Abstract
Regulatory T cells (Tregs) are a subset of CD4+ T cells that are characterized by the expression of CD25 and Foxp3 and are capable of suppressing alloimmune responses. We assessed whether high frequencies of circulating skin or gut tissue–specific Tregs at engraftment could predict acute graft-vs-host disease (aGVHD) incidence and survival in a cohort of hematopoietic cell transplant (HCT) recipients. Tregs were analyzed at engraftment in 74 patients receiving HCT. Treg skin-homing (CLA+) or gut-homing (α4β7+) subsets were identified by flow cytometry, and patients were divided into high CLA+ Tregs or high α4β7+ Tregs groups, using the 75th percentile of tissue-specific Treg percentages as a threshold. At day +100 post-HCT, the cumulative incidence of any stage skin or gut aGVHD was significantly lower in those patients with high CLA+ Tregs or high α4β7+ Tregs at engraftment, respectively (high CLA+ Tregs, 24.0% vs low CLA+ Tregs, 55.1%; p = 0.011 for skin aGVHD or high α4β7+ Tregs, 47.3% vs low α4β7+ Tregs, 74.5%; p = 0.029 for gut aGVHD). The 2-year probabilities of overall survival and nonrelapse mortality were 73.4% and 7.5% among patients with high frequencies of tissue-specific Tregs vs 49.4% and 36.1% for those with both low CLA+ Tregs and low α4β7+ Tregs (p = 0.039, p = 0.010). These results suggest that a threshold value for CLA+ or α4β7+ Tregs could be used to predict important HCT outcomes, and to direct the rationale use of tissue-specific pre-emptive therapies to decrease clinical aGVHD and improve HCT survival.
Acute graft-vs-host disease (aGVHD) is a significant and unpredictable complication after allogeneic hematopoietic cell transplantation (HCT). aGVHD occurs when donor T cells recognize incompatibilities in recipient major or minor histocompatibility antigens. After cell activation and up-regulation of tissue-specific homing receptors, donor T cells migrate from secondary lymphoid organs to recipient target tissues, leading to the clinical manifestations of aGVHD, e.g., dermatitis, gastroenteritis, and cholestatic hepatitis [1–5]. Despite prophylaxis strategies with calcineurin inhibitors and methotrexate or mycophenolate mofetil, about 50% of patients undergoing HCT will develop moderate to severe (Glucksberg grade II–IV) aGVHD [1,2]. Standard treatment of aGVHD with high-dose corticosteroids produces a complete response in only 25% to 50% of patients, with about one third of individuals having persistent symptoms [1,2,6,7]. For patients with steroid refractory aGVHD, there is no standard second-line treatment, and overall prognosis of such individuals is guarded. Due to the unpredictable nature of aGVHD, inferior survival of Glucksberg grade III–IV aGVHD, and the heterogeneous response to initial therapy, the development of a laboratory test that can predict incidence and target organ involvement and severity of aGVHD would be clinically important, and could be used to improve HCT outcomes.
CD25+Foxp3+ regulatory T cells (Tregs) are an important subset of CD4+ T cells that maintain immunological tolerance [8–11]. Tregs are associated with the prevention of aGVHD in murine models of transplantation and in human HCT [12–16]. Similar to conventional T cells, Tregs localize to host secondary lymphoid organs early after HCT and then migrate to the skin, gut, or liver via the expression of selectins, integrins, and chemokine receptors [17, 18]. The expression of these cell-surface molecules can be used to identify unique subsets of Tregs with either a skin-homing (CLA+/CCR4+) or a gut-homing (α4β7+/CCR9+) phenotype [19, 20]. In a limited cohort of 43 patients, our group has shown a relationship between tissue-homing Treg subsets with skin or gut aGVHD [21]. To facilitate future clinical trials, determination of a threshold or cutoff value corresponding to high or protective Treg percentages would be clinically useful. Ideally this biomarker would predict both aGVHD incidence and survival.
We hypothesized that high frequencies of circulating CLA+ or α4β7+ Tregs would be associated with decreased skin or gut aGVHD, respectively, and improved post-HCT survival. The data showed that high or low percentages of CLA+ or α4β7+ Tregs at the time of neutrophil engraftment can be used to predict both aGVHD target organ involvement and post-transplantation survival. These laboratory parameters represent a novel, easily accessible, and pertinent clinical test that can be used to predict aGVHD onset and mortality before their clinical occurrence. Furthermore, this Treg subset threshold represents a target that is potentially modifiable through pharmacological intervention or cellular therapy.
Material and methods
Study design
Patients undergoing HCT were enrolled in a Vanderbilt Institutional Review Board–approved protocol after written informed consent was obtained. All analyzed patients were diagnosed with a hematological malignancy and received either a myeloablative or reduced-intensity conditioning regimen, followed by a T-cell replete related donor or unrelated donor transplant. Patients receiving in vivo T-cell depletion with anti-thymocyte globulin were excluded due to low lymphocyte numbers, which interfered with flow cytometric analysis. All patients received aGVHD prophylaxis with a calcineurin inhibitor and either methotrexate or mycophenolate mofetil. Sirolimus was not administered routinely. No patients received donor lymphocyte infusions during the study period, defined as the first 100 days of transplant. Clinical features of aGVHD were assessed weekly for the first 100 days after HCT by a single individual blinded to the Treg data (M.J.). The recorded features included aGVHD timing, incidence, organ involvement, severity, and recurrence rates. Histologic confirmation of clinical aGVHD was performed for clinical purposes when feasible. Recurrent aGVHD was defined as any increase in aGVHD symptoms or therapy during the first 100 days of transplant after an initial response to treatment or during steroid taper. Recurrent aGVHD was not analyzed beyond day +100. The clinical severity of aGVHD was determined by the overall grade (0–IV) and the individual organ stage (0–4), as defined by the 1994 consensus conference criteria [22].
