.5350) were used. The Bayesian analysis was conducted with the same substitution model, two independent runs of 1,000,000 generations at a sample frequency of 1,000 and an initial burn-in of 25%. The resulting phylogenetic trees of the different analyses methods were combined and graphically edited using the program TreeGraph 2.0.45-197 beta (Stöver and Müller 2010).
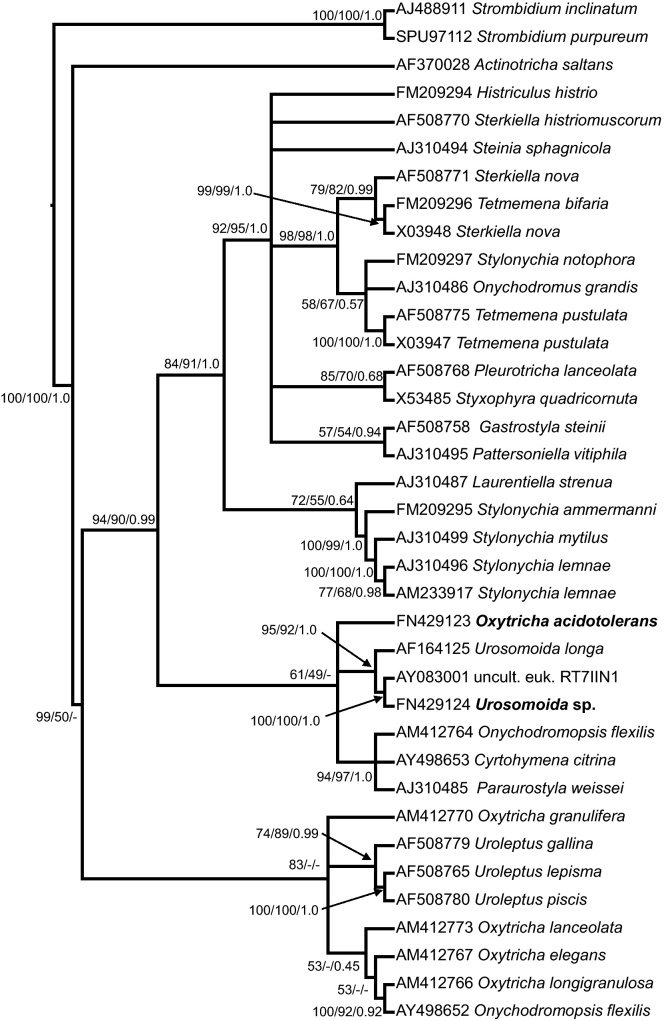
Fig. 5.
Phylogenetic NJ tree of the SSU rDNA of hypotrichous ciliates. The species described and used in this study are shown in bold face. GenBank accession numbers are listed in front of species names. Support values at the nodes represent bootstrap percentages of the Neighbor-joining (first number) and Maximum-likelihood (second number) analyses and posterior probabilities of the Bayesian analysis (third number). Arrows denote support values to corresponding nodes. The names of the following species have been adapted to modern systematics (Berger 2001; Shao et al. 2012): Actinotricha saltans (name in GenBank Oxytricha saltans), Tetmemena bifaria (Stylonychia bifaria), Urosomoida longa (Oxytricha longa).
Experimental design
Experiments were carried out in 50-ml culture tissue flasks in the dark at 17.5 ± 0.2 °C. Experimental volume in each flask was 40 ml. The experimental containers were inoculated with ciliates from exponentially growing stock cultures to yield initial ciliate abundances of 50–100 cells ml−1. The flagellate Chlamydomonas acidophila was used as food at satiating levels (∼106 cells ml−1). The food and ciliate species were stepwise acclimated to the experimental conditions over a period of 2–5 d. The longest period was applied at the extremes of the pH range. Experimental duration ranged from 4–6 d; pH was measured in each flask twice per day. If the pH differed by more than 0.2 from the target pH, it was adjusted by addition of small amounts (15–35 μl) of 0.1 mol l−1 NaOH or HCl. Each treatment was run in triplicate. Subsamples were taken every 24 h, immediately fixed with acid Lugol's solution, and ciliates were counted microscopically. The abundance of C. acidophila was measured electronically by means of an automatic particle counter (CASY 1-model TTC, Schärfe System, Reutlingen, Germany). This device allows precise determination of protist cell numbers and cell size at concentrations >104 cells ml−1 (Weisse and Kirchhoff 1997). Cell length and width of Lugol's fixed ciliates from the experiments were measured by a semi-automatic image analysis system (LUCIA version 4.51 Laboratory Imaging).
Calculation of experimental results and statistical analyses
Specific growth rates (μ, d−1) of the ciliates were calculated from changes in their cell numbers vs. time, assuming exponential growth according to
where N0 and Nt are ciliate numbers at the beginning and time t (in days) of the experiment, respectively. In most treatments in which ciliate cell numbers increased exponentially over several days, results were calculated from a linear regression of ln cell numbers vs. time. The slope of the regression yielded μ. If ciliate abundances did not increase exponentially or even declined in the course of the experiments, μ was calculated from changes between initial and final cell numbers.
All results reported in this study are mean values ± 1 SD. Growth rates measured at the various pH values were tested for significant differences using one-way ANOVA. Multiple linear regression analysis was used to assess significant effects of pH and μ on morphometric parameters of the ciliates. Results were considered significant if P < 0.05. All statistical analyses were performed with Sigma Plot 11.0 and Sigma Stat 2.03 (SPSS Inc.).
Results
Description of Oxytricha acidotolerans sp. nov. and Urosomoida sp.
Oxytricha acidotolerans sp. nov. (Figs 1a–i, 2a–d, 3a–j, Table 1)
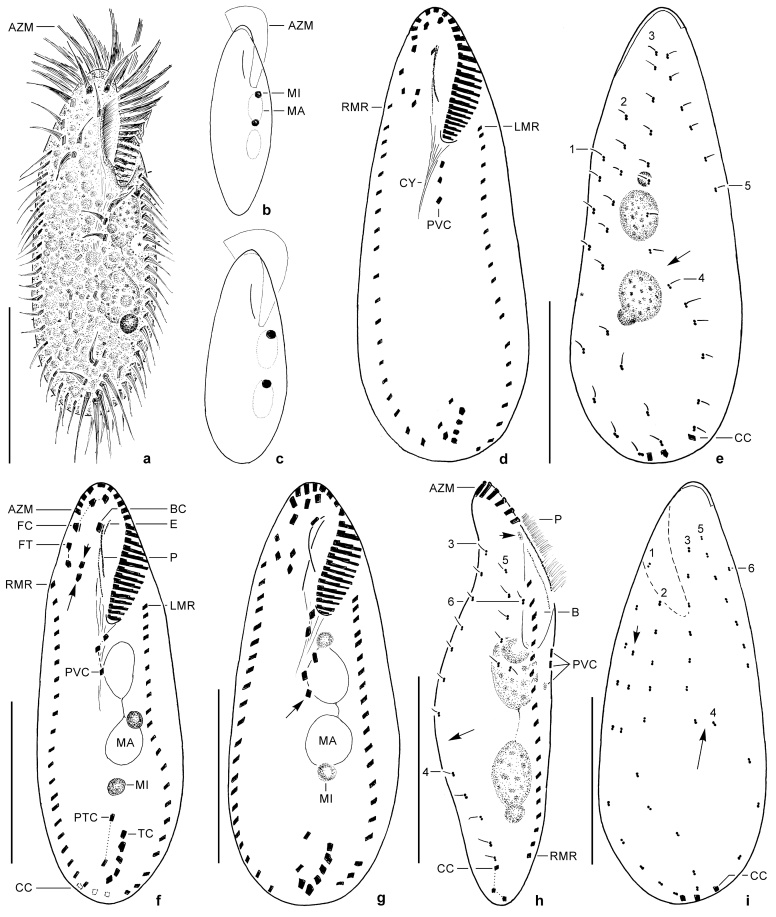
Fig. 1.
