Abstract
Epitopes of the circumsporozoite (CS) protein of Plasmodium falciparum, the most pathogenic species of the malaria parasite, have been shown to elicit protective immunity in experimental animals and human volunteers. The mechanisms of immunity include parasite-neutralizing antibodies that can inhibit parasite motility in the skin at the site of infection and in the bloodstream during transit to the hepatocyte host cell and also block interaction with host cell receptors on hepatocytes. In addition, specific CD4+ and CD8+ cellular mechanisms target the intracellular hepatic forms, thus preventing release of erythrocytic stage parasites from the infected hepatocyte and the ensuing blood stage cycle responsible for clinical disease. An innovative method for producing particle vaccines, layer-by-layer (LbL) fabrication of polypeptide films on solid CaCO3 cores, was used to produce synthetic malaria vaccines containing a tri-epitope CS peptide T1BT* comprising the antibody epitope of the CS repeat region (B) and two T-cell epitopes, the highly conserved T1 epitope and the universal epitope T*. Mice immunized with microparticles loaded with T1BT* peptide developed parasite-neutralizing antibodies and malaria-specific T-cell responses including cytotoxic effector T-cells. Protection from liver stage infection following challenge with live sporozoites from infected mosquitoes correlated with neutralizing antibody levels. Although some immunized mice with low or undetectable neutralizing antibodies were also protected, depletion of T-cells prior to challenge resulted in the majority of mice remaining resistant to challenge. In addition, mice immunized with microparticles bearing only T-cell epitopes were not protected, demonstrating that cellular immunity alone was not sufficient for protective immunity. Although the microparticles without adjuvant were immunogenic and protective, a simple modification with the lipopeptide TLR2 agonist Pam3Cys increased the potency and efficacy of the LbL vaccine candidate. This study demonstrates the potential of LbL particles as promising malaria vaccine candidates using the T1BT* epitopes from the P. falciparum CS protein.
Keywords: malaria vaccines, microparticle, peptide, sporozoite
1. Introduction
Malaria is a leading cause of morbidity and mortality in much of the developing world, affecting 200-500 million people and causing over 1 million deaths each year. There is a general consensus in the malaria community that vaccines will make an important contribution to malaria disease control [1, 2]. Plasmodium parasites have a complex life cycle within the mammalian host that is initiated by injection of sporozoites by the infected mosquito as it takes a blood meal. The parasites enter the blood system and travel to the liver where they invade hepatocytes and undergo a multiplication cycle, ultimately releasing thousands of merozoites from each infected hepatocyte. The merozoites rapidly invade erythrocytes and undergo multiple cycles of replication and erythrocyte invasion, resulting in clinical disease progressing to morbidity and mortality unless treated. Pre-erythrocytic vaccines aim to prevent development of the blood stage parasites responsible for clinical disease by targeting the extracellular sporozoite and the intracellular hepatic stages, both of which express circumsporozoite (CS) protein [3].
Two P. falciparum vaccine candidates which currently show the most promise, attenuated sporozoites and RTS,S, both elicit immune responses to CS protein epitopes. The P. falciparum whole sporozoite vaccine is prepared by dissection of radiation or genetically attenuated parasites from the salivary glands of mosquitoes that have fed on Plasmodium- infected human blood. Numerous logistical hurdles must be overcome for this vaccine candidate, including the use of human blood, inability to grow sporozoites in vitro, limited capacity for scale-up and requirement for cold chain storage [4-6]. RTS,S is a virus-like particle composed of a recombinant protein fusing hepatitis B surface antigen (HBsAg) to a truncated P. falciparum CS protein [7, 8]. Clinical efficacy of RTS,S requires a complex adjuvant formulation containing monophosphoryl lipid A and a purified saponin derivative, QS21, in an oil-in-water emulsion or liposome formulation. In Phase III trials of RTS,S in Africa in infants, vaccine-induced immunity is seen in only 33-55% of the patients and immunity is not sterile as the protected children remain infected with P. falciparum but experience milder clinical disease [9, 10]. Although these two vaccine candidates show promise and validate the CS protein as a viable vaccine antigen, they also demonstrate the need for more efficacious subunit vaccines that are manufactured by a robust and scalable process, elicit immunity comparable to that obtained in sporozoite-immunized hosts, and minimize inflammatory responses related to the use of potent adjuvant formulations. We have constructed synthetic microparticle vaccines made by layer-by-layer (LbL) fabrication [11] and loaded with a designed peptide (DP) containing the T1BT* epitopes of P. falciparum CS protein. In the current study we show that the LbL vaccines elicited neutralizing antibodies and effector T-cells specific for the CS epitopes, and protected immunized mice from mosquito challenge with Plasmodium sporozoites expressing P. falciparum CS repeats [12]. A simple modification of the particles by addition of the TLR2 ligand Pam3Cys increased the potency and efficacy of the vaccine. This study demonstrates that LbL fabrication can yield efficacious malaria vaccines using a scalable process and non-biologic raw materials.
