Abstract
Background
We aimed to describe clinicopathological features, patterns of recurrence, and survival according to breast cancer subtype, with a focus on triple-negative tumors.
Methods
We evaluated 15,204 women presenting to NCCN centers with stage I-III breast cancer between January 2000 and December 2006. Tumors were classified as hormone receptor positive [HR+]/HER2− (ER+ and/or PR+, and HER2−), HER2+ (HER2+, any ER or PR), or triple-negative (ER−, PR−, and HER2−).
Results
Subtype distribution was: triple-negative 17% (n=2,569), HER2+ 17% (n=2,602), HR+/HER2− 66% (n=10,033). Triple-negative subtype was more frequent in African-Americans, compared with Caucasians (adjusted odds ratio [OR] 1.98; p<0.0001). Premenopausal, but not postmenopausal, women with high body mass index had an increased likelihood of triple negative subtype (p=0.02). Women with triple-negative cancers were less likely to present on the basis of an abnormal screening mammogram (29% vs. 48%, p<0.0001), more likely to present with higher T stage, but less likely to have nodal involvement. Relative to HR+/HER2− tumors, triple-negative tumors were associated with a higher risk of brain or lung metastases, and had worse breast cancer-specific and overall survival, even after adjusting for age, stage, race, grade, and receipt of adjuvant chemotherapy (adjusted hazard ratio [HR] for overall survival 2.72, 95% CI 2.39–3.10, p<0.0001). The difference in risk of death by subtype was most dramatic within the first two years after diagnosis (HR for OS for 0 to 2 yrs 6.10 [95% CI 4.81, 7.74]).
Conclusions
Triple-negative tumors are associated with unique risk factors and worse outcomes compared to HR+/HER2− tumors.
Keywords: Triple-negative, basal-like, breast cancer, outcomes, brain metastases, obesity, race
BACKGROUND
Breast cancer is comprised of multiple biological subtypes that can be approximated using standard immunohistochemical (IHC) markers.1 The majority of triple-negative tumors (that is, tumors which are ER−, PR−, and HER2−negative) cluster with the basal subset, and are associated with a high rate of distant relapse.2, 3
Several studies have examined characteristics associated with triple-negative subtype.4–7 Triple-negative cancers comprise a higher proportion of breast cancers in African-American women.4, 7 Other associations with the triple negative subtype include higher parity and lack of breast-feeding; associations with obesity have been inconsistent.5, 8–13 Patients with triple-negative cancers tend to present at a younger age and with more advanced cancer; however, the contribution of tumor subtype to the risk of nodal involvement is less well-defined.4, 6, 14 With respect to patterns of recurrence, central nervous system (CNS) disease is a concern.15, 16
Identification of factors associated with triple-negative subtype is hampered by the absence of data on large populations. With few exceptions, population and hospital cancer registries, a key source of such data, did not routinely collect tumor HER2 status until recently. Since 1997, the National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes Database has collected data on women with newly diagnosed breast cancer presenting to many of its member institutions across the United States. HER2 status by IHC was added to the NCCN data as a routine element in 1999; HER2 status by fluorescence in situ hybridization (FISH) was added in 2001. Demographic, treatment, and outcome information also are available. While not a population-based cohort, the large size of the database and varied patient population allows for investigation of clinical predictors of triple-negative cancer and a detailed description of its behavior.
SUBJECTS AND METHODS
Data Source
Data are collected prospectively within the NCCN Database primarily through review of medical records and institutional tumor registries by trained abstractors. Vital status and cause of death are ascertained from medical records and confirmed using the Social Security Death Index and National Death Index (NDI). If cause of death is unknown based on the medical record, information from the NDI is used in its place. Data are subjected to rigorous quality assurance.17 Institutional review boards from each center approved the study, data collection, transmission, and storage protocols. At centers where institutional review boards require signed informed consent for data collection, only patients who consented are included in the database.