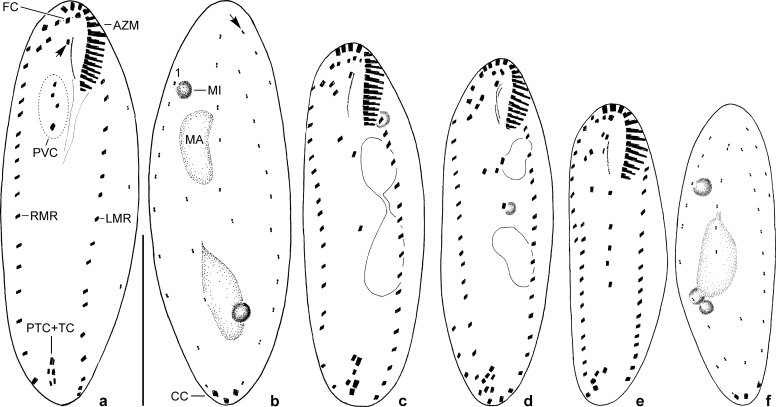
Oxytricha acidotolerans sp. nov. in vivo (a–c) and after protargol impregnation (d–i). a: Ventral view of representative specimen. Asterisk marks contractile vacuole. b, c: Spindle-shaped and slightly oviform shape variants with two rare arrangements of micronuclei. d, e: Infraciliature of ventral and dorsal side and nuclear apparatus of holotype specimen. Asterisk in (e) marks gap in dorsal kinety 1, arrow denotes indistinct “fragmentation” of kinety 3 (details see text). f: Specimen with almost linearly arranged transverse cirri. Short arrow marks frontoventral cirrus III/3, long arrow denotes cirrus IV/3. g: Specimen with five postoral ventral cirri (rearmost marked by arrow) and six transverse cirri. h: Right lateral view. Short arrow marks slightly subjacent buccal cirrus, long arrow marks “fragmentation” of kinety 3. i: Infraciliature of dorsal side of a specimen with an additional (remnant of previous generation?) short row of dorsal bristles (short arrow); kinety 6 is composed of one (rarely two) basal body pair only, if present at all. Long arrow marks “fragmentation” of kinety 3. AZM, adoral zone of membranelles; B, buccal cavity; BC, buccal cirrus; CC, caudal cirri; CY, pharyngeal fibres; E, endoral; FC, frontal cirri; FT, frontoterminal cirri (= cirri VI/3, VI/4); LMR, left marginal row; MA, macronuclear nodule; MI, micronucleus; P, paroral; PTC, pretransverse ventral cirri; PVC, postoral ventral cirri; RMR, right marginal row; TC, transverse cirri; 1–6, dorsal kineties. Scale bars: 30 μm.
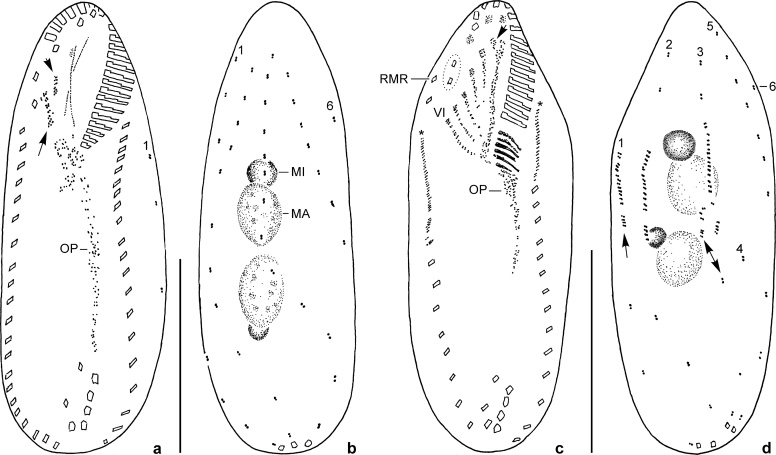
Fig. 2.
Oxytricha acidotolerans sp. nov. after protargol impregnation. a, b: Infraciliature of ventral and dorsal side and nuclear apparatus of early divider. As is usual, anlagen III (short arrow) and IV (long arrow) originate from disorganised parental cirri III/2 and IV/3, respectively. In the area where the nuclear apparatus is located, the oral primordium is not clearly recognisable. c, d: Infraciliature of ventral and dorsal side and nuclear apparatus of middle reorganiser. Arrow in (c) marks new left frontal cirrus originating, as is usual, from anlage I. Asterisks denote marginal row anlagen. Dotted line circles frontoterminal cirri (VI/3, VI/4) which are, as is usual, not involved in primordia formation. Arrow in (d) marks new caudal cirrus on dorsal kinety 1. Obviously this specimen has an additional old and new (double arrow) dorsal kinety 4. MA, macronuclear nodule; MI, micronucleus; OP, oral primordium; RMR, anteriormost cirrus of old right marginal row; VI, frontal-ventral-transverse cirri anlage VI; 1–6, dorsal kineties. Scale bars: 30 μm.
Fig. 3.
Oxytricha acidotolerans sp. nov. in vivo (a, b; differential interference contrast; video frames) and after protargol impregnation (c–j). a: Dorsal view of a freely motile specimen. b: Optical section showing, inter alia, dorsal bristles (about 3 μm) and contractile vacuole (asterisk). c: Rare specimen with a single micronucleus (arrow) in between the two macronuclear nodules. d: Right lateral view showing that the nuclear apparatus is rather close to the ventral side (arrow marks micronucleus). e: Holotype specimen (composite of eight micrographs). White arrow marks rearmost postoral ventral cirrus (V/3), black arrow denotes a caudal cirrus. Asterisk indicates area of contractile vacuole. f: Specimen with micronuclei (arrows) at anterior end of macronuclear nodules. g, h: Posterior end showing, inter alia, pretransverse ventral cirri (arrows in g) and caudal cirri (arrows in h). i: Specimen with anterior postoral ventral cirrus (arrow) slightly shifted leftwards. Frontoventral cirri circled. j: Ventral view of reorganiser (see Fig. 2c). AZM, adoral zone of membranelles; BC, buccal cirrus; DB, dorsal bristles; FC, right frontal cirrus; LMR, left marginal row; MA, macronuclear nodule; MI, micronucleus; RMR, right marginal row; TC, transverse cirri; UM, undulating membranes; VI, frontal-ventral-transverse cirri anlage VI; VI/4, anterior frontoterminal cirrus. Scale bars: 30 μm (a, d–f, i, j), 10 μm (b).
Diagnosis: In vivo about 80 × 28 μm-sized Oxytricha (?) with two macronuclear nodules and two micronuclei and, on average, 24 adoral membranelles and 21 right and 21 left marginal cirri. Dorsal kinety 1 with distinct gap behind mid-body; fragmentation of kinety 3 rather indistinct. In total 40 dorsal bristles on average.
Type locality: Acid mining lake near the village of Langau, Lower Austria (for details, see Material and Methods).
Etymology: The species-group name acidotolerans is a composite of the Latin adjective acid·us (sour, tart; Werner 1972, p. 83), the thematic vowel ·o-, and the Latin adverb tolerans (bearing, enduring, tolerant; Menge 1984, p. 525), and refers to the fact that this species is tolerant to a wide range of low pH. The binomen Oxytricha acidotolerans was already used by Weisse et al. (2011). However, it was disclaimed for nomenclatural purposes referring to Article 8.3 of the ICZN (1999) and therefore the name O. acidotolerans in Weisse et al. (2011) is not available and consequently it is not a homonym of the name introduced above.
Type material: The protargol slide (LI accession number 2012/109) containing the holotype specimen (Figs 1d, e, 3e) and many paratype specimens has been deposited in the Biologiezentrum des Oberösterreichischen Landesmuseums in Linz (LI), Upper Austria. In addition, many paratypes of the type series, distributed on two slides (accession numbers 2012/110, 2012/111), have been deposited in the same museum. Relevant specimens are marked.