2. Materials and methods
2.1. LbL particle fabrication
Peptides were synthesized and analyzed by standard techniques [11]. Figure 1 shows the location and sequence of the T1, B, and T* epitopes in P. falciparum CS protein. Table 1 describes the DP used to make the LbL microparticles. Pam3Cys.T13B5 (DP-2167) was prepared by manual coupling of Pam3Cys-OH (EMD Millipore) to resin-bound DP-2163 (T13B5) in 4:1 N-methylpyrrolidinone/dichloromethane using 2-(1H-benzotriazol-1-yl)- 1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) activation. CaCO3 microparticles (2-4 μm diameter) were obtained from PlasmaChem GmbH (Germany, catalog # PL-CA3). Poly-l-lysine hydrobromide salt (PLL, 15 kDa, catalog # P6516), FITC labeled poly-l-lysine (PLL-FITC, 15-30 kDa, catalog # P3543), poly-l-glutamic acid sodium salt (PGA, 14.5 kDa, catalog # P4636), and 1 M HEPES buffer (catalog #H-3662) were obtained from Sigma-Aldrich (USA). All LbL microparticles (MP) were fabricated as previously reported [11] by alternately layering PGA (negative charge) and PLL (positive charge) on CaCO3 cores to build up a 7-layer base film, and capping with an outermost layer of DP (Table 1). To prepare MP-1141, the base film was chemically crosslinked by treatment with 200 mM EDC and 50 mM sulfo-NHS (Sigma-Aldrich) in 0.2 M phosphate buffer, pH 6.5, for 30 minutes at room temperature prior to layering DP. Following deposition of the DP, the mature LbL microparticles were washed and stored as damp pellets at 4°C. The microcapsule MC-1142 was fabricated by dissolving the solid CaCO3 core of MP-1141 by treatment with 0.5 M EDTA (pH 8.0) for 30 minutes. The microcapsules were recovered by centrifugation (2000g for 5 minutes), washed twice, resuspended, and stored in suspension at 4°C. The final architecture of all constructs was CaCO3:PGA:PLL-FITC:PGA:PLL:PGA:PLL:PGA:DP. PGA, PLL and DP contents were measured by amino acid analysis, and endotoxin content was determined by the Limulus Amebocyte Lysate assay (#50- 647U, Lonza, Walkersville, MD) [11].
Figure 1.
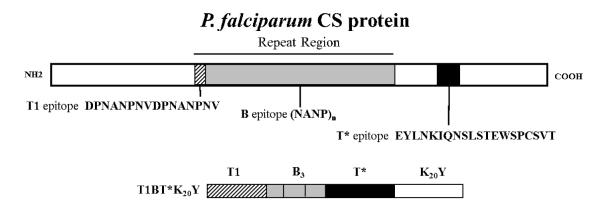
P. falciparum CS protein showing locations and sequences of T1, B, and T* epitopes, and design of T1BT*K20Y peptide.
Table 1.
Designed peptides and LbL particle constructs. MP = microparticle; MC = microcapsule. Pf sequences are from P. falciparum CS protein, Pb sequences are from P. berghei CS protein. K20Y (Lys20Tyr) is the polyelectrolyte tail which drives the assembly of soluble DP into the LbL film. In DP-2062, the B repeat sequence (NANP)3 is flanked by the T1 repeat (N-terminal) and the T* epitope (C-terminal). In DP-2163 and DP-2167, (NANPNVDP)3 is three copies of the T1 repeat sequence, and (NANP)5 is five copies of the B repeat sequence. The SKKKK linker sequence was included in both DP-2163 and DP-2167. All LbL constructs contained 3.2-7.7 μg DP per mg CaCO3 and < 0.05 endotoxin units per μg DP.
| Particle # | DP # | Epitope(s) and source |
Sequence |
|---|---|---|---|
| MP-1140 MP-1141 MC-1142 |
DP-2062 | T1BT* Pf | DPNANPNVDPNANPNV(NANP)3EYLNKIQNSLSTEWSPCSVTSGNGK20Y |
| MP-1182 | DP-2145 | CD4 Pb | LEFVKQIRDSITEEWSQCNVK20Y |
| MP-1183 | DP-2146 | CD8 Pb | KNNNNDDSYIPSAEKILEFVK20Y |
| MP-1184 | DP-2147 | CD8:CD4 Pb | KNNNNDDSYIPSAEKILEFVKQIRDSITEEWSQCNVK20Y |
| MP-1167 | DP-2163 | T13B5 Pf | SKKKK(NANPNVDP)3(NANP)5K20Y |
| MP-1164 | DP-2167 | Pam3.T13B5 Pf | Pam3CSKKKK(NANPNVDP)3(NANP)5K20Y |
2.2. Mice and immunizations
Female C57BL/6J (H-2b) and BALB/cJ (H-2d) mice, 6-8 weeks of age, were obtained from Jackson Laboratories. Mice were housed in microisolator cages and given food and water ad libitum. Animal studies were approved by the Northeast Life Sciences (New Haven, CT) Institutional Animal Care and Use Committee. LbL constructs were resuspended in PBS and diluted to deliver 10 μg DP per dose via the footpad (f.p.). Control mice were immunized with 10 μg DP in CFA (prime) and IFA (boost) or mock-immunized with PBS. Mice were immunized on days 0, 21, and 42 unless otherwise specified in the Figure legends.
Hybridomas secreting anti-CD4 (GK1.5) and anti-CD8 (2.43) monoclonal antibodies were obtained from the American Type Culture Collection and maintained as instructed. Monoclonal antibodies were purified from culture supernatants by ammonium sulfate precipitation, dialyzed into PBS, and stored at -80°C. For in vivo T-cell depletion, mice received a single i.p. injection of anti-CD4 Mab GK1.5 (50 μg), anti-CD8 Mab 2.43 (50 μg), or a cocktail of both antibodies. Flow cytometry analysis of spleen cells confirmed that each antibody depleted >95% of the respective cell phenotype (data not shown).
2.3. Antibody assays
Mice were bled by retro-orbital puncture on the indicated days and sera were tested by ELISA [11] using plates coated with T1B peptide lacking the K20Y tail. Antibody endpoint titers were based on the final serum dilution yielding an OD against T1B peptide greater than twice the OD against unrelated antigen (BSA). Functional antibody responses were measured by the Transgenic Sporozoite Neutralization Assay (TSNA) [13, 14].
