Abstract
Mamajjaka (Enicostemma littorale Auct. non Bl) is a well known folklore medicine frequently used for the treatment of Madhumeha (diabetes mellitus). There is no direct reference available for its antihyperglycaemic activity in Ayurvedic classics. Considering this, a study is planned towards developing pharmaceutical standardization of Mamajjaka Ghana. In this study, five batches of Mamajjaka Ghana were prepared and findings were systematically recorded to maintain the Standard Operating Procedure (SOP). An average of 14.78% Ghana was obtained. The physico-chemical parameters, qualitative test for various functional groups, quantitative estimation of total alkaloids, HPTLC profile, heavy metal analysis and microbial overload were carried out of Mamajjaka Ghana.
Keywords: Enicostemma littorale, Ghana, Kwatha, Mamajjaka
Introduction
Considering the significance of traditional practices in global health care, WHO has been encouraging and promoting traditional practices since past few decades. Hence, the standardization of raw drugs, processing, finished products, verification of the claims, mechanism of action, products that are free from heavy metal and microbial contamination, etc., have became major issues, which are to be taken into consideration in order to increase the global acceptability of herbal drugs and also to prove their respective clinical efficacy. It is very tedious to standardize Ayurvedic herbal formulations due to number of factors. The non availability of reference standards also hurdles the study. In spite of that, the present task is undertaken to evaluate and to compare the formulation with the available physicochemical parameters.
Mamajjaka (Enicostemma littorale Auct. non Bl) is one of the herbs used in Madhumeha traditionally in Gujarat, Madhya Pradesh, and Rajasthan. Ghana is a widely acceptable dosage form in the present scenario due to its advantages like palatability, shelf life, easy administration, etc. Keeping this in view, Mamajjaka Ghana was prepared to develop Standard Operating Procedure (SOP) and analytical profile.
Materials and Methods
Collection of raw materials
The raw drug was procured from Pharmacy, Gujarat Ayurved University, Jamnagar, Gujarat, India, and authenticated at the Pharmacognosy Laboratory, IPGT and RA, Gujarat Ayurved University, Jamnagar. Mamajjaka was powdered in grinding mill and passed through sieve no. 8. Following general principles, 2kg coarse powder of Mamajjaka was added with 16 parts (32 l) of potable water[1] in a stainless steel vessel and the contents were soaked overnight (12 h). The next day, Kwatha was prepared by applying constant mild heat to the mixture to facilitate evaporation and intermediate stirring was carried out till the volume reduced up to one-eighth of the initial quantity. After desirable reduction of volume, the Kwatha was filtered through four folded cotton cloth and collected in a separate vessel for further processing. The residue was discarded.
The prepared Kwatha was subjected to further heating on a gas stove maintaining the temperature between 70°C and 75°C till a semisolid consistency was obtained. Then the material was shifted into a glass tray and placed in a hot-air oven at 45°-50°C for complete drying. After complete drying, the content were grinded in a mixer grinder, collected in duly labeled air-packed glass bottle. Further four additional batches were prepared in order to ensure the Standard Manufacturing Procedure.
Analytical study
Mamajjaka Kwatha Churna, Mamajjaka Kwatha, and Mamajjaka Ghana were analyzed by employing various analytical parameters. Organoleptic characteristics (color, odor, taste) and physico-chemical analysis like loss on drying at 110°C,[2] ash value,[3] acid insoluble ash,[4] pH value,[5] specific gravity at 40°C,[6] total solid content,[7] water soluble extractives,[8] methanol soluble extractives[9] were carried out. Mamajjaka Ghana was subjected to further higher analysis, namely, qualitative test for various functional groups[10, 11] and quantitative estimation of total alkaloids.[12] Tests for presence of Heavy metals[13] (lead, arsenic, and mercury) and Microbial contamination[14] (a bacterial and fungal growth study) were carried out.