Blood cell isolation and flow cytometric analysis
The procedures for collection and storage of peripheral blood mononuclear cells for T-cell immunophenotyping were reported previously [21]. Samples were collected at two time points: neutrophil engraftment (defined as absolute neutrophil count ≥0.5 × 109/L for 3 days) and at day+30 after HCT. After thawing, cells were analyzed using a 10-color multiparametric flow cytometric panel including the following antibodies or dye: CD3-peridinin-chlorophyllprotein complex-Cy5.5, CD4-Alexa700, CD25-allophycocyanin-Cy7, CD45RO-phycoerythrin (PE)-Cy7, CLA-fluorescein isothiocyanate (BD Biosciences, San Jose, CA, USA), CD8-PE-Cy5, CD14-PE-TR, amine viability dye (Invitrogen, Carlsbad, CA, USA), CD127-Pacific Blue (eBioscience, San Diego, CA, USA), and α4β7-PE (kind gift from Millennium Pharmaceuticals, Inc., Cambridge, MA, USA and commercially conjugated by Chromaprobe, Inc., Maryland Heights, MO, USA). After surface staining, cells were fixed and permeabilized with the Human Foxp3 Buffer Set (BD Biosciences, San Jose, CA, USA) as per manufacturer’s instructions followed by intracellular staining with Foxp3-Alexa 647 (clone 259D/C7; BD Biosciences, San Jose, CA, USA). Stained cells were analyzed with a LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). Flow cytometric analysis was performed using FlowJo software version 8.0 (Tree Star, Ashland, OR, USA) by a single expert cytometrist who was blinded to the transplant data. A minimum of 100,000 CD4+ events was acquired. To increase specificity, multiple markers were used to indentify Tregs, including CD4+CD45RO+CD25+Foxp3+CD127lo, and their frequency was expressed as the percentage of positive cells in the total CD4+ gate. CLA+ (considered skin homing) or α4β7+ (considered gut homing) Tregs were identified and quantified as their respective subpopulations within the total population of Tregs (Supplementary Figure E1; online only, available at www.exphem. org). Treg percentages rather that absolute numbers were analyzed due to the infrequency of the T-cell subsets studied and the severity of the lymphopenia at the time of sample collection.
Statistical analysis
Continuous variables were summarized using the mean, median, quartiles, or range. For continuous variables, the mean difference between two independent groups was compared using the Mann-Whitney U test. Categorical variables were described by the percentage or frequency and were compared by the χ2 or Fisher’s exact test. Correlation between continuous variables was determined by Spearman’s rank correlation. The primary aims of this study were to determine if there was an association between high or low percentages of circulating blood CLA+ and α4β7+ Tregs with the occurrence of skin or gut aGVHD, respectively, and to identify if these subsets predict post-HCT survival. Various statistical methods were used to establish a cutoff for high or low Treg percentages, including taking the median and grouping by quartiles. In univariate analysis, χ2 was used to test the association between high or low Treg subset percentages with organ-specific aGVHD incidence or recurrence during the first 100 days of HCT. Multivariate logistic regression was used to evaluate whether high or low Treg subset percentages could independently predict aGVHD outcomes during the first 100 days of HCT, after adjusting for potential confounding variables, including intensity of conditioning regimen (i.e., myeloablative vs reduced-intensity conditioning), donor type (i.e., related vs unrelated), and stem cell source. These clinically important transplant characteristics were selected as covariates for the logistic regression models a priori. Overall survival and disease-free survival were estimated using the Kaplan-Meier method, and cumulative incidence was used to estimate the probability of nonrelapse mortality (NRM). NRM was defined as death in the absence of disease relapse or progression. NRM and relapse were considered competing risks for disease-free survival and NRM, respectively. Survival outcomes between groups were compared with a log-rank test for univariate analysis and a Cox proportional hazards regression for multivariate analysis. The p values were two-tailed and considered significant at p < 0.05. Analyses were performed using SPSS version 18 (SPSS Inc, Chicago, IL, USA) and R version 2.7.0 (Free Software Foundation, Boston, MA, USA).