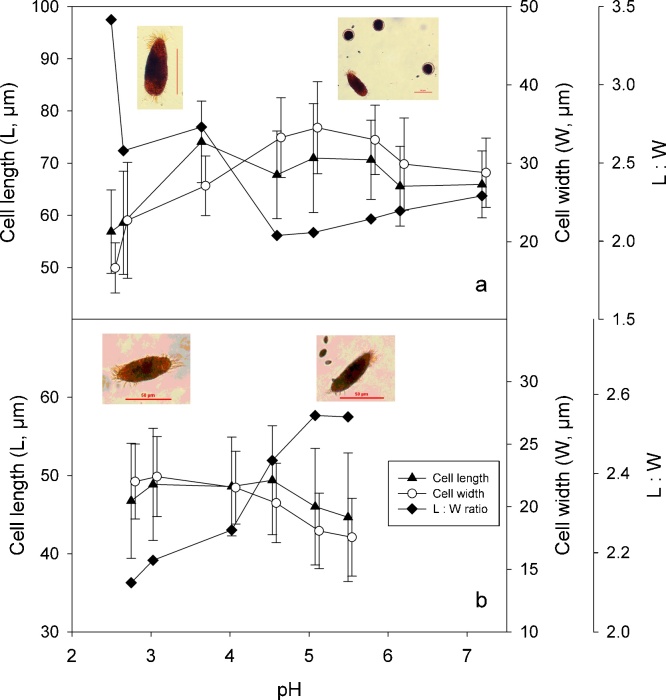
Morphology: Body size in vivo 55–95 × 20–35 μm (n = 5), usually about 80 × 28 μm in life and 77 × 28 μm on average in protargol preparations; length:width ratio thus on average 2.8:1 (2.4:1 to 3.5:1 in protargol preparations) both in vivo and in protargol preparations (Table 1). Body outline usually elliptical to oviform, that is, anterior and especially posterior end broadly rounded (Fig. 1a, c); rarely narrowly elliptical specimens occurred (Fig. 1b). Body flexible, but only very slightly contractile; usually about 1.5:1 flattened dorsoventrally, ventral side roughly flat, area behind buccal vertex sometimes recessed (Figs 1h, 3d); very well-nourished specimens almost not flattened; dorsal side convex in central portion. Cells rather sensitive, that is, cells burst often 1–2 min after placing the coverslip on the preparation. Invariably two macronuclear nodules usually arranged in line behind buccal vertex, that is, slightly left of midline; individual nodules globular (length:width ratio of anterior nodule 1.1:1, of posterior nodule 1.0:1) to ellipsoidal (2.0:1 in posterior nodule) or narrowly ellipsoidal (2.8:1 in anterior nodule); average length:width ratio 1.6:1 (anterior) and 1.3:1 (posterior); nucleoli small; macronuclear nodules usually connected by fine strand (Figs 1a, e–h, 3c–j, Table 1). Almost invariably two micronuclei (Figs 1a–c, e–h, 3d–f), very rarely one (Fig. 3c) or three; both globular and about 4 μm in diameter in vivo and usually one attached to distal portion of each macronuclear nodule; rarely both micronuclei attached to anterior or posterior macronuclear nodule or one of both in between nodules. Contractile vacuole near left body margin at about 45–50% of body length, during diastole without short collecting canals (Figs 1a, 3b, e). Cortical granules lacking. Cytoplasm hyaline and colourless, cultured specimens with many lipid globules about 1–4 μm in diameter, food vacuoles with yellow-brownish content (digested C. acidophila), and ordinary, up to 4 μm-long crystals, so that cells are opaque and relatively dark at low magnification. Movement without peculiarities, that is, glides moderately fast on slide or bottom of culture vessel or rotates around main body axis when freely swimming.
Adoral zone occupies about 32% (Min = 25%; Max = 40%) of body length on average in protargol preparations, composed of 22–28, on average 24 membranelles of usual fine structure, that is, made of two long rows, one slightly shortened, and one very short row of basal bodies. Bases of largest membranelles about 6 μm wide in protargol preparations, cilia up to 15 μm long. Distal portion of adoral zone extends moderately far posteriorly, that is, DE-value 0.24 on average. Buccal cavity moderately wide both in life and in protargol-impregnated specimens. Buccal lip rather inconspicuous, covering only rearmost portion of adoral zone. Undulating membranes arranged in Oxytricha-pattern, that is, both membranes slightly curved and optically more or less distinctly intersecting. Endoral begins somewhat more anteriorly than paroral which is, on average, 3.4 μm shorter than endoral (Figs 1a, d, f, h, 3e, Table 1). Paroral likely composed of dikinetids. Pharyngeal fibres extend obliquely backwards.
Almost invariably 18 frontal-ventral-transverse cirri (Figs 1d, f, 3e–i). Frontal cirri about 10–12 μm long, slightly enlarged, left and middle cirrus in bow formed by distal portion of adoral zone, right one immediately behind distal end of adoral zone. Buccal cirrus right of anterior end of paroral. Frontoventral cirri form V-shaped (hook-shaped) pattern, rearmost cirrus (= IV/3) about at same level as anterior end of right marginal row. Postoral ventral cirri close behind buccal vertex, form almost straight or slightly curved mixed row. Anterior pretransverse ventral cirrus (= cirrus V/2) on average 2 μm more anteriorly than frontmost transverse cirrus (Table 1), distinctly smaller than transverse cirri. Transverse cirri about 15–20 μm long, distinctly projecting beyond rear body end, usually arranged in ordinary hook-shaped (J-shaped) pattern, that is, rightmost cirrus (VI/1) slightly more anteriorly than cirrus V/1; sometimes, however, cirri form almost straight to slightly curved pseudorow (Fig. 1f). Rarely occur specimens with five, zigzagging arranged postoral ventral cirri and six transverse cirri which is obviously due to formation of an additional anlage producing two postoral ventral cirri and a transverse cirrus (Fig. 1g). Each marginal row composed on average of 21, about 8–10 μm long cirri; right row commences about at level of rearmost frontoventral cirrus, extends along right body margin and ends – like left row – about at level of rearmost transverse cirrus; left row begins left of proximal portion of adoral zone.
Dorsal bristles about 3 μm long in vivo, almost invariably arranged in five (eight of 21 specimens analysed morphometrically; Table 1) or six kineties when break in kinety 3 is interpreted as Oxytricha-fragmentation (Fig. 1e, h, i). Kinety 1, like kinety 2, usually slightly to distinctly shortened anteriorly, with very distinct break somewhat behind mid-body; kinety 2 with inconspicuous break at same level. Kinety 3 usually slightly shortened anteriorly, extends along midline and curves more or less distinctly rightwards slightly behind mid-body, strongly indicating that this is an (indistinct) fragmentation as in other oxytrichids; thus, rear portion of kinety 3 is termed kinety 4. Kinety 5, undoubtedly a dorsomarginal row, commences about at same level as kinety 3, terminates about in mid-body or somewhat ahead of it (Fig. 1e). Kinety 6, if present at all (see above), rather indistinct because composed of one (rarely two) bristles only (Fig. 1h, i). Rarely short rows between two ordinary kineties (Fig. 1i). Each one caudal cirrus on kineties 1, 2, and 4; inconspicuous in vivo because not more protruding than marginal and transverse cirri. Distance between left and middle cirrus usually smaller than that between middle and right cirrus (Fig. 1e, f, h, i).
Cyst (Fig. 8a, inset): The following observations are from Lugol-fixed material. Resting cysts globular, about 25–35 μm in diameter. Cyst wall about 2 μm thick, slightly corrugated, likely almost smooth in vivo.
Fig. 8.
Morphometric parameters of Oxytricha acidotolerans (a) and Urosomoida sp. (b) in relation to pH. Symbols represent means of triplicates, the error bars denote 1 SD. The microphotographs (insets) show the typical shape of Lugol's fixed ciliates at low and near-to-neutral pH. Also shown are cysts of O. acidotolerans (a, right photograph) and several cells of the food alga, Chlamydomonas acidophila (b, right photograph). Scale bars: 50 μm.
Notes on cell division and physiological reorganisation (Figs 2a–d, 3j): We found an early divider and a middle reorganiser in the protargol preparations. In the divider the oral primordium extends from near the anterior most transverse cirrus to the area right of the buccal vertex; the postoral ventral cirri are obviously involved in primordia formation. In addition, the frontoventral cirri III/2 and IV/3 are modified to anlagen. No distinct buccal cirrus is present, indicating that it also contributes to primordia formation (Fig. 2a, b). As is usual, the frontoterminal cirri are not involved in primordia formation.
In a middle reorganiser the proximal portion of the adoral zone is newly formed and the ordinary six frontal-ventral-transverse cirri anlagen are clearly recognisable. No anlagen are recognisable for the new dorsomarginal rows. Within parental kineties 1, 2, and 3, each one anlage occurs (Figs 2c, d, 3j). However, the reorganiser shown seems to have no normal kinety pattern because each two old and new caudal cirri and two kineties 4 seem to be present.
Urosomoida sp. from Lusatia (Fig. 4a–f)
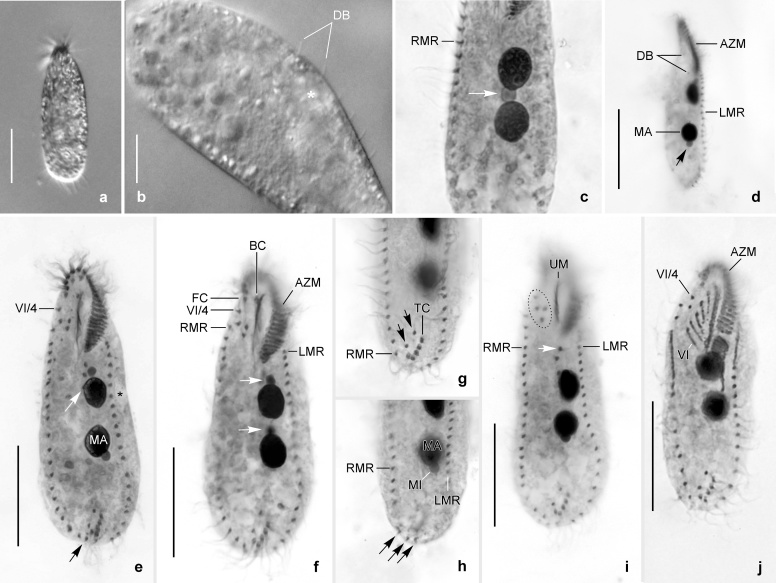
Fig. 4.