Patient Selection
Patients were included if they presented with newly diagnosed, stage I–III, unilateral, invasive breast cancer between January 1, 2000 and December 31, 2006 at one of eight NCCN institutions: Arthur G. James Cancer Hospital at Ohio State University (Columbus, OH), City of Hope Comprehensive Cancer Center (Duarte, CA), Dana-Farber Cancer Institute (Boston, MA), Fox Chase Cancer Center (Philadelphia, PA), H. Lee Moffitt Cancer Center (Tampa, FL), Roswell Park Cancer Institute (Buffalo, NY), The University of Texas M.D. Anderson Cancer Center (Houston, TX), and University of Michigan Comprehensive Cancer Center (Ann Arbor, MI). From 17,510 potentially eligible patients, we excluded patients with previous malignancies (n=1,336), unknown ER, PR, HER2 status (n=868), or who did not have invasive cancer within the breast (n=102), leaving an analysis cohort of 15,204 patients.
Variables of Interest
Tumor characteristics
The database contains information on tumor size, nodal status, grade, lymphovascular invasion, extensive intraductal component, ER and PR status, and HER2 status, as abstracted from pathology reports. Stage is assigned according to the version of the American Joint Committee on Cancer Staging Manual applicable at the time of diagnosis. For this analysis, tumor grade was categorized as high (according to histologic grade, or, if not available, by nuclear grade) or low-intermediate.
Data collected on HER2 status has changed over time. Prior to March 1, 2001, the only information recorded was the result of IHC, categorized as positive or negative. Since March 2001, both IHC (recorded on a scale from 0 to 3+) and FISH (recorded as positive or negative) results have been collected. We used the FISH result, if available. If only IHC was available, 3+, “high positive”, or “positive NOS” were considered HER2−positive; while 2+, 1+, 0, or “negative” were considered HER2−negative. Of note, only ~2% of patients in the database were coded as 2+ IHC without available FISH results.
Patient characteristics
The following variables were collected by chart review: age at diagnosis, height and weight, sites of recurrence, treatment types, and vital status. Body mass index (BMI) was calculated as weight[kg]/{height[m]2} and grouped according to categories defined by the National Heart, Lung, and Blood Institute as follows: <18.5 kg/m2 underweight; 18.5–24.9 normal; 25–29.9 overweight, ≥30 obese.
Data on race, ethnicity, and menopausal status came from patient surveys conducted at the time of initial presentation to the NCCN center. Patients were considered postmenopausal if they were amenorrheic for > 6 months prior to breast cancer diagnosis, were taking hormone replacement therapy, or were ≥ 50 years of age without a documented menopausal status in their medical record or baseline patient survey.
Definition of Breast Cancer Subtypes
Triple-negative tumors were defined as tumors that were ER−, PR−, and HER2−negative. HER2−positive tumors included both ER-positive and ER-negative tumors. HR+/HER2− tumors were defined as ER-positive and/or PR-positive, and HER2−negative.
Statistical Analyses
Clinico-pathological variables were tabulated by tumor subtype and proportions across subtypes were compared using Chi-Square tests. We constructed univariate followed by multivariable logistic regression models to identify factors associated with triple-negative subtype and for the risk of node positivity. Univariate logistic regression estimated risk of sites of recurrence among those diagnosed with a recurrence. Follow up for survival analysis was defined as time in years from tumor diagnosis to date of death or last known vital status date. Breast cancer specific survival (BCSS) was determined by identifying cause of death due to breast cancer based on International Statistical Classification of Disease codes. Kaplan-Meier (KM) analysis was utilized to compare OS and BCSS between triple-negative versus HR+/HER2− tumors. Cox proportional hazards regression was used to calculate hazard ratios (HRs) and their associated 95% confidence interval (95% CI) to estimate risk of any death and breast cancer specific death for triple-negative versus HR+/HER2− tumors adjusting for age (<50, ≥50), stage (I, II, III), race (Caucasian, AA, Other), adjuvant chemotherapy (Y/N), tumor size (≤2cm, >2cm), histologic grade (Low/Int, High, Unknown) and nodal status (positive, negative). It is known that risk of death over time in these tumor subtypes is non-proportional. Several techniques were applied to verify the non-proportionality of tumor subtype and to assess the proportionality of each of the model covariates. Since risk of death between tumor subtypes was not proportional, HRs were calculated for the entire follow-up period in addition to the following time windows, 0 to 2 years from diagnosis, 2 to 6 years and 6 or more years from diagnosis to the end of the follow up period. These time points were chosen based on review of KM survival curves comparing tumor subtype. All statistical analyses were performed using SAS 9.2.