Results
Patients
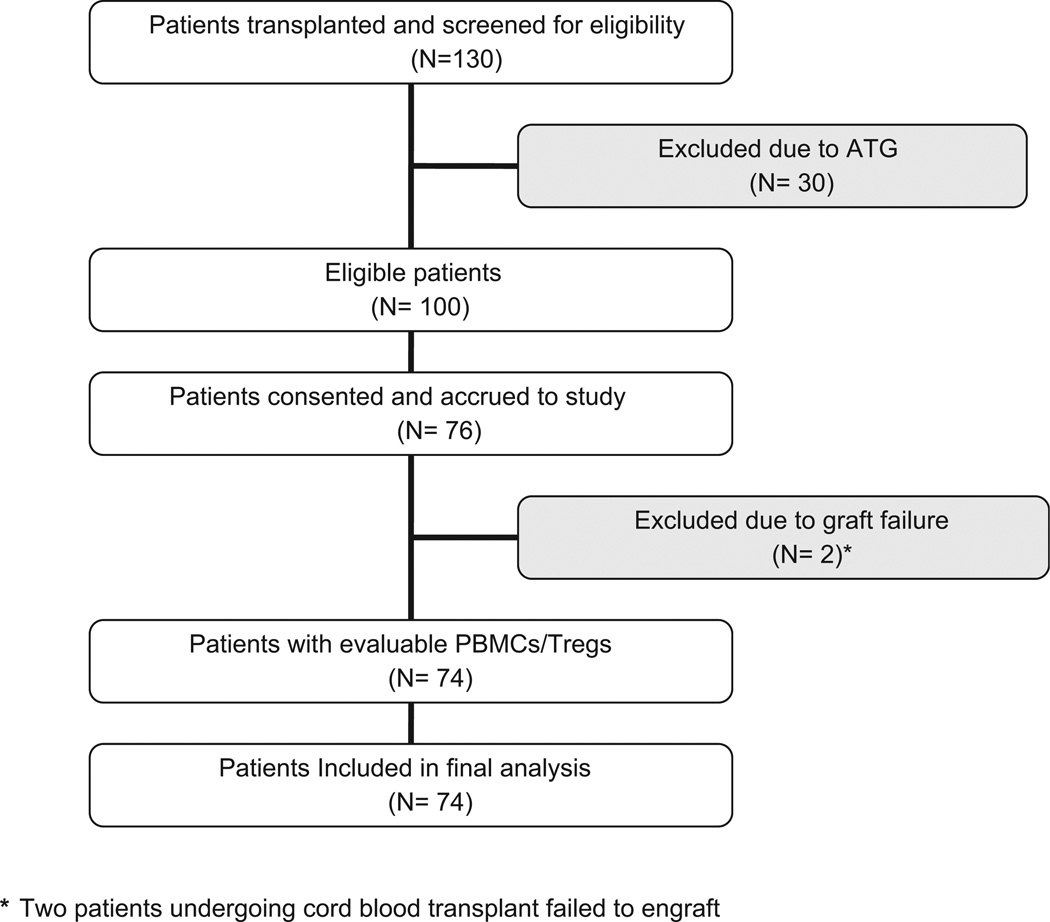
From February 2, 2007 to March 3, 2009, 130 patients (aged 18 years or older) underwent HCT at a single institution (Vanderbilt University Medical Center, Nashville, TN, USA) and 74 of these patients had peripheral blood mononuclear cells collected for Treg analysis (Fig. 1). The clinical characteristics of this cohort are summarized in Table 1. aGVHD events were recorded until day +100. Grade II to IV aGVHD occurred in 57 (77%) patients at a median of 28 days post-HCT (range, 7–93 days) and was biopsy proven in 51 (91%) of those individuals. aGVHD affected the skin, gut, and liver in 33 (44.6%), 50 (67.6%), and 2 (2.7%) subjects, respectively and was grade III–IV in 13 (17.6%) of the HCT recipients. Forty-five (60.8%) patients received systemic corticosteroids as part of their aGVHD treatment. Despite treatment, grade II–IV aGVHD recurred in 32 (68%) of these individuals during the first 100 days. Actuarial survival at day +100 was 95.9%; causes of death included relapse of malignancy (n = 2) and infection (n = 1). No patients died from complications related to aGVHD during the first 100 days.
Figure 1.
Number of patients who underwent allogeneic hematopoietic cell transplantation, accrued to study, and were included in the final Treg analysis. ATG, antithymocyte globulin; PBMCs, peripheral blood mononuclear cells.
Table 1.
Characteristics of total cohort, 74 patients undergoing HCT
| Patients with characteristic (n), stratified by aGVHD organ involvement (%) |
||||
|---|---|---|---|---|
| Characteristic | Skin only aGVHD |
Gut only aGVHD |
Multi-organ* aGVHD |
No aGVHD |
| Total no. of patients | 11 | 29 | 22 | 12 |
| Age (y) | ||||
| Median | 43 | 47 | 44 | 49 |
| Range | 33–65 | 24–61 | 21–70 | 34–65 |
| Sex | ||||
| Male | 6 (55) | 13 (45) | 9 (41) | 8 (67) |
| Female | 5 (45) | 16 (55) | 13 (59%) | 4 (33) |
| Diagnosis | ||||
| Acute leukemia + MDS | 6 (55) | 18 (62) | 14 (64) | 3 (25) |
| CML + MPD | — | 3 (10) | — | 2 (17) |
| NHL + HL + CLL + MM | 5 (45) | 7 (24) | 7 (32) | 7 (58) |
| Other | — | 1 (4) | 1 (4) | — |
| Conditioning regimen | ||||
| Myeloablative | 6 (55) | 20 (69) | 14 (64) | 5 (42) |
| Reduced intensity | 5 (45) | 9 (31) | 8 (36) | 7 (58) |
| Donor | ||||
| Related | 8 (73) | 21 (72) | 11 (50) | 10 (83) |
| Unrelated | 3 (27) | 8 (28) | 11 (50) | 2 (17) |
| Stem cell source | ||||
| Peripheral blood | 8 (73) | 24 (83) | 13 (59) | 10 (83) |
| Other | 3 (27) | 5 (17) | 9 (41) | 2 (17) |
| HLA | ||||
| Matched | 8 (73) | 27 (93) | 20 (91) | 11 (92) |
| Mismatched | 3 (27) | 2 (7) | 2 (9) | 1 (8) |
| Donor/recipient sex | ||||
| Matched | 8 (73) | 13 (45) | 15 (68) | 9 (75) |
| Mismatched | 3 (27) | 16 (55) | 7 (32) | 3 (25) |
| Female to male | 1 (9) | 4 (14) | 1 (5) | 1 (8) |
| CMV serostatus | ||||
| Recipient/donor | ||||
| Negative/negative | 4 (36) | 8 (28) | 2 (9) | 2 (17) |
| Positive/negative | 3 (27) | 10 (34) | 8 (36) | 2 (17) |
| Negative/positive | 1 (9) | 2 (7) | 3 (14) | 4 (33) |
| Positive/positive | 3 (27) | 9 (31) | 9 (41) | 4 (33) |
| CD34+ (×106/kg) | ||||
| Median | 5.56 | 5.94 | 6.34 | 6.23 |
| Range | 0.04–9.68 | 0.16–10.1 | 0.09–9.99 | 0.56–10.4 |
| aGVHD prophylaxis | ||||
| CSA + methotrexate | 5 (46) | 18 (62) | 13 (59) | 5 (42) |
| CSA/FK506 + MMF | 6 (54) | 11 (38) | 9 (41) | 7 (58) |
| Day + 100 disease status | ||||
| CR or PR | 8 (73) | 26 (90) | 18 (82) | 9 (75) |
| Relapse or progression | 3 (27) | 3 (10) | 4 (18) | 3 (25) |
| Day + 100 survival | ||||
| Alive | 10 (91) | 28 (97) | 22 (100) | 11 (92) |
| Dead | 1 (9) | 1 (3) | — | 1 (8) |
CLL = chronic lymphocytic leukemia; CML = chronic myeloid leukemia; CMV = cytomegalovirus; CR = complete response; CSA = cyclosporine; FK506 = tacrolimus; HL = Hodgkin’s lymphoma; MDS = myelodysplastic syndrome; MM = multiple myeloma; MMF = mycophenolate mofetil; MPD = myeloproliferative disorder; NHL = non-Hodgkin lymphoma; PR = partial response.