2.4. T-cell assays
Splenic single-cell suspensions were analyzed in IFNγ and IL-5 ELISPOT using commercial reagents (eBioscience) and plates (Millipore Corporation) and following the manufacturers’ instructions. Cytotoxic effector cells were detected by an in vivo CTL assay as described [11]. Target cells were pulsed with 5 μg/ml of the specified target peptide and labeled with 10 μM of CFSE (Invitrogen, C34554); control target cells were not pulsed with peptide and labeled with 1 μM CFSE.
2.5. Protection from parasite challenge
PfPb is a recombinant P. berghei (mouse pathogen) carrying a transgene containing the entire repeat region of P. falciparum CS protein (human pathogen); antibodies to P. falciparum T1B protect mice from challenge with PfPb sporozoites [12, 14]. Naive and immunized mice were challenged by exposure to bites of PfPb-infected mosquitoes on day 56 (5-15 bites per mouse); this challenge protocol has been shown to reliably elicit patent infection in naive mice [14]. Forty hours post-challenge, the mice were sacrificed and livers were harvested and homogenized in TriReagent (Molecular Research Center, Inc.) using a Polytron PowerGen 500 (Fisher Scientific). RNA was isolated using QIAgen Mini Prep Kit and converted to cDNA using iScript Reverse Transcription Supermix (BioRad), both according to the manufacturers’ directions. Parasite burden was monitored by quantifying P. berghei 18S rRNA levels in qPCR [13].
2.6. Statistical analyses
Comparisons between multiple treatment groups were analyzed by the non-parametric Kruskal-Wallis test. Pair-wise comparisons were analyzed by the non-parametric Wilcoxon rank sum test with Bonferroni correction; P≤0.05 was considered statistically significant. Statistical analyses were performed using SAS v. 9.3 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Immunogenicity
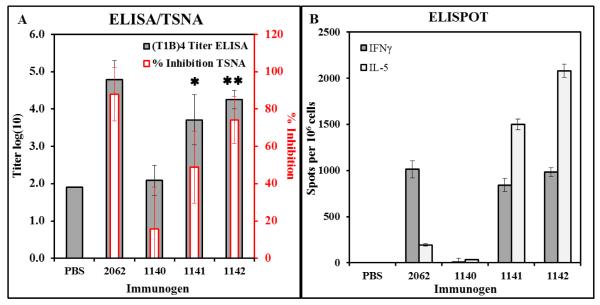
C57BL/6J mice were immunized with MP-1140, MP-1141, or MC-1142, each loaded with DP 2062 (T1BT*K20Y). Antibody responses were tested by ELISA and TSNA, while T-cell responses were tested by ELISPOT. MP-1141 and MC-1142 were the most potent LbL constructs, eliciting antibody titers (Figure 2A) and IFNγ+ responses (Figure 2B) comparable to the positive control mice. Figure 2A also shows that the T1B ELISA results correlate with the level of functional antibody activity measured in the TSNA (r2=0.79, P=0.0004 by Pearson Correlation Coefficient analysis of individual serum titers in both assays), demonstrating the utility of the ELISA as a rapid screening method for measuring functional anti-T1B antibody responses.
Figure 2.
Immunogenicity and efficacy of LbL constructs containing malaria CS epitopes. Constructs are defined in Table 1. C57BL/6 mice were immunized with doses adjusted to deliver 10 μg of DP on days 0, 21, and 42. (A) Sera were collected on day 28 and tested for T1B-specific IgG titers by ELISA and functional antibodies by TSNA. Results show mean±SD of 5 mice per group for antibody titer (grey bar) and % inhibition (red bar); pooled sera from 5 mock-immunized (PBS) mice were used for both assays.  P<0.05 for ELISA compared to 1140;
P<0.05 for ELISA compared to 1140;  P<0.05 for ELISA and TSNA compared to 1140. (B) Spleen cells were restimulated with T1BT* peptide in IFNγ and IL-5 ELISPOT plates, and the number of spot-forming cells on each plate was counted in an AID ViruSpot Reader. Results show mean±SD of 3 mice per group.
P<0.05 for ELISA and TSNA compared to 1140. (B) Spleen cells were restimulated with T1BT* peptide in IFNγ and IL-5 ELISPOT plates, and the number of spot-forming cells on each plate was counted in an AID ViruSpot Reader. Results show mean±SD of 3 mice per group.
3.2. Efficacy
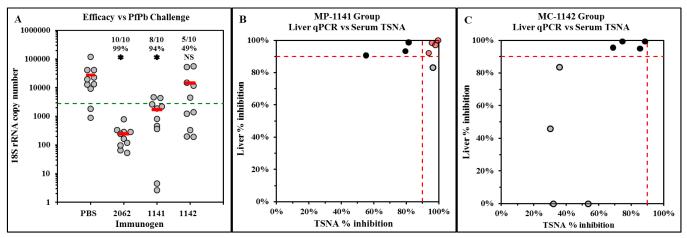
Mice were immunized with MP-1141 or MC-1142 and challenged by exposure to bites of PfPb-infected mosquitoes. Forty hours post-challenge, parasite burden in livers was monitored by quantifying P. berghei 18S rRNA levels via qPCR. Protection is defined as ≥90% reduction in parasite burden compared to naïve, challenged mice. Immunization with MP-1141 protected 8 of 10 mice and resulted in a 94% reduction in average parasite burden in the treatment group (P<0.05, Wilcoxon rank sum test), comparable to control mice immunized with DP 2062 in Freund’s adjuvant (Figure 3A). Immunization with MC-1142 protected half of the mice but did not result in a significant reduction in the group average parasite burden compared to PBS control.