Urosomoida sp. from Lusatia (protargol impregnation). Ventral and dorsal view of four specimens documenting very high variability of infraciliature and nuclear apparatus (drawn to scale). Arrow in (a) marks buccal cirrus, arrow in (b) denotes the dorsomarginal kinety. AZM, adoral zone of membranelles; CC, caudal cirri; FC, frontal cirri; LMR, left marginal row; MA, macronuclear nodule; MI, micronucleus; PVC, postoral ventral cirri; PTC+TC, pretransverse ventral cirri and transverse cirri; RMR, right marginal row; 1, dorsal kinety 1. Scale bar: 30 μm.
Remarks: Already life observations indicated that the morphology of this population is highly variable when it was studied morphologically in detail in 2009, that is, about seven years after sampling. The extraordinary high variability of the cirral pattern and nuclear apparatus was confirmed by protargol impregnation. This unusual variability prevents a detailed description. Thus, the population is only briefly characterized morphologically. Since the protargol preparations of this population are of mediocre quality only, some particulars (e.g. exact arrangement of undulating membranes and dorsal kineties) could not be analysed in every detail.
Brief morphological characterisation: Body size in vivo about 60–100 × 20–35 μm (n = 4). Body outline usually slender elliptical to spindle-shaped. Body flexible, about 2:1 flattened dorsoventrally. Usually two macronuclear nodules slightly left of midline, sometimes only one macronucleus or three nodules present. Number (mostly 1–3) and arrangement of micronuclei rather variable; micronuclei about 3–4 μm across in vivo (Fig. 4b–d, f); relatively often a single micronucleus in between the two macronuclear nodules. Contractile vacuole about in mid-body close to left cell margin. Cortical granules lacking. Movement without peculiarities, that is, moderately fast gliding in culture vessel. Adoral zone rather short, occupying only about 20–24% of body length and composed of 13–17 membranelles of ordinary fine structure in specimens illustrated (Fig. 4a, c–e); bases of largest membranelles about 6 μm wide. Undulating membranes likely in Oxytricha-pattern, that is, both membranes slightly curved, about of equal length and slightly intersecting optically. Arrangement of frontal-ventral-transverse cirri extremely variable so that no “typical” pattern can be described; Fig. 4a, c–e show only a small range of patterns. Usually one buccal cirrus right of anterior portion of paroral. Mostly one right and one left marginal row. Right row commences about at level of buccal cirrus, composed of about 17–23 cirri; left row begins left of proximal end of adoral zone, composed of about 17–19 cirri in specimens illustrated; rows usually distinctly separated posteriorly. Dorsal bristles about 2–4 μm long in vivo, obviously arranged in Urosomoida-pattern, that is, three more or less bipolar kineties each bearing a caudal cirrus and one dorsomarginal kinety (Fig. 4b, f). Caudal cirri distinct in vivo, about 14–18 μm long.
Voucher material: Two slides (LI accession numbers 2012/112, 2012/113) with protargol-impregnated specimens have been deposited in the Biologiezentrum des Oberösterreichischen Landesmuseums in Linz (LI), Upper Austria.
Phylogenetic relationships of Oxytricha acidotolerans and Urosomoida sp. (Fig. 5)
The NJ tree resulting from the 18S rRNA gene sequence analyses revealed that Urosomoida sp. from Lusatia is very closely related to an unidentified Oxytricha-like species from the acidic Spanish River Rio Tinto (strain RT7IIN1; Amaral Zettler et al. 2002) and to Urosomoida longa (Fig. 5). The next relatives are Oxytricha acidotolerans and a cluster composed of three flexible oxytrichids with typical kinety 3 fragmentation (Onychodromopsis flexilis; Cyrtohymena citrina; Paraurostyla weissei). Although the other phylogenetic analyses yielded a somewhat different tree topology, they confirmed the general structure (data not shown). Sequence identity between the two hypotrich species used in this study is 97%.
Growth response in relation to pH (Figs 6–8)
Fig. 6.
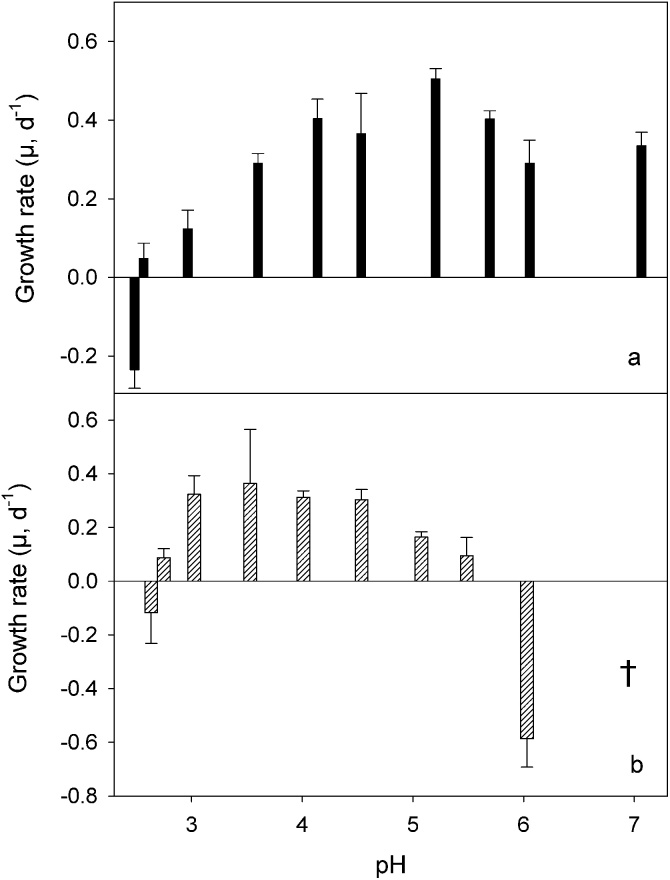
Specific growth rate of Oxytricha acidotolerans (a) and Urosomoida sp. (b) in relation to pH. Bars represent means of triplicates, the error bars denote 1 SD.
Fig. 7.
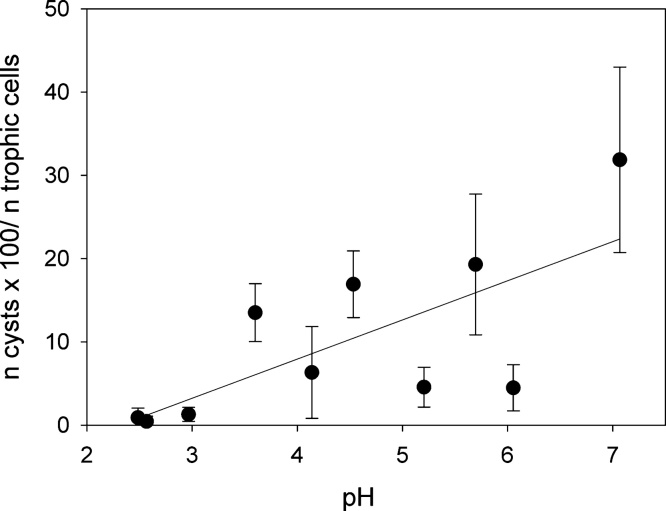
The percentage of cysts in the population of Oxytricha acidotolerans in relation to pH. The symbols represent means of triplicates, the error bars denote 1 SD; the line represents the linear regression of cysts (%) vs. pH (y = −10.88 + 4.70x, r2 = 0.513).
Oxytricha acidotolerans showed positive population growth over pH ranging from about 2.5 to >7 (Fig. 6a). Highest specific growth rates (μmax) were reached at pH 5.2 (0.51 d−1). The lower limit (pessimum) of its pH tolerance was close to 2.5; the growth rate was positive at pH 2.57, but negative at pH 2.48. At circumneutral pH, cell numbers of O. acidotolerans doubled every second day (μ = 0.335 d−1). We observed in our experiments that cyst formation was positively related to increasing pH (Figs 7, 8a, inset). At pH 7.0, more than 20% of the trophic cells encysted in the course of the experiment (Fig. 7). The x-axis intercept of the linear regression suggests that cysts formation ceases at pH ∼ 2.3, that is, close to the pH where positive population growth is no longer feasible. The lower pH pessimum of Urosomoida sp. isolated from Lusatia was slightly shifted to higher pH, i.e. we measured negative growth rates at pH 2.64 (Fig. 6b). Positive growth was obtained at pH 2.75 and μ peaked at pH ranging from 3.0 to 4.5. More importantly, Urosomoida sp. did not tolerate neutral pH conditions. There was no statistical difference between growth rates measured at pH 3.0, 3.5, 4.0, and 4.5 (one-way ANOVA). Maximum average growth rates (Fig. 6b) of the isolate from Lusatia (μmax = 0.37 d−1) were significantly lower (t-test, P < 0.001) than those of O. acidotolerans (Fig. 6a). However, in individual experimental treatments we measured a μmax of up to 0.51 d−1 for the isolate from Lusatia (at pH 3.5, Table 2). We observed only very few encysted cells of Urosomoida sp. in our experiments and could, therefore, not relate cyst formation to pH for this species.