Liver aGVHD occurred in two patients with multi-organ aGVHD involvement.
Treg analysis
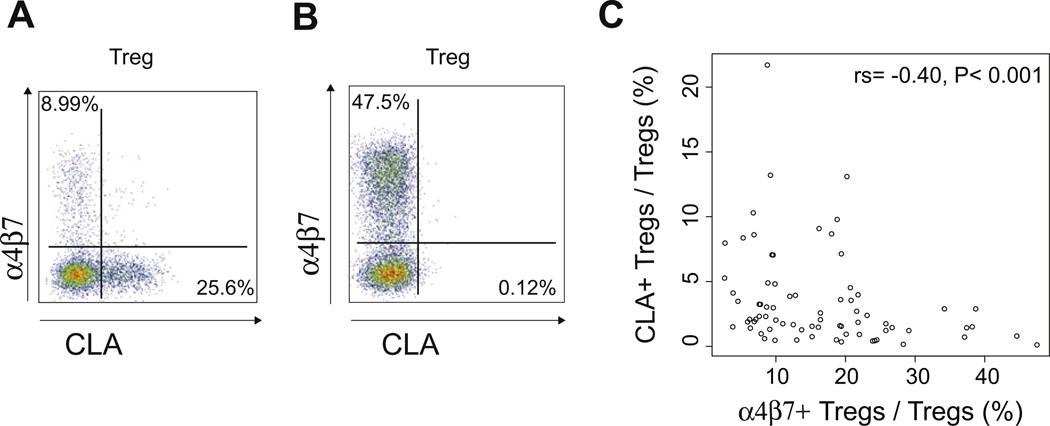
Median time from stem cell infusion to initial Treg analysis was 19 days (range, 10–34 days). Treg subsets could be quantified 7–10 days before development of grade II–IVaGVHDor the start of systemic steroids. Using multiparametric flow cytometry, we were able to identify populations of CLA+ (skin homing) or α4β7+ (gut homing) Tregs in all patients at the time of neutrophil engraftment and as early as 10 days post-HCT (Supplementary Figure E1; online only, available at www.exphem.org). As expected, these Foxp3+ tissue-homing subsets were primarily CD45RO+ and CD127lo. These subpopulations of Tregs appeared mutually exclusive with few cells positive for both CLA+ and α4β7+ (median percentage of CLA+α4β7+ Tregs at engraftment and at day +30 was 0.13% [range, 0–1.12%] and 0.12% [range, 0–1.10%], respectively). Treg expression of CLA was inversely related to expression of α4β7, with a negative correlation noted at engraftment (rs = −0.40; p < 0001) (Fig. 2C). Thus, it appeared that some patients exhibited preferential expansion of a single organ-specific subset of Tregs (Fig. 2A, B).
Figure 2.
CLA+ and α4β7+ Tregs can be identified at the time of neutrophil recovery after allogeneic hematopoietic cell transplantation. (A) Patient with preferential expansion of skin-homing (CLA+) Tregs (CD4+CD45RO+CD25+Foxp3+CD127lo cells). (B) Patient with preferential expansion of gut-homing (α4β7+) Tregs (CD4+CD45RO+CD25+Foxp3+CD127lo cells). (C) Treg expression of CLA and α4β7 are inversely related.
Donor chimerisms were not studied at the time of neutrophil engraftment. Day +30 restriction fragment length polymorphism data were obtained from bone marrow in all evaluable patients (n = 73), and circulating CD3+ sorted T cells in patients undergoing cord blood or reduced-intensity conditioning HCT (n = 33). The median percentage of donor cells in the marrow or within the peripheral blood T-cell compartment was 99% (range, 50–100%) and 83.5% (range, 39–100%), respectively. These data indicate that the majority of circulating hematopoietic cells early after transplantation were derived from the donor.