RESULTS
Description of Study Cohort
We identified 15,204 women who were eligible for inclusion. Subtype distribution was: triple-negative 17% (n=2,569), HER2+ 17% (n=2,602), HR+/HER2− 66% (n=10,033). As shown in Table 1, 82% of patients identified themselves as Caucasian/non-Hispanic, 8% as African-American, 7% as Hispanic, and 3% as Asian/Pacific Islander. Mean follow-up time was 3.06 years (median 2.6 years, range 0–8.5 years).
Table 1.
Patient Demographics and Clinicopathological Characteristics
| Characteristic | All Cases | Triple-Negative | HER2+ | HR+/HER2− | p-value |
|---|---|---|---|---|---|
|
| |||||
| N (%) | 15,204 | 2,569 (17) | 2,602 (17) | 10,033 (66) | |
|
| |||||
| Age, mean (SD), y | 55 (12) | 52 (12) | 52 (12) | 56 (12) | <0.001 |
|
| |||||
| Years of Follow-up from Presentation, mean (SD), y | 3.1 (2.0) | 2.9 (2.0) | 3.1 (2.0) | 3.1 (2.1) | <0.001 |
|
| |||||
| Race/Ethnicity, N (%) | <0.001 | ||||
|
| |||||
| Caucasian | 12,406 (82) | 1,953 (76) | 2,059 (79) | 8,394 (84) | |
|
| |||||
| African-American | 1,142 (8) | 330 (13) | 174 (8) | 618 (6) | |
|
| |||||
| Hispanic | 995 (7) | 185 (7) | 209 (8) | 601 (6) | |
|
| |||||
| Asian/Pacific Islander | 443 (3) | 67 (3) | 97 (4) | 279 (3) | |
|
| |||||
| Other/Unknown | 218 (1) | 34 (1) | 43 (2) | 141 (1) | |
|
| |||||
| Menopausal status, N (%) | <0.001 | ||||
|
| |||||
| Premenopausal | 6,175 (41) | 1,137 (44) | 1,216 (47) | 3,822 (38) | |
|
| |||||
| Postmenopausal | 9,029 (59) | 1,432 (56) | 1,386 (53) | 6,211 (62) | |
|
| |||||
| Body Mass Index at Presentation, N (%) | <0.001 | ||||
|
| |||||
| <18.5 kg/m2 (Underweight) | 228 (1.5) | 34 (1) | 36 (1) | 158 (2) | |
|
| |||||
| 18.5 to <25 kg/m2 (Normal) | 5,606 (37) | 879 (34) | 1,019 (39) | 3,708 (37) | |
|
| |||||
| 25 to <30 kg/m2 (Overweight) | 4,442 (29) | 740 (29) | 736 (28) | 2,966 (30) | |
|
| |||||
| ≥30 kg/m2 (Obese) | 4,366 (29) | 835 (33) | 713 (27) | 2,818 (28) | |
|
| |||||
| Missing | 562 (4) | 81 (3) | 98 (4) | 383 (4) | |
|
| |||||
| Method of Detection | <0.001 | ||||
|
| |||||
| Abnormal screening mammogram | 6,472 (43) | 735 (29) | 883 (34) | 4,854 (48) | |
|
| |||||
| Symptoms | 8,158 (54) | 1,745 (68) | 1,591 (61) | 4,822 (48) | |
|
| |||||
| Other | 466 (3) | 67 (3) | 107 (4) | 292 (3) | |
|
| |||||
| Unknown | 108 (<1) | 22 (<1) | 21 (<1) | 65 (<1) | |
|
| |||||
| Tumor size, mean (SD), cm | 1.9 (1.6) | 2.2 (1.8) | 2.0 (1.8) | 1.8 (1.5) | <0.001 |
|
| |||||
| T stage, category, N (%) | <0.001 | ||||
|
| |||||
| T1 | 9,258 (61) | 1,187 (46) | 1,338 (51) | 6,733 (67) | |
|
| |||||
| T2 | 4,504 (30) | 1,036 (40) | 892 (34) | 2,576 (26) | |
|
| |||||
| T3 | 818 (5) | 192 (7) | 196 (8) | 430 (4) | |
|
| |||||
| T4 | 613 (4) | 151 (6) | 171 (7) | 291 (3) | |
|
| |||||
| Unknown | 11 (<1) | 3 (<1) | 5 (<1) | 3 (<1) | |