Figure 3.
Protective efficacy of T1BT* microparticles. In a repeat experiment, immunized mice were challenged by exposure to the bites of PfPb-infected mosquitoes on day 56. (A) Parasite burden in the livers was measured by qPCR 2 days post-challenge. Results show parasite rRNA copy number of individual mice (gray circles) and mean value for each group (red bars); insets show number of mice per group that were protected (≥90% reduction of parasite rRNA), group % reduction of parasite rRNA, and  P<0.05, all compared to PBS control group; NS = not significant. (B and C) Comparison of in vivo protection from parasite challenge (Y-axis) and in vitro neutralizing activity of sera (X-axis). Eight randomly-selected individual sera from (A) were tested in each group. Dotted lines indicate ≥ 90% efficacy in vivo (Y-axis) or neutralizing activity in vitro (X-axis). Red = mice with ≥ 90% activity in both measurements; black = mice with ≥ 90% in vivo protection and ≤ 90% in vitro neutralizing activity; gray = mice with ≤ 90% in vivo protection.
P<0.05, all compared to PBS control group; NS = not significant. (B and C) Comparison of in vivo protection from parasite challenge (Y-axis) and in vitro neutralizing activity of sera (X-axis). Eight randomly-selected individual sera from (A) were tested in each group. Dotted lines indicate ≥ 90% efficacy in vivo (Y-axis) or neutralizing activity in vitro (X-axis). Red = mice with ≥ 90% activity in both measurements; black = mice with ≥ 90% in vivo protection and ≤ 90% in vitro neutralizing activity; gray = mice with ≤ 90% in vivo protection.
Sera collected from the mice prior to challenge were tested in the TSNA to measure parasite-neutralizing activity that effectively blocked sporozoite invasion of human hepatoma cells in vitro, defined as >90% reduction of parasite rRNA levels in HepG2 cells measured by qPCR. A comparison of TSNA activity with in vivo efficacy showed that efficacy was associated with potent neutralizing antibody activity in half of the MP-1141-immunized mice (Figure 3B, red circles). However, there were several mice in both immunized groups that were protected from parasite challenge in vivo while mounting only modest neutralizing antibody responses (Figure 3B and 3C, black circles), suggesting that cellular mechanisms may also be involved in protection.
3.3. Role of cellular immunity in efficacy of LbL particles
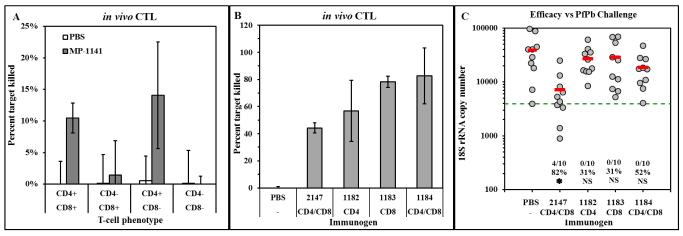
The detection of IFNγ-secreting cells in ELISPOT (Figure 2B) suggests potential activation of cytotoxic effector T-cells following LbL particle immunization, as found in our previous study [11]. The generation of malaria-specific cytotoxic effector cell responses was examined in an in vivo CTL assay using BALB/c mice since C57BL/6J mice fail to develop strong CTL responses to CS protein and there is a known H-2d restricted CD8+ T-cell epitope contained within the T* epitope [15]. Mice were immunized with PBS or MP-1141, and 7 days later were depleted of CD4+, CD8+, or both T-cell phenotypes by administration of the relevant monoclonal antibodies. The next day, in vivo CTL activity was measured [11]. Figure 4A shows that a modest level of killing of T*-loaded target cells was detected in the immunized mice with intact T-cell populations. Depletion of CD8+ cells did not decrease the in vivo CTL activity while depletion of CD4+ cells completely prevented effector activity, indicating that immunization with LbL MP bearing the T1BT* antigen elicits CD4+ cytotoxic effector cells, in agreement with published results demonstrating CD4+ effector activity in human volunteers [16, 17].
Figure 4.
Cellular immunity and efficacy induced by LbL constructs containing malaria CS epitopes. (A) BALB/c mice were mock-immunized with PBS or immunized with MP-1141, and 7 days later were depleted of CD4+ or CD8+ cells or both. In vivo CTL activity was measured the day following depletion. Results show mean±SD percent peptide-specific killing in 3 mice per group. X-axis shows phenotype of T-cells remaining in the mice on day of challenge. (B and C) BALB/c mice were immunized on days 0 and 28 with DP 2147 (Pb CD4+:CD8+ fusion peptide) in Freund’s adjuvant or MP loaded with Pb CD4+ peptide (MP-1182), CD8+ peptide (MP-1183) or CD4+:CD8+ fusion peptide (MP-1184) as indicated (10 μg of DP in each dose). (B) On day 35, in vivo CTL activity was measured in three mice per group. Results show mean±SD percent specific killing of cells pulsed with target peptide. (C) The remaining 10 mice per group were challenged by exposure to PfPb-infected mosquitoes, and parasite burden in the liver 40 hours later was measured by qPCR. Results are shown as described in the legend to Figure 3A.