Treg subsets and organ-specific aGVHD outcomes
Because the majority of initial aGVHD events occurred before day +30, we focused our analysis on the frequency of Tregs at engraftment as predictive markers for development of aGVHD. After examining the distribution of Treg subset percentages among patients with or without aGVHD in our original cohort, the 75th percentile or higher was established as the cutoff point to define high or protective percentages of Treg cell subsets (Tregs/CD4+ ≥14.0%), CLA+ Tregs (CLA+ Tregs/Tregs ≥3.25%), or α4β7+ Tregs (α4β7+ Tregs/Tregs ≥21.8%) [21]. This cutoff was then applied to the total cohort (n = 74) for analysis, and 13 (17.6%), 25 (33.8%) and 19 (25.7%) patients had high frequencies of Tregs, CLA+ Tregs, or α4β7+ Tregs, respectively.
We noted a reciprocal regulation of the skin and gut-coming subsets in peripheral blood. Consistent with this trend, only one patient in the cohort was classified as having both high CLA+ and high α4β7+ circulating Tregs. Next, we examined whether the clinical characteristics as outlined in Table 1 influenced the occurrence of high CLA+ or high α4β7+ Treg cell subsets. Patients classified as high α4β7+ Treg producers were younger (median 5 42 vs 47 years; p = 0.047) and were more likely to have received human leukocyte antigen–mismatched grafts (31.6% vs 3.64%; p = 0.001). Patients with high α4β7+ Tregs also tended to have received myeloablative conditioning (78.9% vs 54.5%; p = 0.060) and transplants using bone marrow or cord blood (42.1% vs 20%; p = 0.057). Definite associations were not found between high CLA+ Tregs and the clinical characteristics listed in Table 1.
The proportion of patients developing any skin aGVHD during the first 100 days of HCT was significantly lower in those with high CLA+ Tregs vs those with low CLA+ Tregs (24.0% [6/25] vs 55.1% [27/49]; p = 0.011). Similar results were found with respect to high α4β7+ Tregs with any stage gut aGVHD occurring in 47.3% (9 of 19) as compared to 74.5% (41 of 55) in patients with low α4β7+ Tregs (p = 0.029). The analysis was repeated after excluding the three patients who died before day +100 and similar results were found with high CLA+ Tregs and high α4β7+ Tregs associated with the prevention of skin aGVHD (p = 0.009) or gut aGVHD (p = 0.043), respectively (data not shown). During the first 100 days after transplantation, high frequencies of circulating tissue-specific Tregs at engraftment were also associated with prevention of repeat episodes of aGVHD involving the same organ. Among patients with a history of aGVHD involving the skin (n = 33) or gut (n = 50), 20 (60.6%) and 19 (38%) of those individuals had recurrent symptoms of either skin or gut aGVHD, respectively. Patients with high α4β7+ Tregs had decreased recurrent gut aGVHD episodes (high α4β7+ Tregs, 0% [0 of 19] vs low α4β7+ Tregs, 34.5% [19 of 55]; p = 0.003), while HCT recipients with high CLA+Tregs had a nonsignificant decrease in recurrent skin aGVHD (high CLA+Tregs, 16% [4 of 25] vs low CLA+ Tregs, 32.6% [16 of 49]; p = 0.127). Thus, the frequency of Treg subsets at engraftment predicted not only the incidence of organ-specific aGVHD, but also identified individuals who may require prolonged exposure to systemic steroids, which in turn could affect survival. In contrast to the organ-specific Treg percentages, the total number of circulating Treg cells (not accounting for homing properties) was not a predictive factor. Total Treg percentages were not associated with grade II–IV aGVHD (p = 0.474), any stage skin aGVHD (p = 0.176), or any stage gut aGVHD (p =0.888) (data not shown).
In multivariate logistic regression, patients with high percentages of CLA+ Tregs or α4β7+ Tregs at the time of neutrophil engraftment continued to have a significantly decreased odds of skin (odds ratio [OR] = 0.27; 95% confidence interval [CI], 0.09–0.81; p = 0.020) or gut (OR = 0.20; 95% CI, 0.06–0.69; p = 0.011) aGVHD, respectively, during the first 100 days of transplant (Table 2).
Table 2.
Logistic regression models for development of any stage skin or gut aGVHD
| Target organ | Factor | aGVHD/patients at risk |
Odds ratio (95% CI) | p Value |
|---|---|---|---|---|
| Skin aGVHD | ||||
| Low CLA+ Tregs | 27/49 | 1 | 0.020 | |
| High CLA+ Tregs* | 6/25 | 0.27 (0.09–0.81) | ||
| Myeloablative | 20/45 | 1 | 0.430 | |
| Reduced intensity | 13/29 | 0.63 (0.20–1.97) | ||
| Related donor | 19/50 | 1 | 0.750 | |
| Unrelated donor | 14/24 | 1.32 (0.24–7.36) | ||
| Peripheral blood stem cells | 21/55 | 1 | 0.300 | |
| Bone marrow or cord blood | 12/19 | 2.53 (0.44–14.6) | ||
| Gut aGVHD | ||||
| Low α4β7+Tregs | 41/55 | 1 | 0.011 | |
| High α4β7+ Tregs† | 9/19 | 0.20 (0.06–0.69) | ||
| Myeloablative | 33/45 | 1 | 0.145 | |
| Reduced intensity | 17/29 | 0.41 (0.12–1.36) | ||
| Related donor | 32/50 | 1 | 0.716 | |
| Unrelated donor | 18/24 | 1.43 (0.21–9.69) | ||
| Peripheral blood stem cells | 36/55 | 1 | 0.833 | |
| Bone marrow or cord blood | 14/19 | 1.24 (0.17–9.16) |
High CLA+ Tregs: CLA+ Tregs/Tregs ≥3.25%.
High α4β7+ Tregs: α4β7+ Tregs/Tregs ≥21.8%.