|
| |||||
| Nodal status | <0.001 | ||||
|
| |||||
| Positive | 5,953 (39) | 975 (38) | 1,162 (45) | 3,816 (38) | |
|
| |||||
| Negative | 9,233 (61) | 1,593 (62) | 1,438 (55) | 6,202 (62) | |
|
| |||||
| Nodes not assessed | 18 (<1) | 1 (<1) | 2 (<1) | 15 (<1) | |
|
| |||||
| AJCC Stage | <0.001 | ||||
|
| |||||
| I | 6,688 (44) | 840 (33) | 883 (34) | 4,965 (49) | |
|
| |||||
| II | 6,306 (41) | 1,274 (50) | 1,146 (44) | 3,886 (39) | |
|
| |||||
| III | 2,210 (15) | 455 (18) | 573 (22) | 1,182 (12) | |
|
| |||||
| Histology | <0.001 | ||||
|
| |||||
| Invasive ductal | 11,942 (79) | 2,379 (93) | 2,359 (91) | 7,204 (72) | |
|
| |||||
| Invasive lobular | 1,379 (9) | 59 (2) | 91 (3) | 1,229 (12) | |
|
| |||||
| Mixed ductal/lobular | 1,260 (8) | 47 (2) | 118 (5) | 1,095 (11) | |
|
| |||||
| Other (tubular, colloid, medullary, adenocystic) | 623 (4) | 84 (3) | 34 (1) | 505 (5) | |
|
| |||||
| Histologic Grade | <0.001 | ||||
|
| |||||
| Low/intermediate | 7,896 (52) | 347 (14) | 704 (27) | 6,845 (68) | |
|
| |||||
| High | 6,583 (43) | 2,123 (83) | 1,783 (69) | 2,677 (27) | |
|
| |||||
| Other | 5 (<1) | 3 (<1) | 1 (<1) | 1 (<1) | |
|
| |||||
| Unknown | 720 (5) | 96 (4) | 114 (4) | 510 (5) | |
|
| |||||
| Presence of LVI | <0.001 | ||||
|
| |||||
| Yes | 3,755 (25) | 663 (26) | 933 (36) | 2,159 (22) | |
|
| |||||
| No | 11,031 (73) | 1,814 (71) | 1,591 (61) | 7,626 (76) | |
|
| |||||
| Unknown | 418 (3) | 92 (4) | 78 (3) | 248 (2) | |
|
| |||||
| Presence of EIC | <0.001 | ||||
|
| |||||
| Yes | 1,861 (12) | 235 (9) | 482 (19) | 1,144 (11) | |
|
| |||||
| No | 13,343 (88) | 2,334 (91) | 2,120 (81) | 8,889 (89) | |
|
| |||||
| Chemotherapy | <0.001 | ||||
| Neoadjuvant only | 1,901 (13) | 520 (20) | 452 (17) | 929 (9) | |
| Adjuvant only | 6,859 (45) | 1,457 (57) | 1,400 (54) | 4,002 (40) | |
| Both neoadjuvant and adjuvant | 629 (4) | 158 (6) | 221 (8) | 250 (2) | |
| None | 5,815 (38) | 434 (17) | 529 (20) | 4,852 (48) | |
Note: Due to rounding error, some of the percentages do not total 100. LVI, lymphovascular invasion; EIC, extensive intraductal component.
Presenting Characteristics
Compared to patients with HR+/HER2− tumors, patients with triple-negative tumors were less likely to present on the basis of an abnormal screening mammogram (48% vs 29%, p<0.0001) (Table 1). Over two-thirds of patients with triple negative tumors initially presented with symptoms, most commonly, a self-detected breast mass. Patients with triple-negative tumors were also less likely to present with T1 disease (46% vs. 67% for HR+/HER2− tumors, p<0.001). Lymphovascular invasion and extensive intraductal component were less common in triple-negative tumors, and were more frequently present in association with HER2−positive tumors.