In light of the T-cell responses detected in ELISPOT (Figure 2B) and in vivo CTL assay (Figure 4A), and the apparent discordance between efficacy and TSNA titers in several of the immunized mice (Figure 3B and 3C), we examined the contribution of cellular immunity to efficacy of LbL microparticles. To test the efficacy of cellular responses alone, in the absence of T1B-specific antibody responses, we constructed MP loaded with T-cell epitopes from the CS protein of P. berghei, the mouse pathogen (Table 1). BALB/c were used in this study since both the CD4+ and CD8+ T-cell epitopes are recognized in H-2d mice. Mice were immunized on days 0 and 28 with MP containing P. berghei CD4+ T-cell epitopes (MP-1182), CD8+ T-cell epitopes (MP-1183), a fusion peptide containing both T-cell epitopes (MP-1184), or DP fusion peptide in Freund’s adjuvant. On day 35, an in vivo CTL experiment was performed using target cells loaded with the immunizing epitope(s). Immunization with MP loaded with either P. berghei T-cell epitope elicited effector activity against target cells loaded with the immunizing peptide (Figure 4B). However, the CTL activity was not sufficient to protect the mice against challenge with PfPb sporozoites which express the P. berghei T-cell epitopes (Figure 4C), suggesting that the efficacy reported in Figure 3A was antibody-mediated.
3.4. Improved vaccine potency by Pam3Cys modification
Clinical trials of malaria peptide vaccines have demonstrated that adjuvants can significantly increase antibody and cellular responses, but frequently at the cost of increased reactogenicity [18]. The use of TLR agonists that more precisely target innate immunity may help avoid excessive inflammatory responses associated with potent adjuvants. Pam3Cys, a synthetic lipopeptide TLR2 agonist, is an especially attractive innate immune stimulator for the LbL approach since it can be incorporated directly into DP. We designed a repeat peptide modified by coupling Pam3Cys to the amino terminal residue. Microparticles were fabricated with T13B5K20Y (MP-1167) or Pam3Cys.T13B5K20Y (MP-1164) on the outermost layer. We used multiple copies of the repeat sequences (T13B5) in order to increase spatial separation between the lipid moiety and the epitopes, thus reducing the possibility that Pam3Cys modification of the DP might mask the target epitopes in the MP-1164 construct.
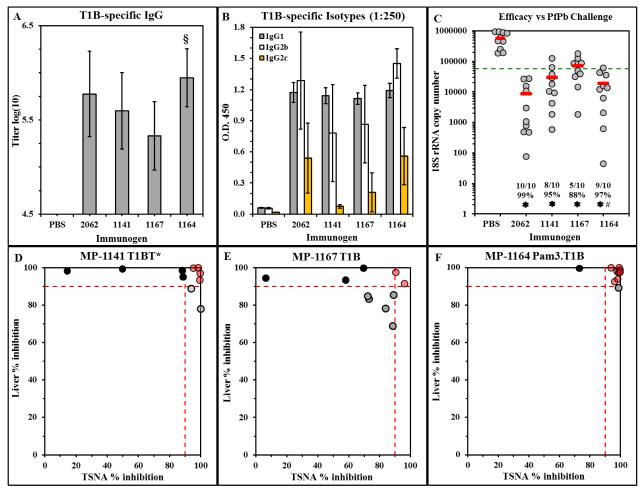
C57BL/6 mice were immunized with MP-1141, MP-1167, or MP-1164; mice immunized with PBS or with DP-2062 (T1BT*) in CFA were included as positive controls. ELISA analysis of sera collected on day 28 shows that MP-1164 containing the Pam3Cys-modified DP was comparable to the positive control DP-2062 (T1BT*) in Freund’s adjuvant and statistically more potent than MP-1167 containing the same DP without Pam3Cys (P=0.02, Wilcoxon rank sum test) (Figure 5A). MP-1164 also yielded an antibody isotype profile identical to that in the positive control group, including the Th1-associated IgG2c isotype that was minimally induced by MP-1167 or MP-1141 (Figure 5B), each of which lacks Pam3Cys. The Pam3Cys-modified MP-1164 was as efficacious as DP 2062 peptide/CFA positive control group, protecting 90% of the mice from liver stage infection (Figure 5C). Protection correlated with neutralizing antibody most strongly in the MP-1164 group (Figure 5F), modestly in the MP-1141 group (Figure 5D), and weakly in the MP-1167 group (Figure 5E). Thus, a simple Pam3Cys modification of the DP yields an improved LbL vaccine that elicits more potent antibody responses and provides a higher level of protection from parasite challenge.
Figure 5.

Immunogenicity and efficacy of Pam3Cys-modified Pf repeat MP. C57BL/6 mice were immunized with the indicated treatments on days 0, 21 and 42. (A) On day 28, sera were tested in ELISA against T1B peptide. Results show the mean±SD anti-T1B IgG antibody titer of 10 mice per group. § P < 0.05 compared to the MP-1167 group. (B) The T1B ELISA was repeated with a 1:250 dilution of individual sera, and each serum was probed with isotype-specific detection antibodies. Results show the mean±SD of 10 mice per group. (C) On day 56, mice were challenged by exposure to PfPb-infected mosquitoes, and parasite burden in the liver 40 hours later was measured by qPCR. Results are shown as described in the legend to Figure 3A. # P = 0.05 compared to the MP-1167 group. (D, E, F) Comparison of in vitro neutralizing activity of sera and in vivo protection from parasite challenge. Each circle represents an individual mouse from (C). Results depict in vitro neutralizing antibody activity on the X-axis compared to in vivo protection on the Y-axis, 10 mice per group. Dotted lines indicate ≥ 90% in vivo efficacy (Y-axis) or neutralizing activity in vitro (X-axis). Red, black, and gray circles are defined in the legend to Figure 3B and 3C.