Table 2.
pH tolerance, pH optimum (pHopt), body size (from Lugol-fixed cells), and maximum growth rate (μmax) of Oxytricha acidotolerans sp. nov. and Urosomoida sp.
| Parameter | Oxytricha acidotolerans | Urosomoida sp. |
|---|---|---|
| pH tolerance | 2.5 to >7.1 | 2.5 to ∼5.6 |
| pHopt a | 5.2 | 3.5 |
| Cell length at pHopt (μm) | 71.0 ± 9.0 | 48.9 ± 7.2 |
| Cell width at pHopt (μm) | 34.5 ± 5.9 | 22.4 ± 3.2 |
| Cell length:width ratio at pHopt | 2.1 | 2.2 |
| μmax (d−1) | 0.51b (0.53)c | 0.37b (0.51)c |
pH at μmax.
Average of three replicates.
Highest individual growth rate.
Cell size and shape of both species varied in relation to changing pH (Fig. 8a, b). Cell length and width of both species were correlated with each other and tended to be highest in the pH range where their growth rates peaked. For O. acidotolerans, cell length, width, and the length:width ratio (Fig. 8a) were all significantly affected by growth rate, while the effect of pH was insignificant (multiple linear regressions with pH and μ as independent variables). Accordingly, the seeming pH effect was indirectly mediated via changing growth rate. In contrast, there was a significant direct pH effect on cell width and the length:width ratio in Urosomoida sp. from Lusatia. The cell shape changed significantly, i.e. the cells became more elongated with increasing pH (Fig. 8b). Table 2 summarises the cell dimensions and ecophysiological characteristics of the two species in relation to pH.
Discussion
Systematics of Oxytricha acidotolerans sp. nov. from Langau
According to the ventral infraciliature (18 frontal-ventral-transverse cirri; frontoventral cirri V-shaped arranged; undulating membranes in Oxytricha-pattern; one right and one left marginal row), Oxytricha acidotolerans unequivocally belongs to Oxytricha (Berger 1999; Berger and Foissner 1997; Shao et al. 2012). By contrast, the dorsal kinety pattern of O. acidotolerans does not allow an unmistakable generic assignment because the kinety 3 fragmentation – the main morphological apomorphy of the oxytrichids according to Berger (1999, 2006) – is rather indistinct (Fig. 1e, h, i), whereas it is very conspicuous in Oxytricha granulifera, type of Oxytricha and the oxytrichids (e.g. Foissner and Adam 1983). This lack of clarity in O. acidotolerans is reminiscent of O. africana, O. elegans, and O. opisthomuscorum where the posterior end of kinety 3 and the anterior end of kinety 4 are also only indistinctly separated (Figs 14d, 15c, f in Foissner 1999; Figs 48–51 in Petz and Foissner 1997). In spite of that, we suppose that the inconspicuous break in these species is homologous to the fragmentation present in O. granulifera and therefore we preliminary classify the Langau population as new species in Oxytricha (for separation from morphologically similar and molecular biologically related taxa, see next paragraphs). This notoriously difficult genus is still an inhomogeneous group according to morphological (rather different dorsal kinety patterns; Berger 1999; Shao et al. 2012) and molecular data (e.g. Hu et al. 2011; Paiva et al. 2009; Schmidt et al. 2007). Even various populations of O. granulifera or morphologically very similar species (O. granulifera and O. longigranulosa) occur in different clusters demonstrating that we are still far away from a sophisticated understanding of this group and the hypotrichs in general (Shao et al. 2012).
Oxytricha elegans, which has a similar size (55–90 × 20–30 μm in life) and also lacks cortical granules (Foissner 1999), is easily distinguished from O. acidotolerans by the (i) number and arrangement of the micronucleus (single micronucleus in between two macronuclear nodules vs. usually one micronucleus attached to each macronuclear nodule); (ii) arrangement of the frontoventral cirri (not V-shaped and rearmost cirrus behind level of buccal vertex vs. V-shaped and distinctly ahead of buccal vertex), postoral ventral cirri (distinctly behind buccal vertex vs. rather close), and pretransverse ventral cirrus V/2 (distinctly displaced anteriad vs. close to transverse cirri); (iii) number of adoral membranelles (on average 16 vs. 24); (iv) number of marginal cirri (on average 8.8 right and 10.7 left vs. 20.6 in both rows); (v) length and number of dorsal bristles (3–5 μm and 25 and 28 in specimens illustrated vs. about 3 μm and 38 and 39); and (vi) habitat (discovered in forest soil in Africa vs. acid mining lake in Austria).
Oxytricha africana is, inter alia, somewhat larger (80–115 × 30–40 μm vs. 55–95 × 20–35 μm), has somewhat fewer right marginal cirri (15 vs. 21), lives in soil (vs. acid mining lake), and – most important – has longer (10 μm vs. about 3 μm) and more dorsal bristles (about 93 in specimen illustrated vs. about 39; Foissner 1999).
Oxytricha opisthomuscorum occurs in terrestrial and limnetic habitats (for review, see Berger 1999). It can be easily distinguished from O. acidotolerans in life by the nuclear apparatus (single micronucleus between macronuclear nodules) and the dorsal bristles which are 8–15 μm long (Berger 1999; Petz and Foissner 1997).
Oxytricha arabica is a further species which very closely resembles our population (Foissner et al. 2008). However, they can be distinguished by the following features: position of postoral ventral cirrus IV/2 (extremely close, almost right of buccal vertex vs. distinctly behind buccal vertex as in other Oxytricha species); fragmentation of kinety 3 (distinct vs. indistinct); arrangement of bristles in dorsal kineties, especially in rear portion (very narrowly spaced vs. ordinary to widely spaced); number of dorsal bristles (72 and 73 in specimens illustrated vs. 38 and 39); length of dorsal bristles (about 5 μm vs. 3 μm); habitat (discovered in Saudi Arabian sand dune soil vs. acid mining lake in Austria). Perhaps there are also some meticulous differences in the cirral pattern and morphometrics, for example, the arrangement of the frontoventral cirri and the number of adoral membranelles (27–32, on average 30 vs. 22–28, 24; in both cases n = 21).
Recently, Shao et al. (2012) discussed four Oxytricha species (O. islandica, O. lanceolata, O. pseudosimilis, and O. setigera) which have the Urosomoida dorsal kinety pattern, that is, they unequivocally lack kinety 3 fragmentation. Since they invariably have 18-frontal-ventral-transverse cirri, Shao et al. (2012) refrained from a transfer to Urosomoida (see next paragraph). They preliminary classified them as incertae sedis in Oxytricha. Oxytricha lanceolata is very similar to O. acidotolerans (for review, see Berger 1999). However, this species is larger (about 90–110 × 30–50 μm vs. 55–95 × 20–35 μm), has many more dorsal bristles (73, 75, and 81 in specimens illustrated vs. about 40), and lives in terrestrial habitats demonstrating that they are not identical. This is supported by the gene sequence data where the two species are widely separated (Fig. 5). Oxytricha pseudosimilis can be distinguished from O. acidotolerans even in vivo, namely by the length of the dorsal bristles (8–10 μm vs. about 3 μm). The terrestrial O. islandica has four macronuclear nodules while the limnetic O. setigera is rather small (40–60 × 16–21 μm) and has 10–15 μm long dorsal bristles and invariably a single micronucleus in between the two macronuclear nodules (for reviews, see Berger 1999).
Several limnetic Oxytricha species are not described in detail hampering a thorough comparison (all reviewed by Berger 1999). Oxytricha variabilis, discovered in a Sphagnum pond in France (details, see discussion of Urosomoida sp. from Lusatia), has a rather variable cirral pattern (Grolière 1975). Unfortunately, the dorsal kinety pattern is only superficially described (five kineties, dorsal bristles short) so that it can be separated from O. acidotolerans, at the present state of knowledge, only via some details of the cirral pattern (see Figs 14–18 in Grolière 1975), namely the position of the buccal cirrus (distinctly behind level of right frontal cirrus vs. about at same level), position of cirrus III/2 (left of area between frontoterminal cirri vs. left of back frontoterminal cirrus), position of cirrus IV/3 (likely distinctly set off from remaining frontoventral cirri vs. rather close), and number of marginal cirri (about 32 right and 27 left in specimen illustrated vs. 16–24 and 17–23, Table 1) and adoral membranelles (28–32 vs. 22–28, Table 1). In addition, in O. variabilis usually two micronuclei are attached to each of the two macronuclear nodules while in O. acidotolerans in total only two micronuclei are present. Oxytricha minor is very slender (50–70 × 10–12 μm) and has, according to the redescription by Grolière (1975), only three dorsal kineties (vs. at least four when kineties 3 and 4 are not counted separately) and a right marginal row which commences about at the level of the right frontal cirrus (vs. distinctly behind). In Oxytricha matritensis, discovered in a moss habitat at a spring, the right marginal row begins far anteriorly, the frontal-ventral-transverse cirri are rather prominent according to the original illustration, and caudal cirri are perhaps lacking; unfortunately, the dorsal kinety pattern is not known. Oxytricha halophila was discovered in a pond in China and has, according to the description, “long and fine” dorsal bristles which are, however, only 4 μm long according to the illustration (Fig. 84c in review by Berger 1999). The caudal cirri are rather long and protrude distinctly beyond the rear end of the cell indicating that O. halophila is not identical with O. acidotolerans. Oxytricha parallela is larger (usually 120–200 μm long) and has about 10 μm long dorsal bristles and four distinct caudal cirri. Oxytricha similis, whose dorsal kinety pattern is not known, has two, rather long and therefore prominent caudal cirri. Oxytricha bimembranata is distinctly larger (150–200 μm) and therefore obviously not identical with O. acidotolerans.