Predictors of Triple-Negative Subtype
On univariate analysis, African-American race, premenopausal status, and obesity were each associated with a higher risk of triple-negative subtype. Triple-negative subtype comprised 33% of tumors in premenopausal, African-American women, and 26% of tumors in postmenopausal, African-American women, compared to 17% and 15% of breast cancers in pre- and post-menopausal Caucasian women, respectively (p<0.001 for association of tumor subtype and menopausal status within Caucasian; p=0.04 for African-American).
When race and BMI were entered into a logistic regression model including stage and menopausal status (Table 2), African-American race remained significantly associated with triple-negative subtype (adjusted OR 1.98, 95% CI 1.72–2.27; p<0.0001). BMI retained borderline significance overall (p=0.052); however, there was a significant interaction between BMI and menopausal status (p-value for interaction=0.02). Among obese premenopausal women, 24% of breast tumors were triple-negative, compared to 16% of normal weight premenopausal women; there was no apparent effect of BMI on risk of triple-negative subtype in postmenopausal women (Table 3).
Table 2.
Results of All Main Effects Logistic Regression Model to Test for Risk of Triple-Negative Breast Cancer
| Variable | Stratum | Sample Size | Adjusted OR (95% CI) | Type 3 p-value |
|---|---|---|---|---|
| Race | Caucasian | 12,406 | baseline | <0.001 |
| African-American | 1,142 | 1.98 (1.72, 2.27) | ||
| Other | 1,656 | 1.05 (0.91, 1.20) | ||
| BMI | 18.5 to <25 kg/m2 | 5,606 | baseline | 0.052 |
| 25 to < 30 kg/ m2 | 4,442 | 1.04 (0.94, 1.16) | ||
| ≥ 30 kg/ m2 | 4,366 | 1.16 (1.04, 1.29) | ||
| < 18. 5 kg/ m2 | 228 | 0.94 (0.64, 1.36) | ||
| Missing | 562 | 0.92 (0.72, 1.19) | ||
| AJCC Stage | I | 6,688 | baseline | <0.001 |
| II | 6,306 | 1.70 (1.54, 1.87) | ||
| III | 2,210 | 1.66 (1.46, 1.89) | ||
| Menopausal status | Premenopausal | 6,175 | baseline | 0.003 |
| Postmenopausal | 9,029 | 0.88 (0.80, 0.96) |
Note: A model including all main effects and an interaction term for menopausal status and BMI was run and found to be statistically significant (p-value for interaction=0.02).
Table 3.
Distribution of Breast Cancer Subtypes by Menopausal Status and BMI
| Menopausal Status at Diagnosis | Body Mass Index at Presentation | All cases | Triple-Negative | All Other | p-value |
|---|---|---|---|---|---|
| Premenopausal | <18.5 kg/m2 | 121 | 20 (17) | 101 (83) | <0.001 |
| 18.5 to <25 kg/m2 | 2,835 | 462 (16) | 2,373 (84) | ||
| 25 to <30 kg/m2 | 1,643 | 298 (18) | 1,345 (82) | ||
| ≥30 kg/m2 | 1,403 | 335 (24) | 1,068 (76) | ||
| Missing | 173 | 22 (13) | 151 (87) | ||
| Postmenopausal | <18.5 kg/m2 | 107 | 14 (13) | 93 (87) | 0.35 |
| 18.5 to <25 kg/m2 | 2,771 | 417 (15) | 2,354 (85) | ||
| 25 to <30 kg/m2 | 2,799 | 442 (16) | 2,357 (84) | ||
| ≥30 kg/m2 | 2,963 | 500 (17) | 2,463 (83) | ||
| Missing | 389 | 59 (15) | 330 (85) |
Relationship Between Nodal Status and Tumor Subtype
To explore the relationship between tumor subtype and nodal status, we constructed a logistic regression model to control for tumor size. For patients who received neoadjuvant chemotherapy, we used clinical T stage at initial presentation. For patients who did not receive neoadjuvant therapy, we used pathological T stage. Across all subtypes, the likelihood of positive nodes increased by tumor size (Table 4a). Compared to HR+/HER2−tumors as the referent group, triple-negative subtype was associated with a lower risk of node positivity, (adjusted OR 0.88, 95% CI 0.80, 0.97; p<0.001) (Table 4b). HER2−positive subtype was associated with the highest risk of nodal involvement.