4. Discussion
The goal of developing a vaccine to eliminate the pre-erythrocytic stages of P. falciparum, thus preventing the blood stage infection responsible for clinical disease, was triggered by studies showing that immunization with attenuated sporozoites elicited protective immunity in experimental rodents, monkeys, and human volunteers [19]. The CS protein was the first protective antigen identified using immune sera and cells obtained from these sporozoite-immunized and protected hosts. The central repeat region of CS contains the protective B-cell epitope, namely the (NANP)n repeat sequence, which elicited sterile immunity in a small clinical trial of patients immunized with (NANP)3-TT tetanus toxoid conjugate [20]. T-cell epitopes of CS include the T1 epitope, which is conserved in all P. falciparum strains of diverse geographical origin [21], and T*, a “universal” T-cell epitope recognized by multiple HLA class II molecules [22]. In a small clinical trial, a tri-epitope Pam3Cys-modified T1BT* branched peptide elicited sporozoite-neutralizing antibodies and polyfunctional CD4+ T-cell clones [23, 24], including clones that directly lysed target cells pulsed with CS peptide, as was found with clones from P. falciparum sporozoite-immunized volunteers [16, 17]. These trials provided the first demonstration that CS peptide vaccines can elicit human antibody and CD4+ T-cells with fine specificity and potential effector function comparable to those elicited by attenuated P. falciparum sporozoites [23, 25].
The difficult synthesis of the tetrabranched (T1BT*)4-Pam3Cys peptide precluded the scale-up necessary to produce quantities needed to prevent the >500,000 deaths caused by P. falciparum malaria each year [26, 27]. The value of the CS epitopes as vaccine targets necessitated the search for more efficient ways to manufacture efficacious vaccines. For this purpose, we incorporated the T1BT* epitope into synthetic microparticles made by LbL fabrication of multilayer films on solid supports, using the methodology previously applied to LbL nanoparticle vaccines [11]. Mice immunized with the T1BT* LbL microparticles, in the absence of exogenous adjuvant, generated parasite-neutralizing antibody responses that correlated with protection from parasite invasion of the liver cells following challenge. This is a key finding, since the first immune effector mechanism identified in Plasmodium sporozoite-immunized hosts was sporozoite-neutralizing antibodies specific for the CS central repeat region [28-30], represented by the P. falciparum T1B epitopes contained in the current constructs.
T1BT* LbL-immunized mice also developed specific cellular responses including CD4+ T-cell cytotoxic effector activity, in agreement with isolation of cytotoxic CD4+ T-cell clones from human volunteers immunized with either T1BT* peptides or live attenuated P. falciparum sporozoites [16]. We found that immunization with LbL microparticles containing only T-cell epitopes failed to provide protection even though robust cytotoxic effector activity was detected. Thus, it appears that parasite-neutralizing antibodies elicited by T1BT*-LbL vaccination were required for efficacy.
Previous studies showed that a T1BT* branched peptide modified with Pam3Cys elicited high titers of anti-repeat antibodies in mice and humans [24, 31]. LbL microparticles loaded with a Pam3Cys-modified T13B5 DP elicited anti-repeat antibody responses that were quantitatively (titer) and qualitatively (opsonizing isotype) superior to those elicited by microparticles loaded with T13B5 DP. Protection from liver stage infection correlated more strongly with neutralizing antibody responses in the mice immunized with the Pam3Cys-modified particle compared to mice immunized with unmodified particles. These results demonstrate that a simple Pam3Cys modification of the DP yields an LbL microparticle vaccine candidate that can elicit high titer protective antibody responses.
Incorporation of antigenic epitopes in particulate vaccines elicits improved T-cell and antibody responses to model antigens, tumors, and numerous pathogens [32-43], including malaria [44-51]. Enhanced immunogenicity of particle vaccines is concomitant with efficient phagocytosis of the particles and activation of DC [42, 43, 52], initiating the immunological reactions that culminate in antigen-specific adaptive immunity which can be augmented by inclusion of innate immune stimulators [53, 54]. LbL microparticles offer several advantages for development of particulate vaccines: they are readily fabricated using straightforward methodologies, are stable at room temperature in lyophilized form (unpublished observations), can include multiple target antigen epitopes, and can be modified to include innate immune stimulants that increase vaccine potency. The current study demonstrates the utility of synthetic LbL microparticles as a potent delivery platform for malaria vaccines that elicit protective immune responses including both cellular and humoral components.
Acknowledgements
The authors thank Dr. Donald Masters and Mr. Thomas Malone for critical review of the manuscript, Rita Altszuler for excellent technical assistance, and Dr. James Dziura for expert advice in statistical analysis. This work was supported by grant R43AI091089 from the National Institutes of Allergy and Infectious Diseases.