Treg subsets and survival analysis
Median follow-up was 2.5 years (range, 0.5–4 years) from time of HCT for surviving patients (n = 45). The major cause of death was relapse or progression of malignancy (n = 19). NRM occurred in 10 (13.5%) patients. Causes of death included infection (n = 5), acute blood loss from gastrointestinal GVHD (n = 1), diffuse alveolar hemorrhage (n = 1), thrombotic thrombocytopenic purpura (n = 1), secondary malignancy (n = 1), and myocardial infarction (n = 1). The majority of patients with NRM were on systemic immunosuppression (n = 9), had active GVHD (overlap chronic GVHD, n = 5; recurrent aGVHD, n = 2; and classic chronic GVHD, n = 1), and had low frequencies of CLA+ Tregs and α4β7+ Tregs (n = 8).
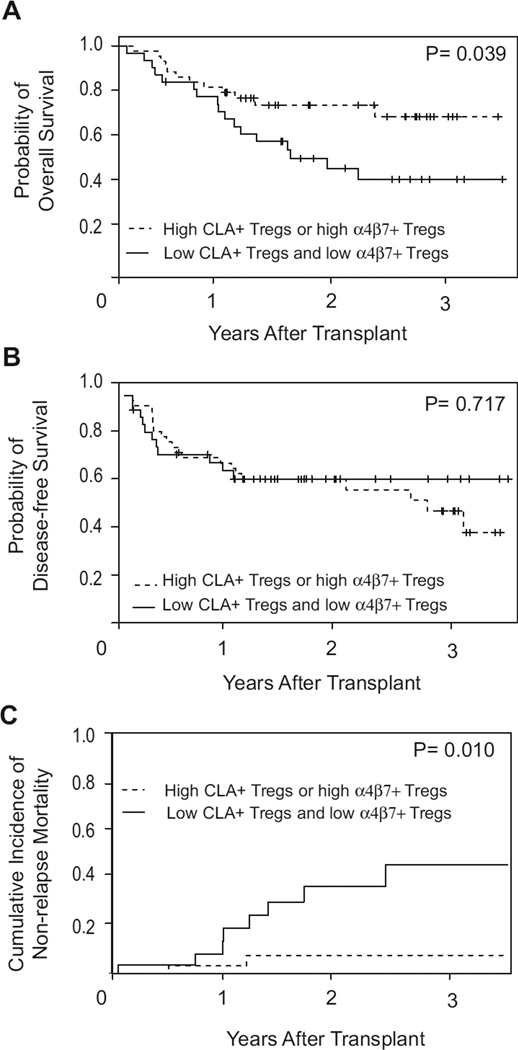
To determine whether tissue-specific Tregs were associated with survival after HCT, patients were stratified into two groups. Patients with the favorable phenotype of either high CLA+ Tregs or high α4β7+ Tregs were collapsed into a single group (n = 43) and were compared to patients with both low CLA+ Tregs and low α4β7+ Tregs (n = 31). The estimated 2-year overall survival was 73.4% (95% CI, 59.7–87.1%) for patients with either high CLA+ Tregs or high α4β7+ Tregs and was 49.4% (95% CI, 31.2–67.6%) for individuals with decreased frequencies of tissue-specific Tregs (p =0.039). This survival benefit was primarily due to a decreased 2-year NRM among patients with the favorable Treg phenotype when compared to patients with both low CLA+ Tregs and low α4β7+ Tregs (7.5%; 95% CI, 2.7–17.7% vs 36.1%; 95%CI, 14.1–58.1%; p = 0.010, respectively). No difference in disease-free survival was seen between the groups (Fig. 3).
Figure 3.
Clinical outcomes stratified by Treg tissue-homing subsets. Probabilities of (A) overall survival, (B) disease-free survival, and (C) non-relapse mortality based on high CLA+ or high α4β7+ Tregs (n = 43) vs low CLA+ and low α4β7+ Tregs (n = 31). High CLA+ Tregs: CLA+ Tregs/Tregs ≥3.25%; High α4β7+ Tregs: α4β7+ Tregs/Tregs ≥21.8%.
Cox proportional hazards regression models were constructed utilizing the same covariates used in the previous logistic regression models, along with adjustment for grade II–IV aGVHD. In multivariate analysis, patients with either high CLA+ Tregs or high α4β7+ Tregs had a 56% reduction in the risk for all-cause mortality (hazard ratio = 0.44; 95% CI, 0.20–0.99; p = 0.046) and specifically an 88% decrease in the risk for NRM (hazard ratio = 0.12; 95% CI, 0.02–0.61; p = 0.011) (Table 3).
Table 3.
Cox proportional hazard regression models for overall survival and nonrelapse mortality
| Overall survival |
Nonrelapse mortality |
|||||
|---|---|---|---|---|---|---|
| Factor | Deaths/patients at risk |
HR (95% CI) | p Value | Deaths/patients at risk |
HR (95% CI) | p Value |
| Low CLA+ and low α4β7+ Tregs | 17/31 | 1 | 8/31 | 1 | ||
| High CLA+* or high α4β7+ Tregs† | 12/43 | 0.44 (0.20–0.99) | 0.046 | 2/43 | 0.12 (0.02–0.61) | 0.011 |
| Myeloablative | 14/45 | 1 | 6/45 | 1 | ||
| Reduced intensity | 15/29 | 1.23 (0.53–2.90) | 0.630 | 4/29 | 1.09 (0.22–5.53) | 0.918 |
| Related donor | 22/50 | 1 | 6/50 | 1 | ||
| Unrelated donor | 7/24 | 0.24 (0.05–1.22) | 0.085 | 4/24 | 0.22 (0.02–3.02) | 0.255 |
| Peripheral blood stem cells | 22/55 | 1 | 6/55 | 1 | ||
| Bone marrow or cord blood | 7/19 | 3.41 (0.67–17.4) | 0.140 | 4/19 | 9.78 (0.68–140) | 0.093 |
| Grade 0–I aGVHD | 8/17 | 1 | 2/17 | 1 | ||
| Grade II–IV aGVHD | 21/57 | 0.65 (0.28–1.52) | 0.324 | 8/57 | 0.70 (0.14–3.60) | 0.667 |
HR 5 hazard ratio.