Table 4a.
Frequency of Node Positivity by Tumor Size, Stratified by Tumor Subtype (N=15,168)
| Tumor size | No. (%) of Patients with at least 1 positive node | ||
|---|---|---|---|
| Triple Negative | HER2+ | HR+/HER2− | |
| Missing | 33 | 69 | 148 |
| ≤1 cm | 61 (17) | 97 (20) | 382 (14) |
| >1 to ≤ 2 cm | 258 (33) | 324 (40) | 1,340 (34) |
| >2 to ≤ 5 cm | 485 (47) | 542 (60) | 1,431 (56) |
| > 5 cm | 247 (73) | 269 (78) | 477 (70) |
Note: The sample INCLUDES patients who received neoadjuvant chemotherapy or neoadjuvant endocrine therapy (n= 2,641 for total N=15,204) but EXCLUDES patients with nodes not assessed or unknown clinical stage (n=36), resulting in a sample size of 15,168.
Sites of Recurrence
At a median follow-up of 3.06 years, recurrences were recorded among 1,389 women. Relative to patients with HR+/HER2− tumors, women with triple-negative tumors were more likely to experience a first recurrence in brain, lung, or loco-regional sites, and less likely to recur in bone (Table 5). Results were similar for first and subsequent sites of recurrence (data not shown). CNS comprised 62 of 589 sites of recurrence at metastatic presentation among patients with triple negative breast cancer with documented recurrence. Overall, CNS comprised 174 of 1348 sites of involvement at initial or subsequent recurrence among patients with triple negative breast cancer. Thus, the CNS was initially involved in 13% (62/480) and ever-involved in 36% (174/480) of patients with documented recurrences of triple-negative breast cancer.
Table 4b.
Results of All Main Effects Logistic Regression Model to Test for Predictors of Positive Nodes (N=14,918)*
| Variable | Stratum | Sample Size | Adjusted OR (95% CI) | Type 3 p-value |
|---|---|---|---|---|
| Tumor subtype | HR+/HER2− | 9,864 | baseline | <0.001 |
| Triple-negative | 2,526 | 0.88 (0.80, 0.97) | ||
| HER2+ | 2,528 | 1.35 (1.23, 1.48) | ||
| Tumor size | ≤ 1 cm | 3,509 | baseline | <0.001 |
| >1 to ≤ 2 cm | 5,531 | 2.94 (2.64, 3.28) | ||
| > 2 cm | 5,878 | 7.83 (7.03, 8.71) |
Note: The sample INCLUDES patients who received neoadjuvant chemotherapy or neoadjuvant endocrine therapy (n= 2,641), and EXCLUDES nodes not assessed (n=36), or with missing tumor size (n=250), resulting in a sample size of 14,918.
Survival Outcomes
Because of the varying use of trastuzumab across the time period of the study, we chose to limit our survival analysis to patients with either triple-negative or HR+/HER2−tumors. Among the 12,902 women who met these criteria, 1,280 deaths have occurred, of which 1,025 are classified as breast cancer-specific. Triple-negative subtype was associated with worse BCSS (data not shown) and OS (Figure 1) as compared to HR+/HER2− tumors, and retained its poor prognostic significance after adjustment for age, stage, race, use of adjuvant chemotherapy, tumor size, grade, and nodal status (HR for BCSS 2.99 [95% CI 2.59–3.45], p<0.0001; HR for OS 2.72 [2.39–3.10], p<0.0001). The inclusion of race in the model did not appreciably alter the HR for death associated with triple-negative subtype. Of note, there was a dramatic increase in the risk of death within 2 years of diagnosis among the triple negative group, even after adjusting for age, stage, race, use of adjuvant chemotherapy, tumor size, grade, and nodal status (HR for BCSS for 0 to 2 yrs 8.30 [95% CI 6.23, 11.05]; HR for OS for 0 to 2yrs 6.10 [95% CI 4.81, 7.74]); however, the magnitude of the risk increase declined substantially over time (Figure 2).
Figure 1.
Overall Survival by Tumor Subtype (HR+/HER2− versus Triple Negative) Adjusting for Age, Stage, Race, Receipt of Chemotherapy, Tumor Size, Histologic Grade and Nodal Status
Figure 2.