HPTLC profile
Test solution[15]: Five grams of the finely powdered plant material was defatted with 25 ml of solvent ether and then refluxed with 25 ml of methanol for 25 min on water bath consecutively three times; it was filtered and the solvent was removed under reduced pressure. Then 25 mg of the extractive was dissolved in 10 ml of methanol. Toluene: ethyl acetate: formic acid: methanol (6:3:0.1:1% v/v) was selected as the solvent system after multiple trial-and-error method. The developed plate was visualized under visible daylight, short UV (254 nm), long UV (366 nm), and after spraying with the anisaldehyde–sulfuric acid reagent was again observed in daylight.
Results and Discussion
Initially, liquid of the Mamajjaka Kwatha was light brown in color and bitter in taste. Evaporation was started at 70°C, which was aggravated on stirring. An average 9.06 h time was required for the preparation of Mamajjaka Kwatha. After 2 h heating of Mamajjaka Kwatha, mild sticky nature was observed. After drying in the oven, the dark brown semisolid material converted into a blackish brown solid material.
The degree of size reduction depends upon the structure of the drug. Particle size reduction facilitates adequate mass transfer for better extraction. To facilitate further extraction of water soluble constituents, overnight soaking was done, which allow micelles to take up liquid film and tissue swelling. Swelling also results from distension and bursting of thin-walled cells that have taken up the liquid by osmosis. During processing, the application of mild heat is required with occasional stirring to avoid destruction of the components sensitive to higher temperature. During the preparation of Mamajjaka Kwatha, maximum temperature was between 90°C and 95°C.
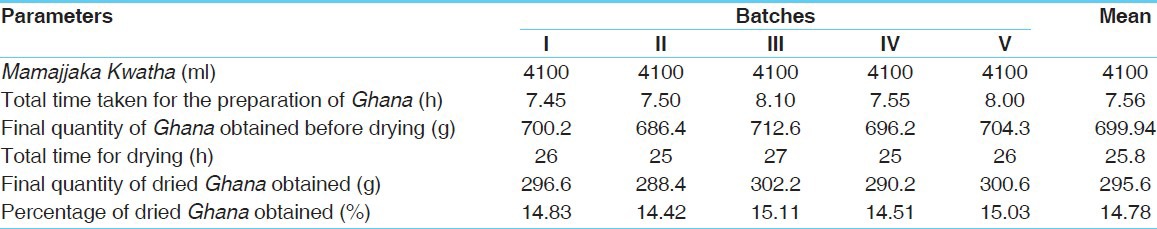
Kwatha was further reheated and converted into a semisolid material, to remove watery portion; in the conversion state, (from semisolid to solid) continuous, mild heat (70-75°C) was applied and stirring was done very cautiously to avoid burning of the material. After 3 h of heating, the liquid became stickier. After attaining semisolid consistency, the contents were transferred into a glass tray and were spreaded as a uniform layer. Finally, for complete drying, the tray was placed in an oven at 45-50°C. After complete drying, the contents were collected and stored in air tight container for further use. The average time required for the preparation of Kwatha was 9.06 h, while for Ghana it was 7.56 h. The average yield of Kwatha and Ghana was 4.15 l and 295.6 g (14.78%), respectively. The observations of Kwatha and Ghana preparation are placed at Tables 1 and 2.
Table 1.
Observation and result obtained during preparation of Mamajjaka Kwatha
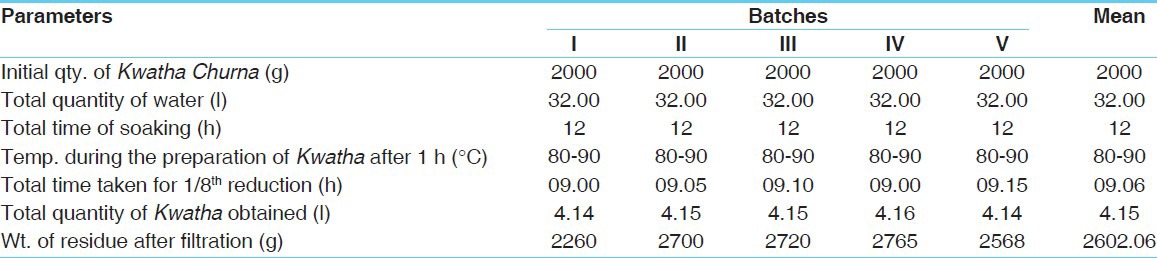
Table 2.