Many Oxytricha species reviewed by Berger (1999) differ from O. acidotolerans by at least one important feature (e.g. single micronucleus in between the two macronuclear nodules, cortical granules present, long dorsal bristles) and are therefore not compared in detail. Oxytricha procera is a little known species discovered by Kahl (1932) in terrestrial mosses from Wisconsin and California. It was also recorded (without support by morphological data) from limnetic habitats and differs from O. acidotolerans, at the present state of knowledge, only in cell length (100–120 μm vs. 55–90 μm), cell shape (slender spindle-shaped vs. usually elliptical), and the length of the caudal cirri (distinctly longer than transverse cirri vs. inconspicuous in life). The terrestrial O. nauplia is only slightly larger (80–100 × 40 μm), has a somewhat different arrangement of the frontoventral cirri, and – most important – distinctly more dorsal bristles (78 in specimen illustrated vs. about 40, Table 1) arranged in four bipolar kineties and one dorsomarginal row (vs. kineties 3 and 4 at least indistinctly separated).
Some Urosomoida species are also difficult to distinguish from Oxytricha acidotolerans, for example, U. longa, originally also classified in Oxytricha (Berger 1999; Gelei and Szabados 1950; Shao et al. 2012). However, U. longa and other Urosomoida species have less than 18 frontal-ventral-transverse cirri and do not even have an indistinct break in kinety 3 (Berger 1999; Foissner 1982; Shao et al. 2012). A distinct separation of U. longa and O. acidotolerans is also indicated by the number of caudal cirri (two vs. three) and the gene sequence data (Fig. 5).
The discussion above demonstrates that the taxonomy of Oxytricha is a difficult task. Foissner et al. (2008) correctly stated that many species of this complex are very difficult to distinguish in life and therefore identifications should be checked in protargol preparations where details of the infraciliature (e.g. exact positions and number of cirri and dorsal kineties, number of dorsal bristles) are clearly recognisable.
The early divider shows no peculiarities, that is the buccal cirrus, cirrus III/2, and cirrus IV/3 form or at least contribute to the anlagen II–IV of the proter, and all postoral ventral cirri are obviously involved in primordia formation of the opisthe (Fig. 2a; Table 4 in Berger 1999). The reorganiser shows that, as is usual, six frontal-ventral-transverse cirri anlagen are formed and the frontoterminal cirri (cirri VI/3 and VI/4) are not involved in primordia formation (Figs 2a, c, 3j). Although we do not have ontogenetic evidence for the origin of dorsal kineties 5 and 6 (Fig. 1c, d), we are convinced that these two rows are dorsomarginal ones as indicated by their position in non-dividers.
Systematics of Urosomoida sp. from Lusatia
There is little doubt that the population from Lusatia is basically an 18-cirri hypotrich, that is, a hypotrich with more or less 18 frontal-ventral-transverse cirri arranged in distinct groups. The high variability described above concerns all important groups of cirri (Fig. 4a, c–e). The variation is much higher than in other 18-cirri hypotrichs, for example, in O. acidotolerans (Table 1) or other species belonging to the Oxytricha complex (e.g., Berger 1999; Foissner et al. 2008; Shao et al. 2012). An unambiguous generic assignment is not possible via the ventral cirral pattern, that is, it is unclear whether the population belongs to Oxytricha or Urosomoida. The dorsal kinety pattern, although difficult to recognise due to increased variability and the somewhat less than perfect quality of the preparations, indicates that it belongs to Urosomoida because a distinct kinety fragmentation is obviously lacking and one dorsomarginal row is present (Fig. 4b, f). This assumption is supported by the fact that in the molecular tree its next identified relative is Urosomoida longa and not, for example, Oxytricha granulifera, type of Oxytricha (Fig. 5; see Shao et al. 2012 for comment on the generic classification of U. longa). Urosomoida longa, a rather common limnetic species, is effectively the most similar species (for review, see Berger 1999, p. 158). However, the type population (Gelei and Szabados 1950) and the populations described by Ganner et al. (1987) have only two caudal cirri while the population from Lusatia has three (Fig. 4b, f). The U. longa population from alkaline soils from arid regions in South Australia described by Niessen (1984) has three caudal cirri but the ordinary 18-cirri pattern.
Urosomoida sp. from Lusatia and Oxytricha acidotolerans sp. nov. from Langau have a sequence similarity of 97% and therefore occur in the same subcluster (Fig. 5). However, in the NJ tree resulting from the 18S rRNA gene sequences, the clusters with O. acidotolerans and Urosomoida sp. had low bootstrap support values within NJ and ML analyses. Especially the basal branch patterns differed between the applied phylogenetic methods and the unequal distribution of parsimony informative sites among the character partitions might be a reason for weak bootstrap support within NJ and ML analyses. We conclude that more sequences from closely related species are needed to reveal the phylogenetic positions of O. acidotolerans and Urosomoida sp. more clearly.
As already discussed above, the Langau population is only preliminary assigned to Oxytricha because the fragmentation of kinety 3 is rather indistinct. Perhaps it belongs, like some other species (e.g. Oxytricha islandica, O. lanceolata; details see Shao et al. 2012), to a so far undescribed genus which is closely related to Urosomoida.
In many 18-cirri species the number of frontal-ventral-transverse cirri is constant or only slightly variable as, for example, in some Urosomoida species (for review, see Berger 1999), and only some gonostomatids show a relatively high variability (Berger 2011). By contrast, the variability of the Lusatian population is extraordinarily high. Grolière (1975) found also a rather high variation in the cirral pattern of an Oxytricha species isolated from a Sphagnum pond with a pH ranging from 4.1 to 5.6 (Grolière and Njine 1973). This species – which he named O. variabilis – has, inter alia, five dorsal kineties (vs. four in the present population) and obviously a higher number of adoral membranelles (28–32 vs. about 13–17; for review, see Berger 1999), indicating that it is not conspecific with our population. These findings suggest that the variability of the cirral pattern is higher at low pH than at circumneutral conditions. However, for the Lusatian population it cannot be ruled out that at least a portion of the variability is due to the long period (∼7 y) of cultivation prior to our description because it is known that morphological differences originating during cultivation might be greater than those observed in natural populations (Wirnsberger-Aescht et al. 1990). Frankel (1973), for example, demonstrated that senile lines of Euplotes showed a reduced average ciliary row number, an increased variation in the row numbers commonly found, and a reduced fidelity in the vegetative propagation of pre-existing row number. However, our ecophysiological experiments (discussed below) demonstrated that this Urosomoida sp. from Lusatia was still vigorously growing in our cultures. Access to this Lusatian AML is currently restricted; if possible, we recommend to investigate freshly collected populations from this AML to reveal whether the high variability is species-specific or mainly due to long cultivation.
Ecology
The habitats of their origin of both study species were similar, extremely acidic mining lakes located approximately 325 km apart. Although we cannot disprove that both and even more hypotrich species occurred in each habitat, our regular monitoring of the plankton community in the study lakes (Moser and Weisse 2011c; Weithoff et al. 2010) suggests that each isolate is the dominant Urosomoida, respectively Oxytricha species in its respective habitat. The ability to form cysts is widespread in hypotrich ciliates and enables many species to withstand harsh environmental conditions. Encystment may be a successful strategy to overcome short periods of unsuitable pH, thus broadening the pH niche width of a species. In contrast to Urosomoida sp., encystment was positively related to pH in O. acidotolerans, with ∼20% of the cells encysting at pH 6–7. Due to the formation of cysts, ciliate growth rates were reduced at circumneutral conditions, relative to pH 5 (Fig. 7a). This immediate loss in fitness may be compensated by the benefit that excystment may provide if the environmental conditions change. The large variation that we observed in cyst formation between pH 3.5–6.0 suggests that encystment of O. acidotolerans may also depend on other environmental factors than pH and/or on intrinsic factors.