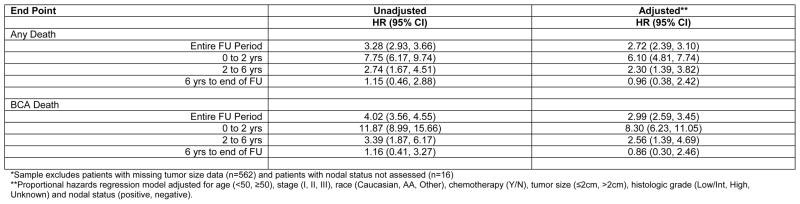
Hazard Ratios for Triple Negative versus HR+/HER2− Tumors (n=12024)*
*Sample excludes patients with missing tumor size data (n=562) and patients with nodal status not assessed (n=16)
**Proportional hazards regression model adjusted for age (<50, ≥50), stage (I, II, III), race (Caucasian, AA, Other), chemotherapy (Y/N), tumor size (≤2cm, >2cm), histologic grade (Low/Int, High, Unknown) and nodal status (positive, negative).
COMMENT
In a cohort of over 15,000 women with stage I-III breast cancer, we found that presenting features, patterns of recurrence, and survival differed significantly by breast cancer subtype.
Our findings are consistent with population-based data indicating a higher frequency of triple-negative tumors in African-American women.4, 7 The extent to which this association explains racial differences in breast cancer mortality is an open question. In a neoadjuvant trial conducted among patients with triple-negative breast cancer, the likelihood of pathological response did not vary by race.18 However, because the benefits of adjuvant chemotherapy are greater in triple-negative than in HR+/HER2− tumors, racial differences in receipt of appropriate therapy could further amplify baseline differences in prognosis.19–21 Of note, including race in our models did not substantially alter our survival estimates by tumor subtype, suggesting that the poor prognosis of the triple-negative subtype we observed was not mediated by an effect of race, either biologically or indirectly through disparities in care.
The biologic basis of the association between race and triple-negative subtype is not well-understood. Obesity has been proposed as a possible contributing factor.9, 10, 12, 14, 22 We found that race remained a significant predictor of triple-negative subtype, independent of BMI. With respect to the relationship between BMI and tumor subtype, several smaller studies have yielded conflicting results.5, 8, 12 Study of this issue has been limited as data on BMI and HER2 status are not available in large population registries. Our study included over 2,500 women with triple-negative breast cancer. We were therefore able to assess the overall effect of BMI and to test for different effects within subgroups. The association between BMI and triple negative subtype did not quite reach statistical significance (p=0.052). However, there was a significant interaction between BMI and menopausal status, such that triple-negative tumors were overrepresented in obese, premenopausal individuals. It is possible that this effect could be mediated by reproductive risk factors or other modifiers of risk, including family history, alcohol consumption, or physical activity. Millikan et al have noted that younger age at menarche, younger age at first full-term pregnancy, higher parity, and shorter duration of breastfeeding are associated with basal-type cancers.9 Of interest, obesity may be associated with increased breast cancer risk among BRCA1/2 carriers.23 Other potential mediators include insulin, insulin-like growth factor-1, inflammatory cytokines, or a pro-angiogenic state.24–26 Because these factors are not collected in the NCCN database, we were unable to assess their contribution to the observed association of BMI and triple negative disease among pre-menopausal women in our cohort. Because we compared proportions among women with a breast cancer diagnosis rather than estimating population-based risk, it is also possible that the true effect of obesity is to reduce the risk of ER-positive breast cancer, leading to an apparent, but not real, increase in the risk of triple-negative breast cancer. While our data do not point to a specific mechanism, they support the importance of assessing clinical and biochemical risk factors separately in younger versus older women, and by tumor subtype.
Our data clearly demonstrate that triple-negative tumors are less likely to be node-positive than either HER2+ or HR+/HER2− tumors, particularly above tumor size greater than 2 cm. This has been an unresolved question in the literature with conflicting results from several smaller studies.4, 6 We also found that the risk of recurrence was elevated relative to HR+/HER2− tumors, particularly in the first two years following diagnosis. Together, these data have direct implications for patient care. Published data indicate a median survival of only approximately one year among women with metastatic, triple negative breast cancer.16, 27 Thus, even among older individuals, the benefits of adjuvant chemotherapy may outweigh the risks. Indeed, in a randomized trial evaluating capecitabine versus standard chemotherapy in women over age 65 with early-stage breast cancer, standard chemotherapy was found to be superior (3-year relapse-free survival 68% vs 85%; overall survival 86% vs 91%), and this effect was driven almost entirely by ER-negative tumors, about 90% of which were triple-negative.