Observations and results obtained during preparation of Mamajjaka Ghana
The organoleptic characters of Mamajjaka Kwatha Churna are tabulated at Table 3. Observations of the analytical parameters are tabulated at Tables 4-8.
Table 3.
Organoleptic characteristics of Mamajjaka Kwatha Churna

Table 4.
Physico-chemical data of Mamajjaka Kwatha Churna
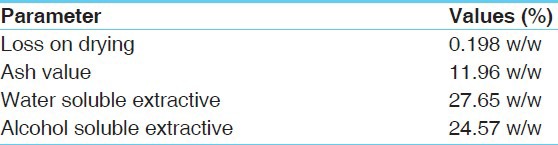
Table 5.
Organoleptic characteristics of Mamajjaka Kwatha
Table 6.
Physico-chemical data of Mamajjaka Kwatha
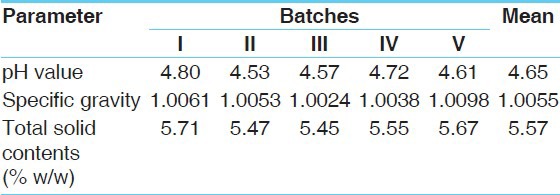
Table 7.
Organoleptic characteristics of Mamajjaka Ghana

Table 8.
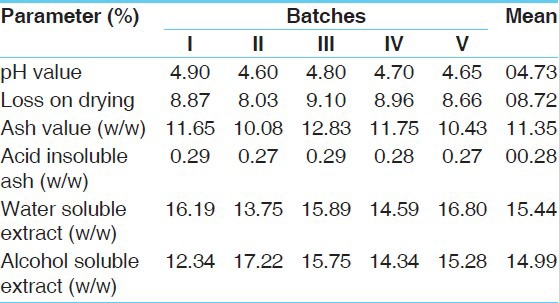
Physicochemical data of Mamajjaka Ghana
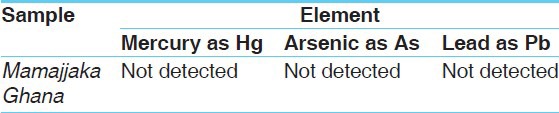
Qualitative tests were done to detect the presence of functional groups. The study reveals the presence of alkaloids, glycosides, saponins and flavonoids in all five batches of both the formulations whereas an absence of starch, tannin, and phenol was observed [Table 9]. The total alkaloid content observed in Mamajjaka Ghana was 0.009% [Table 10]. Observations on microbial contamination and heavy metal analysis were found to be in permissible limits [Tables 11 and 12]
Table 9.
Qualitative tests for various functional groups of Mamajjaka Ghana
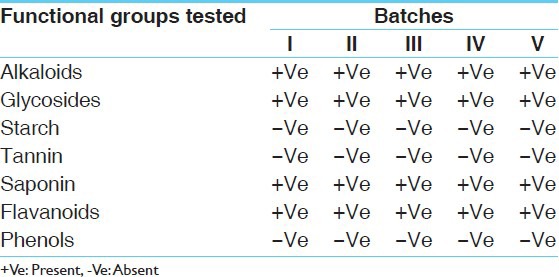
Table 10.
Percentage of the total alkaloid content of Mamajjaka Ghana

Table 11.
Pathogen and total microbial count of Mamajjaka Ghana

Table 12.