Ciliates and other protozoa living in permanently aquatic environments may have lost the ability to encyst (Finlay 1990). Since the Urosomoida sp. culture was older than the relatively fresh O. acidotolerans culture (discussed above), we do not emphasise the differences in cyst formation that we observed between the two species. The ability to form cysts is rapidly lost in many ciliate species when kept in the laboratory under food-replete conditions. However, cell shape of Urosomoida sp. was directly affected by pH, while this was not the case in O. acidotolerans. This may provide another indication that Urosomoida sp. is more sensitive to changing pH than O. acidotolerans. Changes in cell size were found in cryptophytes and ciliates close to their pH pessimum and are, therefore, indicative of limited pH tolerance (Weisse and Stadler 2006). Since we dealt with different species, it is not surprising that their pH niches did not completely overlap. Under our experimental conditions, O. acidotolerans from Langau was the superior performer, with higher μmax and considerably wider pH tolerance than Urosomoida sp. from Lusatia (Table 2). However, Urosomoida sp. showed clearer signs of adaptation to the extreme acidic conditions than the former. We measured population growth rate because it is a direct proxy of fitness in exponentially growing, asexually reproducing ciliates (Weisse 2006a). Fitness of Urosomoida sp. peaked at low pH (∼3.5) and was significantly reduced with increasing pH. This species was unable to survive at circumneutral pH. Accordingly, the Urosomoida sp. from Lusatia is an obligate acidophilic species (sensu Gross 2000). Following the terminology used by this author, O. acidotolerans was acidotolerant, since it did not require acidic conditions to survive. However, it should be noted that the nomenclature used in the literature for the various taxa is not clear (Cuaresma et al. 2006). Some authors would call O. acidotolerans acidophilic, because its growth rate was higher under acidic than at neutral conditions. Irrespective of the definition used, O. acidotolerans was more on the generalist side than Urosomoida sp. along the generalist to specialist continuum. Based upon our experimental results, we reject our initial hypothesis that the shape of the pH reaction norm would not differ between closely related species isolated from similar habitats. Obviously, similar habitats can be inhabited by closely related, but unequivocally different protist species. Since AML are man-made, isolated water bodies with reduced rates of dispersal, compared to natural lakes, the chances for local adaptation and ecological speciation are particularly high in such environments (Weisse et al. 2011). Although AML are of limited global significance, the findings from our case study add to the rapidly growing evidence that biodiversity of aquatic microorganisms is higher, and maybe even orders of magnitude higher, than assumed until recently (Caron et al. 2009; Finlay et al. 2006; Hahn 2006; Weisse 2006b).
Acknowledgements
We thank W. Foissner (University of Salzburg) for taxonomic advice and two anonymous reviewers for their constructive comments. Molecular and phylogenetic analyses were performed by D. Barth (University of Leipzig) and S. Krenek and C. Burkart (TU Dresden). This work was supported by the Austrian Science Fund FWF (projects P20118-B17 to T.W. and P23415-B17 to H.B.).
References
- Amaral Zettler L.A., Gómez F., Zettler E., Keenan B.G., Amils R., Sogin M.L. Eukaryotic diversity in Spain's River of Fire. Nature. 2002;417:137. doi: 10.1038/417137a. [DOI] [PubMed] [Google Scholar]
- Beaver J.R., Crisman T.L. Acid precipitation and the response of ciliated protozoans in Florida lakes. Verh. int. Verein Limnol. 1981;21:353–358. [Google Scholar]
- Berger H. 1999. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monographiae biol. 78. i–xii, 1–1080. [Google Scholar]
- Berger H. Verlag Helmut Berger; Salzburg: 2001. Catalogue of Ciliate Names 1. Hypotrichs. [Google Scholar]
- Berger H. 2006. Monograph of the Urostyloidea (Ciliophora, Hypotricha). Monographiae biol. 85. i–xvi, 1–1303. [Google Scholar]
- Berger H. 2008. Monograph of the Amphisiellidae and Trachelostylidae (Ciliophora, Hypotricha). Monographiae biol. 88. i–xvi, 1–737. [Google Scholar]
- Berger H. 2011. Monograph of the Gonostomatidae and Kahliellidae (Ciliophora, Hypotricha). Monographiae biol. 90. i–xiv, 1–741. [Google Scholar]
- Berger H., Foissner W. Cladistic relationships and generic characterization of oxytrichid hypotrichs (Protozoa, Ciliophora) Arch. Protistenkd. 1997;148:125–155. [Google Scholar]
- Caron D.A., Worden A.Z., Countway P.D., Demir E., Heidelberg K.B. Protists are microbes too: a perspective. ISME J. 2009;3:4–12. doi: 10.1038/ismej.2008.101. [DOI] [PubMed] [Google Scholar]
- Cuaresma M., Garbayo I., Vega J.M., Vílchez C. Growth and photosynthetic utilization of inorganic carbon of the microalga Chlamydomonas acidophila isolated from Tinto river. Enzyme Microb. Technol. 2006;40:158–162. [Google Scholar]
- Drummond, A., Ashton, B., Buxton, S., Cheung, M., Cooper, A., Heled, J., Kearse, M., Moir, R., Stones-Havas, S., Sturrock, S., Thierer, T., Wilson, A., 2010. Geneious v5.1. [DOI] [PMC free article] [PubMed]
- Finlay B.L. Physiological ecology of free-living protozoa. In: Marshall K.C., editor. vol. 11. Plenum Publishing Corporation; New York: 1990. pp. 1–35. (Advances in Microbial Ecology). [Google Scholar]
- Finlay B.J., Esteban G.F., Brown S., Fenchel T., Hoef-Emden K. Multiple cosmopolitan ecotypes within a microbial eukaryote morphospecies. Protist. 2006;157:377–390. doi: 10.1016/j.protis.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Foissner W. Ökologie und Taxonomie der Hypotrichida (Protozoa: Ciliophora) einiger österreichischer Böden. Arch. Protistenkd. 1982;126:19–143. [Google Scholar]
- Foissner W. Notes on the soil ciliate biota (Protozoa, Ciliophora) from the Shimba Hills in Kenya (Africa): diversity and description of three new genera and ten new species. Biodivers. Conserv. 1999;8:319–389. [Google Scholar]
- Foissner W., Adam H. Morphologie und Morphogenese des Bodenciliaten Oxytricha granulifera sp. n. (Ciliophora, Oxytrichidae) Zool. Scr. 1983;12:1–11. [Google Scholar]
- Foissner W., Agatha S., Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib desert. Part I: Text and line drawings. Part II: Photographs. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W., Al-Rasheid K. A unified organization of the stichotrichine oral apparatus, including a description of the buccal seal (Ciliophora: Spirotrichea) Acta Protozool. 2006;45:1–16. [Google Scholar]
- Foissner W., Berger H., Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3/99:1–793. [Google Scholar]
- Foissner W., Qintela-Alonso P., Al-Rasheid K. Soil ciliates from Saudi Arabia, including descriptions of two new genera and six new species. Acta Protozool. 2008;47:317–352. [PMC free article] [PubMed] [Google Scholar]
- Frankel J. Dimensions of control of cortical patterns in Euplotes: the role of pre-existing structure, the clonal life cycle, and the genotype. J. exp. Zool. 1973;183:71–94. [Google Scholar]
- Ganner B., Foissner W., Adam H. Morphogenetic and biometric comparison of four populations of Urosomoida agiliformis (Ciliophora, Hypotrichida) Annls Sci. nat., Zoologie. 1987;13 Série 8:199–207. (years 1986/1987) [Google Scholar]
- Gelei J., Szabados M. Tömegprodukció városi esövízpocsolyában (Massenproduktion in einer städtischen Regenwasserpfütze) Annls biol. Univ. szeged. 1950;1:249–294. (in Hungarian with German translation and Russian summary) [Google Scholar]
- Geller W., Klapper H., Schultze M. Natural and anthropogenic sulphuric acidification of lakes. In: Geller W., Klapper H., Salomons W., editors. Acid Mining Lakes. Springer; Berlin: 1998. pp. 3–14. [Google Scholar]
- Gerloff-Elias A., Spijkerman E., Pröschold T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acid lake (pH 2.6) Plant Cell Environ. 2005;28:1218–1229. [Google Scholar]
- Grolière C.-A. Descriptions de quelques ciliés hypotriches des tourbières a sphaignes et des étendues d’eau acides. Protistologica. 1975;11:481–498. [Google Scholar]
- Grolière C.-A., Njine T. Étude comparée de la dynamique des populations de ciliés dans différents biotopes d’une mare de forêt pendant une année. Protistologica. 1973;9:5–16. [Google Scholar]