Abbreviations
- CFA
complete Freund’s adjuvant
- CFSE
carboxyfluorescein succinimide ester
- CS
circumsporozoite
- DP
designed peptide
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- HBTU
2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- IFA
incomplete Freund’s adjuvant
- LbL
layer-by-layer
- MC
microcapsule
- MP
microparticle
- NP
nanoparticle
- OVA
ovalbumin
- PGA
poly-l-glutamic acid
- PLL
poly-l-lysine
- PLL-FITC
PLL conjugated to fluorescein isothiocynate
- sulfo-NHS
3-sulfo-N-hydroxysuccinimide
- TSNA
Transgenic Sporozoite Neutralization Assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Targett GA, Greenwood BM. Malaria vaccines and their potential role in the elimination of malaria. Malar J. 2008;7(Suppl 1):S10. doi: 10.1186/1475-2875-7-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, et al. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008 Apr;118(4):1266–76. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nardin EH, Nussenzweig RS. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- [4].Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003 Nov;206(Pt 21):3803–8. doi: 10.1242/jeb.00644. [DOI] [PubMed] [Google Scholar]
- [5].Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009 Jul 30;361(5):468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- [6].Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011 Oct 28;334(6055):475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- [7].Casares S, Brumeanu TD, Richie TL. The RTS,S malaria vaccine. Vaccine. 2010 Jul 12;28(31):4880–94. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- [8].Regules JA, Cummings JF, Ockenhouse CF. The RTS,S vaccine candidate for malaria. Expert Rev Vaccines. 2011 May;10(5):589–99. doi: 10.1586/erv.11.57. [DOI] [PubMed] [Google Scholar]
- [9].Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011 Nov 17;365(20):1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- [10].Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012 Dec 13;367(24):2284–95. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Powell TJ, Palath N, Derome ME, Tang J, Jacobs A, Boyd JG. Synthetic nanoparticle vaccines produced by layer-by-layer assembly of artificial biofilms induce potent protective T-cell and antibody responses in vivo. Vaccine. 2011 Jan 10;29(3):558–69. doi: 10.1016/j.vaccine.2010.10.001. [DOI] [PubMed] [Google Scholar]
- [12].Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002 Dec 15;169(12):6681–5. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- [13].Kumar KA, Oliveira GA, Edelman R, Nardin E, Nussenzweig V. Quantitative Plasmodium sporozoite neutralization assay (TSNA) J Immunol Methods. 2004 Sep;292(1-2):157–64. doi: 10.1016/j.jim.2004.06.017. [DOI] [PubMed] [Google Scholar]
- [14].Othoro C, Johnston D, Lee R, Soverow J, Bystryn JC, Nardin E. Enhanced immunogenicity of Plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic Toll-like receptor 7 agonist, imiquimod. Infect Immun. 2009 Feb;77(2):739–48. doi: 10.1128/IAI.00974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blum-Tirouvanziam U, Beghdadi-Rais C, Roggero MA, Valmori D, Bertholet S, Bron C, et al. Elicitation of specific cytotoxic T cells by immunization with malaria soluble synthetic polypeptides. J Immunol. 1994 Nov 1;153(9):4134–41. [PubMed] [Google Scholar]
- [16].Frevert U, Moreno A, Calvo-Calle JM, Klotz C, Nardin E. Imaging effector functions of human cytotoxic CD4+ T cells specific for Plasmodium falciparum circumsporozoite protein. Int J Parasitol. 2009 Jan;39(1):119–32. doi: 10.1016/j.ijpara.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moreno A, Clavijo P, Edelman R, Davis J, Sztein M, Herrington D, et al. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int Immunol. 1991 Oct;3(10):997–1003. doi: 10.1093/intimm/3.10.997. [DOI] [PubMed] [Google Scholar]
- [18].Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nussenzweig RS, Nussenzweig V. Development of sporozoite vaccines. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1984 Nov 13;307(1131):117–28. doi: 10.1098/rstb.1984.0113. [DOI] [PubMed] [Google Scholar]
- [20].Nardin E. The past decade in malaria synthetic peptide vaccine clinical trials. Hum Vaccin. 2010 Jan;6(1):27–38. doi: 10.4161/hv.6.1.9601. [DOI] [PubMed] [Google Scholar]
- [21].Nardin EH, Herrington DA, Davis J, Levine M, Stuber D, Takacs B, et al. Conserved repetitive epitope recognized by CD4+ clones from a malaria-immunized volunteer. Science. 1989 Dec 22;246(4937):1603–6. doi: 10.1126/science.2480642. [DOI] [PubMed] [Google Scholar]
- [22].Calvo-Calle JM, Hammer J, Sinigaglia F, Clavijo P, Moya-Castro ZR, Nardin EH. Binding of malaria T cell epitopes to DR and DQ molecules in vitro correlates with immunogenicity in vivo: identification of a universal T cell epitope in the Plasmodium falciparum circumsporozoite protein. J Immunol. 1997 Aug 1;159(3):1362–73. [PubMed] [Google Scholar]
- [23].Calvo-Calle JM, Oliveira GA, Nardin EH. Human CD4+ T cells induced by synthetic peptide malaria vaccine are comparable to cells elicited by attenuated Plasmodium falciparum sporozoites. J Immunol. 2005 Dec 1;175(11):7575–85. doi: 10.4049/jimmunol.175.11.7575. [DOI] [PubMed] [Google Scholar]
- [24].Nardin EH, Calvo-Calle JM, Oliveira GA, Nussenzweig RS, Schneider M, Tiercy JM, et al. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. J Immunol. 2001 Jan 1;166(1):481–9. doi: 10.4049/jimmunol.166.1.481. [DOI] [PubMed] [Google Scholar]
- [25].Moreno A, Clavijo P, Edelman R, Davis J, Sztein M, Sinigaglia F, et al. CD4+ T cell clones obtained from Plasmodium falciparum sporozoite-immunized volunteers recognize polymorphic sequences of the circumsporozoite protein. J Immunol. 1993 Jul 1;151(1):489–99. [PubMed] [Google Scholar]
- [26].Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012 Feb 4;379(9814):413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- [27].World Malaria Report 2012. 2012.