Heavy metal analysis of Mamajjaka Ghana,
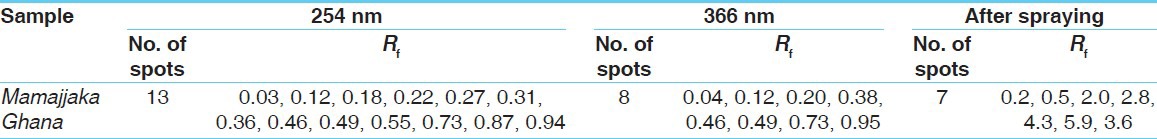
In the absence of the marker compound, the HPTLC profile of Mamajjaka Ghana was attempted [Figures 1 and 2]. Visual spots and Rfvalues were recorded [Table 13]. This particular study can be considered as standard for further research work.
Figure 1.
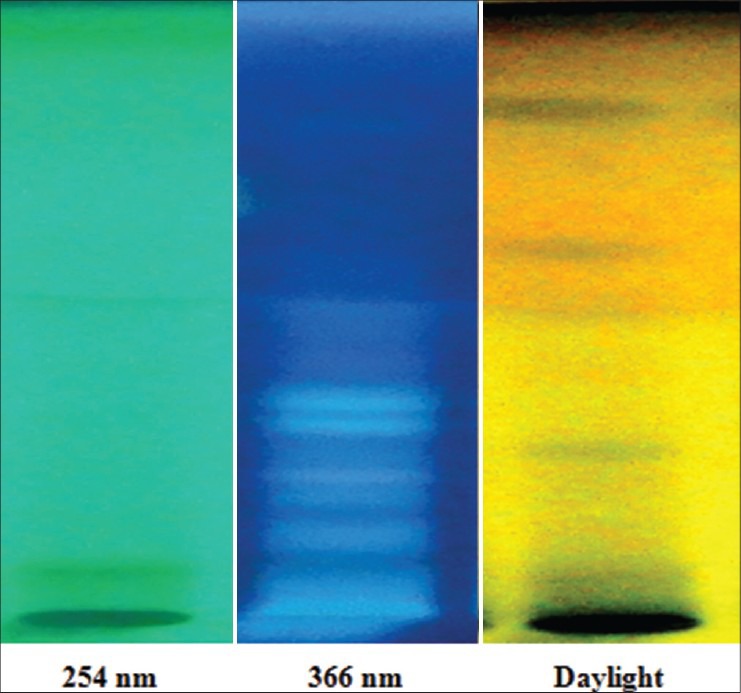
Visualization of Mamajjaka Ghanaat 254 nm, 366 nm, and in daylight
Figure 2.
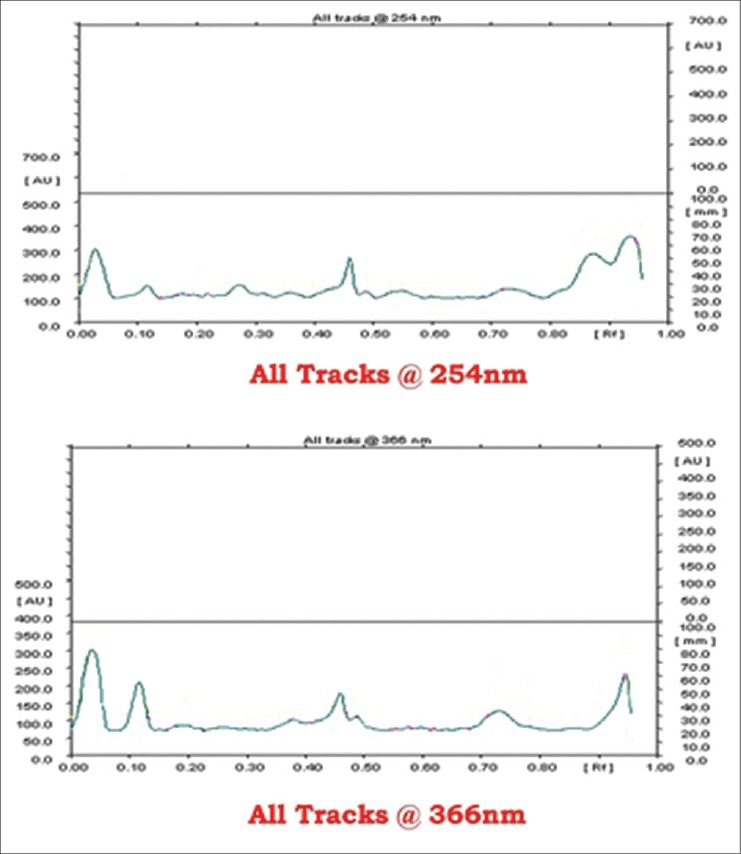
Densitometry of Mamajjaka Ghanaat 254 nm and 366 nm
Table 13.