High CLA+ Tregs: CLA+ Tregs/Tregs ≥3.25%.
High α4β7+ Tregs: α4β7+ Tregs/Tregs ≥21.8%.
Discussion
Tregs are recognized as an important lymphocyte population for the prevention of aGVHD in human HCT [16, 19]. Our data support this concept while further exploring the role of Treg tissue-homing subsets with organ-specific aGVHD incidence and transplant survival. The data show that increased frequencies of circulating, skin-homing (CLA+) or gut-homing (α4β7+) Tregs at engraftment are associated with a reduced incidence of skin or gut aGVHD, respectively. Furthermore, the level of expansion of either CLA+ or α4β7+ Tregs appears to be inversely related to each other, and the relative proportions of these subsets may represent an individualized set point for Treg frequencies post-transplantation. This Treg set point represents a target that is potentially modifiable via pharmacologic intervention or by cellular therapy. Indeed, our data suggested that α4β7+ Tregs subsets could be influenced by human leukocyte antigen disparity and stem cell graft source. Furthermore, these novel results also demonstrated that early expansion of CLA+ or α4β7+ Tregs was associated with improved survival and decreased NRM in patients receiving T cell replete HCT. Remarkably, the biomarkers predicted both aGVHD incidence and survival in a heterogenous patient population and the results remained significant, even after adjustment for important transplant characteristics.
These data suggest that Tregs are not a single, uniform subset of T cells, but rather a highly diversified cell population, each with distinct characteristics and functional properties [19, 20,23]. Similar to other T cell subsets, the current studies indicate that Tregs can be divided into several tissue-specific groups, including skin- or gut-homing populations. This compartmentalization appears to occur during the early phases of immune reconstitution, which may have important implications for determining aGVHD organ involvement and long-term morbidity and mortality after HCT.
It is increasingly clear that Tregs must migrate from the circulation to either secondary lymphoid organs or to other peripheral tissues to maintain their regulatory control over the immune system. Currently, there is ongoing debate about where Tregs exert their suppressive effect during HCT. In animal models of transplantation, Tregs initially accumulate in secondary lymphoid organs and prevent proliferation of alloreactive T cells there, and then they migrate to aGVHD target tissues [17]. In addition, it was previously shown that only the CD62L+ (lymph node– homing) population of Tregs prevented aGVHD in the mouse, thus further supporting the lymph node as the principal biological site for aGVHD suppression [13, 14].
Human studies suggest that Treg localization in cutaneous or gut tissues is important for the suppression of aGVHD at those sites [24, 25]. Presumably, these cells migrated from secondary lymphoid organs to skin or gut as directed by expression of adhesion molecules, however, tissue-specific homing markers were not specifically analyzed in those studies. In our current analysis, high frequencies of circulating CLA+ or α4β7+ Tregs were associated with the prevention of skin or gut aGVHD, which supports the hypothesis that aGVHD suppression occurs, at least in part, at a local tissue level in humans. Alternatively, because Tregs activated in peripheral lymph nodes up-regulate CLA, while those activated in mesenteric lymph nodes more often express α4β7our analysis may simply reflect the secondary lymph node location in which the Treg was initially induced to acquire suppressive function, as opposed to the final destination of the Treg. To help answer this question, future experiments should determine whether circulating CLA+ or α4β7+ Tregs in the blood of HCT recipients correlate with the proportion of Foxp3+ cells infiltrating skin or gut biopsies of patients with aGVHD, respectively. Adding another layer of complexity, it was shown recently in an islet allograft model that Tregs needed to migrate from the transplanted tissue to the draining lymph node to suppress graft rejection [26]. Tregs failing to first localize in the transplanted tissue due to lack of homing receptors did not prolong graft survival. These data imply that Treg trafficking to both secondary lymphoid organs and to peripheral tissues could be important for determining aGVHD outcomes. The molecular basis for control of this migration requires further elucidation.
Although we identified a relationship between Tregs and organ-specific aGVHD outcomes, not all studies have arrived at the same conclusions. In a recent report, neither the circulating percentage of Tregs nor the infiltration pattern of Foxp3+ cells in gut biopsies correlated with the prevention of gastrointestinal aGVHD [27]. There were significant differences between the studies with respect to Treg subset identification. We focused our analysis on organ-specific subsets of Tregs to identify the association. However, when investigating the total number of circulating Treg cells in a way that did not account for homing properties, the results were similar with the other study. No relationship was found for total number of circulating Tregs with either skin or gut aGVHD. As we move forward with therapeutic clinical trials aimed at increasing Treg numbers post-transplantation as a means to prevent or treat aGVHD, it will be important that we understand how specific Treg populations influence aGVHD outcomes and long-term survival.
The majority of adult patients undergoing HCT at this institution were entered into the trial. Within this population, we report a high incidence of grade II–IV aGVHD with a predominance of gastrointestinal involvement. Other groups also have reported increasing diagnosis of gut aGVHD, likely due to early esophagogastroduodenoscopy and less reliance on total parenteral nutrition [28, 29]. However, the possibility that gut aGVHD was overdiagnosed while cutaneous aGVHD was underdiagnosed in this study certainly exists, thus underscoring the need for prospective multicenter trials in aGVHD.