- [28].Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–13. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980 Jan 4;207(4426):71–3. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- [30].Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, et al. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982 Jul 1;156(1):20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nardin EH, Calvo-Calle JM, Oliveira GA, Clavijo P, Nussenzweig R, Simon R, et al. Plasmodium falciparum polyoximes: highly immunogenic synthetic vaccines constructed by chemoselective ligation of repeat B-cell epitopes and a universal T-cell epitope of CS protein. Vaccine. 1998 Apr;16(6):590–600. doi: 10.1016/s0264-410x(97)00238-7. [DOI] [PubMed] [Google Scholar]
- [32].Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004 Sep 1;173(5):3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- [33].Fifis T, Mottram P, Bogdanoska V, Hanley J, Plebanski M. Short peptide sequences containing MHC class I and/or class II epitopes linked to nano-beads induce strong immunity and inhibition of growth of antigen-specific tumour challenge in mice. Vaccine. 2004 Nov 25;23(2):258–66. doi: 10.1016/j.vaccine.2004.05.022. [DOI] [PubMed] [Google Scholar]
- [34].Uto T, Wang X, Akagi T, Zenkyu R, Akashi M, Baba M. Improvement of adaptive immunity by antigen-carrying biodegradable nanoparticles. Biochem Biophys Res Commun. 2009 Feb 6;379(2):600–4. doi: 10.1016/j.bbrc.2008.12.122. [DOI] [PubMed] [Google Scholar]
- [35].Makidon PE, Bielinska AU, Nigavekar SS, Janczak KW, Knowlton J, Scott AJ, et al. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS ONE. 2008;3(8):e2954. doi: 10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007 Jan-Feb;4(1):73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- [37].Audran R, Peter K, Dannull J, Men Y, Scandella E, Groettrup M, et al. Encapsulation of peptides in biodegradable microspheres prolongs their MHC class-I presentation by dendritic cells and macrophages in vitro. Vaccine. 2003 Mar 7;21(11-12):1250–5. doi: 10.1016/s0264-410x(02)00521-2. [DOI] [PubMed] [Google Scholar]
- [38].Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Polymer nanoparticles for immunotherapy from encapsulated tumor-associated antigens and whole tumor cells. Mol Pharm. 2007 Jan-Feb;4(1):47–57. doi: 10.1021/mp060107e. [DOI] [PubMed] [Google Scholar]
- [39].Hamdy S, Elamanchili P, Alshamsan A, Molavi O, Satou T, Samuel J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly(D,L-lactic-co-glycolic acid) nanoparticles. J Biomed Mater Res A. 2007 Jun 1;81(3):652–62. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- [40].Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, et al. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc Natl Acad Sci U S A. 2011 Nov 1;108(44):E989–97. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H, et al. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J Control Release. 2012 Feb 10;157(3):354–65. doi: 10.1016/j.jconrel.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, et al. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007 Mar 1;178(5):2979–86. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- [43].Yang YW, Hsu PY. The effect of poly(D,L-lactide-co-glycolide) microparticles with polyelectrolyte self-assembled multilayer surfaces on the cross-presentation of exogenous antigens. Biomaterials. 2008 Jun;29(16):2516–26. doi: 10.1016/j.biomaterials.2008.02.015. [DOI] [PubMed] [Google Scholar]
- [44].Schodel F, Wirtz R, Peterson D, Hughes J, Warren R, Sadoff J, et al. Immunity to malaria elicited by hybrid hepatitis B virus core particles carrying circumsporozoite protein epitopes. J Exp Med. 1994 Sep 1;180(3):1037–46. doi: 10.1084/jem.180.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Allsopp CE, Plebanski M, Gilbert S, Sinden RE, Harris S, Frankel G, et al. Comparison of numerous delivery systems for the induction of cytotoxic T lymphocytes by immunization. Eur J Immunol. 1996 Aug;26(8):1951–9. doi: 10.1002/eji.1830260841. [DOI] [PubMed] [Google Scholar]
- [46].Gilbert SC, Plebanski M, Harris SJ, Allsopp CE, Thomas R, Layton GT, et al. A protein particle vaccine containing multiple malaria epitopes. Nat Biotechnol. 1997 Nov;15(12):1280–4. doi: 10.1038/nbt1197-1280. [DOI] [PubMed] [Google Scholar]
- [47].Birkett A, Lyons K, Schmidt A, Boyd D, Oliveira GA, Siddique A, et al. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infect Immun. 2002 Dec;70(12):6860–70. doi: 10.1128/IAI.70.12.6860-6870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mata E, Carcaboso AM, Hernandez RM, Igartua M, Corradin G, Pedraz JL. Adjuvant activity of polymer microparticles and Montanide ISA 720 on immune responses to Plasmodium falciparum MSP2 long synthetic peptides in mice. Vaccine. 2007 Jan 15;25(5):877–85. doi: 10.1016/j.vaccine.2006.09.036. [DOI] [PubMed] [Google Scholar]
- [49].Kaba SA, Brando C, Guo Q, Mittelholzer C, Raman S, Tropel D, et al. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009 Dec 1;183(11):7268–77. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pusic K, Xu H, Stridiron A, Aguilar Z, Wang A, Hui G. Blood stage merozoite surface protein conjugated to nanoparticles induce potent parasite inhibitory antibodies. Vaccine. 2011 Nov 8;29(48):8898–908. doi: 10.1016/j.vaccine.2011.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):1080–5. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kwon YJ, Standley SM, Goh SL, Frechet JM. Enhanced antigen presentation and immunostimulation of dendritic cells using acid-degradable cationic nanoparticles. J Control Release. 2005 Jul 20;105(3):199–212. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schlosser E, Mueller M, Fischer S, Basta S, Busch DH, Gander B, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008 Mar 20;26(13):1626–37. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- [54].Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009 May 18;27(23):3013–21. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]