HPTLC profi le of Mamajjaka Ghana
Conclusion
Mamajjaka is a widely used traditional herb in the present era for its antidiabetic and antipyretic attributes. The pharmaceutical standardization of this herb ensures the uniformity of the preparation, thereby proving its global acceptance. Physicochemical and phyto-chemical data as well as HPTLC, heavy metal profile, and microbial overload are essential parameters followed to develop SOP. The data generated by this particular work can be taken as standard for the preparation of Mamajjaka Ghana at a laboratory scale.
Acknowledgment
The authors are thankful to Shrey Pathology Lab. (ISO 9001:2008 Certified Lab.), and SICART (Anand, Gujarat) for the help provided in performing relevant parts of the study.
References
- 1.Sharangadhara Sharangadhara Samhita, Madyama Khanda, (6th ed) 2005;2(1) [Google Scholar]; Pandit Parasuram Shastri, Vidyasagar, Chaukhamba Orientalia. Varanasi; p. 144. [Google Scholar]
- 2.Anonymus. The Ayurvedic Pharmacopoeia of India, Ministry of Health and Family Welfare, Gov.of India, Part 1, 1999:214(2.2.9). [Google Scholar]
- 3.Ibid. The Ayurvedic Pharmacopoeia of India, Part -1. 1(Appendix-2):213(2.2.3). [Google Scholar]
- 4.Ibid. The Ayurvedic Pharmacopoeia of India, Part -1. 1(Appendix-2):213(2.2.4). [Google Scholar]
- 5.Ibid. The Ayurvedic Pharmacopoeia of India, Part -1. 1(Appendix-2):213(3.3). [Google Scholar]
- 6.Ibid. The Ayurvedic Pharmacopoeia of India, Part -1. 1(Appendix-2):213(3.7). [Google Scholar]
- 7.Ibid. The Ayurvedic Pharmacopoeia of India, Part -1. 1(Appendix-2):213(2.2.9). [Google Scholar]
- 8.Ibid. The Ayurvedic Pharmacopoeia of India, Part -1. 1(Appendix-2):213(2.2.7). [Google Scholar]
- 9.Ibid. The Ayurvedic Pharmacopoeia of India, Part -1. 1(Appendix-2):213(2.2.6). [Google Scholar]
- 10.Baxi AJ, Shukla VJ, Bhatt UB. Methods of qualitative testing of some Ayurvedic formulations, 1st ed. Jamnagar: Gujarat Ayurved University; 2001. pp. 5–12. [Google Scholar]
- 11.Khandelwal KR. Practical Pharmacognosy Techniques and Experimental Nirali Prakashan. 16th ed. Pune: 2006. pp. 149–56. [Google Scholar]
- 12.Sim Stephen K. Medicinal plant alkaloids: An introduction for Pharmacy students. 2nd ed. University of Toronto press; 1965. p. 44. [Google Scholar]
- 13.Anonymous. Quality control methods for medicinal plant materials, 1. W.H.O., Pharm,; 1992. pp. 46–48. [Google Scholar]
- 14.Anonymous. Quality control methods for medicinal plant materials, W.H.O. New Delhi: AITBS Publishers and Distributors (Regd); Quality control methods for medicinal plant materials, WHO, (An Authorized publication of World Health Organization, Geneva); 2002. pp. 48–55. [Google Scholar]
- 15.Quality standards of Indian Medicinal Plants. 1st ed. New Delhi: Indian Council of Medical Research; 2005. p. 208. [Google Scholar]