- Gross W. Ecophysiology of algae living in highly acidic environments. Hydrobiologia. 2000;433:31–37. [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hahn M.W. The microbial diversity of inland waters. Curr. Opin. Biotech. 2006;17:256–261. doi: 10.1016/j.copbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hu X., Hu X., Al-Rasheid K.A.S., Al-Farraj S.A., Song W. Insights into the phylogeny of sporadotrichid ciliates (Protozoa, Ciliophora: Hypotricha) based on genealogical analyses of multiple molecular markers. Chin. J. Oceanol. Limnol. 2011;29:96–102. [Google Scholar]
- ICZN (The International Commission on Zoological Nomenclature) International Trust for Zoological Nomenclature; London: 1999. International Code of Zoological Nomenclature. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 3. Spirotricha. Tierwelt Dtl. 1932;25:399–650. [Google Scholar]
- Kamjunke N., Gaedke U., Tittel J., Weithoff G., Bell E.M. Strong vertical differences in the plankton composition of an extremely acidic lake. Arch. Hydrobiol. 2004;161:289–306. [Google Scholar]
- Lackey J.B. The flora and fauna of surface waters polluted by acid mine drainage. Publ. Hlth Rep., Wash. 1938;53:1499–1507. [Google Scholar]
- Lackey J.B. Aquatic life in waters polluted by acid mine waste. Publ. Hlth Rep., Wash. 1939;54:740–746. [Google Scholar]
- Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lynn D.H. 3rd ed. Springer; Heidelberg: 2008. The Ciliated Protozoa. Characterization, Classification, and Guide to the Literature. [Google Scholar]
- Mattern D., Schlegel M. Molecular evolution of the small subunit ribosomal DNA in woodlice (Crustacea, Isopoda, Oniscidea) and implications for Oniscidean phylogeny. Mol. Phylogenet. Evol. 2001;18:54–65. doi: 10.1006/mpev.2000.0861. [DOI] [PubMed] [Google Scholar]
- McConathy J.R., Stahl J.B. Rotifera in the plankton and among filamentous algal clumps in 16 acid strip-mine lakes. Trans. Ill. Acad. Sci. 1982;75:85–90. [Google Scholar]
- Menge H. Langenscheidt; Berlin/München/Wien/Zürich: 1984. Langenscheidts Taschenwörterbuch der Lateinischen und deutschen Sprache. [Google Scholar]
- Moser M., Weisse T. The outcome of competition between the two chrysomonads Ochromonas sp. and Poterioochromonas malhamensis depends on pH. Eur. J. Protistol. 2011;47:79–85. doi: 10.1016/j.ejop.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Weisse T. Combined stress effect of pH and temperature narrows the niche width of flagellates in acid mining lakes. J. Plankton Res. 2011;33:1023–1032. doi: 10.1093/plankt/fbr014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Weisse T. The most acidified Austrian lake in comparison to a neutralized mining lake. Limnologica. 2011;42:303–315. doi: 10.1016/j.limno.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen, R., 1984. Taxonomische und ökologische Untersuchungen an Ciliaten aus Salzböden. Diploma Thesis, University of Bonn.
- Packroff G. Protozooplankton in acidic mining lakes with special respect to ciliates. Hydrobiologia. 2000;433:157–166. [Google Scholar]
- Packroff G., Woelfl S. A review on the occurrence and taxonomy of heterotrophic protists in extreme acidic environments of pH values <3. Hydrobiologia. 2000;433:153–156. [Google Scholar]
- Paiva T.S., Borges B.N., Harad M.L., Silva-Neto I.D. Comparative phylogenetic study of Stichotrichia (Alveolata: Ciliophora: Spirotrichea) based on 18S-rDNA sequences. Genet. Mol. Res. 2009;8:223–246. doi: 10.4238/vol8-1gmr529. [DOI] [PubMed] [Google Scholar]
- Petz W., Foissner W. Morphology and infraciliature of some soil ciliates (Protozoa, Ciliophora) from continental Antarctica, with notes on the morphogenesis of Sterkiella histriomuscorum. Polar Rec. 1997;33:307–326. [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schindler D.W. Effects of acid rain on freshwater ecosystems. Science. 1988;239:149–157. doi: 10.1126/science.239.4836.149. [DOI] [PubMed] [Google Scholar]
- Schmidt S.L., Bernhard D., Schlegel M., Foissner W. Phylogeny of the Stichotrichia (Ciliophora; Spirotrichea) reconstructed with nuclear small subunit rRNA gene sequences: discrepancies and accordances with morphological data. J. Eukaryot. Microbiol. 2007;54:201–209. doi: 10.1111/j.1550-7408.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- Shao C., Song W., Al-Rasheid K.A.S., Berger H. Redefinition and reassignment of the 18-cirri genera Hemigastrostyla, Oxytricha, Urosomoida, and Actinotricha (Ciliophora, Hypotricha), and description of one new genus and two new species. Acta Protozool. 2012;50 (year 2011):263–287. [Google Scholar]
- Steinberg C.E.W., Wright R.F. Wiley; Chichester: 1994. Acidification of Freshwater Ecosystems: Implications for the Future. [Google Scholar]
- Stöver B.C., Müller K.F. TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics. 2010;11 doi: 10.1186/1471-2105-11-7. Article No. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittel J., Bissinger V., Zippel B., Gaedke U., Bell E., Lorke A., Kamjunke N. Mixotrophs combine resource use to outcompete specialists: implications for aquatic food webs. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12776–12781. doi: 10.1073/pnas.2130696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKNCC (Catalogue of the UK National Culture Collection) UK National Culture Collection; Windermere and Oban: 2001. List of Algae and Protozoa. [Google Scholar]
- Wallengren H. Zur Kenntnis der vergleichenden Morphologie der hypotrichen Infusorien. Bih. K. svensk VetenskAkad. 1900;26:1–31. [Google Scholar]
- Weisse T. The significance of inter- and intraspecific variation in bacterivorous and herbivorous protists. Antonie van Leeuwenhoek. 2002;81:327–341. doi: 10.1023/a:1020547517255. [DOI] [PubMed] [Google Scholar]
- Weisse T. Freshwater ciliates as ecophysiological model organisms – lessons from Daphnia, major achievements, and future perspectives. Arch. Hydrobiol. 2006;167:371–402. [Google Scholar]
- Weisse T. Biodiversity of freshwater microorganisms – achievements, problems, and perspectives. Pol. J. Ecol. 2006;54:633–652. [Google Scholar]
- Weisse T., Kirchhoff B. Feeding of the heterotrophic freshwater dinoflagellate Peridiniopsis berolinense on cryptophytes: analysis by flow cytometry and electronic particle counting. Aquat. Microb. Ecol. 1997;12:153–164. [Google Scholar]
- Weisse T., Stadler P. Effect of pH on growth, cell volume, and production of freshwater ciliates, and implications for their distribution. Limnol. Oceanogr. 2006;51:1708–1715. [Google Scholar]
- Weisse T., Scheffel U., Stadler P., Foissner W. Local adaptation among geographically distant clones of the cosmopolitan freshwater ciliate Meseres corlissi. II. Response to pH. Aquat. Microb. Ecol. 2007;47:289–297. [Google Scholar]
- Weisse T., Berendonk T., Kamjunke N., Moser M., Scheffel U., Stadler P., Weithoff G. Significant habitat effects influence protist fitness: evidence for local adaptation from acidic mining lakes. Ecosphere. 2011;2 Article 134. [Google Scholar]
- Weithoff G., Moser M., Kamjunke N., Gaedke U., Weisse T. Lake morphometry strongly shapes the plankton community structure in acidic mining lakes. Limnologica. 2010;40:161–166. doi: 10.1016/j.limno.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner F.C. Suhrkamp; Baden-Baden: 1972. Wortelemente lateinisch-griechischer Fachausdrücke in den biologischen Wissenschaften. [Google Scholar]
- Wetzel R.G. Academic Press; San Diego: 2001. Limnology – Lake and River Ecosystems. [Google Scholar]
- Wirnsberger-Aescht E., Foissner W., Foissner I. Natural and cultured variability of Engelmanniella mobilis (Ciliophora, Hypotrichida); with notes on the ultrastructure of its resting cyst. Arch. Protistenkd. 1990;138:29–49. [Google Scholar]
- Wollmann K., Deneke R., Nixdorf B., Packroff G. Dynamics of planktonic food webs in three mining lakes across a pH gradient (pH 2–4) Hydrobiologia. 2000;433:3–14. [Google Scholar]
- Wylezich C., Meisterfeld R., Meisterfeld S., Schlegel M. Phylogenetic analyses of small subunit ribosomal RNA coding regions reveal a monophyletic lineage of euglyphid testate amoebae (order Euglyphida) J. Eukaryot. Microbiol. 2002;49:108–118. doi: 10.1111/j.1550-7408.2002.tb00352.x. [DOI] [PubMed] [Google Scholar]