Conclusions
These data supports our hypothesis that Treg tissue compartmentalization is important for determining both organ-specific aGVHD incidence and survival after T-cell replete HCT. In this analysis, high frequencies of CLA+ or α4β7+ Tregs at engraftment were associated with prevention of skin or gut aGVHD during the first 100 days of transplant, respectively. Despite no definite correlation between Tregs and aGVHD severity, high frequencies of CLA+ or α4β7+ Tregs continued to be associated with improved survival and decreased NRM. If validated, a level of < or ≥75th percentile of CLA+ or α4β7+ Tregs could be used to stratify a patient’s risk for either skin or gut aGVHD, or even NRM. The mechanism by which Tregs may decrease mortality is not clear because not all studies have shown a correlation between reduced aGVHD incidence and superior survival [30, 31].
The purpose of the current study was to identify a cutoff value corresponding to high or protective Treg frequencies to facilitate future clinical trials examining Tregs in the post-transplantation setting. Ideally, if subclinical aGVHD could be identified, then prophylaxis strategies could be intensified or directed to a particular tissue at an earlier stage, thereby preventing clinical manifestations of aGVHD, limiting patient exposure to high-dose corticosteroids, and improving transplant outcomes. In addition, a level of ≥75th percentile of CLA+ or α4β7+ Tregs at engraftment could be used as an end point or benchmark in future clinical trials examining novel therapeutic strategies aimed at increasing Tregs, preventing aGVHD, and improving HCT survival.
Supplementary Material
Supplementary Figure E1. Identification of CLA+ and α4β7+ Tregs using 10-color multiparametric flow cytometry. (A) Peripheral blood mononuclear cells were isolated at the time of neutrophil recovery after allogeneic hematopoietic cell transplantation. Cells staining with the viability marker or CD14 were excluded and CD3+ cells were selected (data not shown). Live CD4+CD8− T cells were identified for additional immunophenotyping. (B) The total CD4+ T-cell population (from A) was used to generate serial two-parameter comparisons between Foxp3 and CD127, CD45RO and CD127, and α4β7 and CLA. (C) CD25+Foxp3+ Tregs were isolated from the CD4+ population (A). The gating strategies generated from (B) were then sequentially applied to the CD25+Foxp3+ Treg population to determine percentages of Treg tissue-homing subsets.
Acknowledgments
Funding disclosure
This work was supported by the National Institutes of Health/National Cancer Institute Grant K12 CA090625, the American Cancer Society–Institutional Research Grant (#IRG-58-009-48) and the Sartain-Lanier Family Foundation.
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.exphem.2012.08.002.
Conflict of interest disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Arai S, Vogelsang GB. Management of graft-versus-host disease. Blood Rev. 2000;14:190–204. doi: 10.1054/blre.2000.0137. [DOI] [PubMed] [Google Scholar]
- 2.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 3.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi-Bin C, Kim HT, McDonough S. Up-regulation of α4β7 integrin on peripheral T cell subsets correlates with the development of acute intestinal graft-versus-host disease following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1066–1076. doi: 10.1016/j.bbmt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 7.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Hauben E, Bacchetta R, Roncarolo MG. Utilizing regulatory T cells to control alloreactivity. Cytotherapy. 2005;7:158–165. doi: 10.1080/14653240510018154. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus- host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 13.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 14.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JL, Salomon BL. Therapeutic potential of CD4+CD25+ regulatory T cells in allogeneic transplantation. Cytotherapy. 2005;7:166–170. doi: 10.1080/14653240510018145. [DOI] [PubMed] [Google Scholar]
- 16.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 18.Kim CH. Migration and function of FoxP3+ regulatory T cells in the hematolymphoid system. Exp Hematol. 2006;34:1033–1040. doi: 10.1016/j.exphem.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt BG, Crowe JE., JR Homing in on acute graft vs. host disease: tissue-specific T regulatory and Th17 cells. Curr Top Microbiol Immunol. 2010;341:121–146. doi: 10.1007/82_2010_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelhardt BG, Jagasia M, Savani BN, et al. Regulatory T cell expression of CLA or alpha(4)beta(7) and skin or gut acute GVHD outcomes. Bone Marrow Transplant. 2010;46:436–442. doi: 10.1038/bmt.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przepiorka D,Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 23.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 25.Fondi C, Nozzoli C, Benemei S, et al. Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant. 2009;15:938–947. doi: 10.1016/j.bbmt.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord JD, Hackman RC, Gooley TA, et al. Blood and gastric FOXP3(+) T cells are not decreased in human gastric graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:486–496. doi: 10.1016/j.bbmt.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 30.Chao NJ, Schmidt GM, Niland JC, et al. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–1230. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- 31.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure E1. Identification of CLA+ and α4β7+ Tregs using 10-color multiparametric flow cytometry. (A) Peripheral blood mononuclear cells were isolated at the time of neutrophil recovery after allogeneic hematopoietic cell transplantation. Cells staining with the viability marker or CD14 were excluded and CD3+ cells were selected (data not shown). Live CD4+CD8− T cells were identified for additional immunophenotyping. (B) The total CD4+ T-cell population (from A) was used to generate serial two-parameter comparisons between Foxp3 and CD127, CD45RO and CD127, and α4β7 and CLA. (C) CD25+Foxp3+ Tregs were isolated from the CD4+ population (A). The gating strategies generated from (B) were then sequentially applied to the CD25+Foxp3+ Treg population to determine percentages of Treg tissue-homing subsets.