Consistent with other studies, we observed an increased risk of CNS relapse among patients with triple-negative or HER2+ tumors.15, 28–31 CNS metastases comprised a significant fraction of the documented recurrence events among women with these tumor subtypes. Unfortunately, the prognosis after CNS relapse in patients with triple-negative breast cancer is particularly poor.32, 33 Efforts to improve the outcomes of patients with HER2+ or triple-negative cancer will likely require attention to the CNS, either by identifying patients at highest risk for prevention/prophylaxis trials and/or developing brain-permeable agents in order to effectively treat micro-metastatic disease.
Our study had several limitations. First, we did not directly assess tumors for molecular subtype. Although most triple-negative breast cancers cluster with the basal subtype, concordance rate across studies varies from 70–100%.2, 34 We did not have information on percent staining of ER or PR by IHC, nor did we have information on cytokeratin or epidermal growth factor receptor staining; these variables may influence the proportion of patients with true basal subtype, and separate triple-negative tumors into different prognostic groups.35, 36 Second, our analysis was limited to patients who presented to NCCN centers. The median age for our cohort was 55 years, or approximately 6 years younger than the median age of patients with breast cancer in the United States, suggesting a referral bias.37 However, the distribution of subtypes in our database is similar to that in population-based registries.4, 7,38 In addition, there is no a priori reason to believe that the relationships between tumor characteristics and clinical phenotype that are the primary focus of our analysis would be systematically different in a population-based sample. Our definition of menopausal status also may have misclassified some women. However, because we were analyzing data from an existing registry that surveyed patients on cessation of menses in the 6 months prior to diagnosis, we are unable to assess alternate definitions of menopause.
Another limitation was the relatively short follow-up time. Given the long natural history of HR+/HER2− breast cancer, it is likely that survival estimates will evolve over time in this subset.39 In contrast, recurrences tend to occur early in patients with triple-negative tumors, and survival after a diagnosis of metastatic disease is only about one year.6, 16, 28 Indeed, despite the short follow up, 19% of patients with triple-negative breast cancer in our dataset had a recorded recurrence event, and the greatest hazard of death occurred in the first 2 years after initial diagnosis.6 Therefore, we believe that our description of the natural history of triple-negative breast cancer is likely to be a reasonably accurate reflection of outcomes.
In conclusion, we provide a comprehensive portrait of the presenting features and clinical outcomes of patients with triple-negative breast cancer, relative to other breast cancer subtypes, within the NCCN. Future analyses will hone in on the prognostic significance of tumor size and nodal status in the triple-negative subset, and on variations in patterns of care. It is our hope that these and other studies will aid in the planning and conduct of subtype-specific clinical trials for the prevention, detection, and treatment of this aggressive tumor subtype.
Table 5.
Univariate Logistic Regression for First Site(s) of Recurrence
| Site | Triple-Negative vs. HR+/HER2− | HER2+ vs HR+/HER2− | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Locoregional vs. Other | 1.32 (1.01, 1.74) | 0.045 | 1.12 (0.83, 1.51) | 0.45 |
| Lung vs Other | 2.17 (1.47, 3.21) | <0.001 | 1.73 (1.13, 2.66) | 0.012 |
| Brain vs Other | 3.50 (2.10, 5.85) | <0.001 | 3.97 (2.35, 6.72) | <0.001 |
| Bone vs Other | 0.26 (0.19, 0.36) | <0.001 | 0.39 (0.29, 0.54) | <0.001 |
| Liver vs Other | 1.09 (0.74, 1.61) | 0.67 | 1.58 (1.07, 2.33) | 0.02 |
Analysis based on cohort of 1,389 patients with documented recurrence (Triple-negative, n=480; HER2+, n=373; HR+/HER2−, n=536). HR+/HER2− cohort used as the referent group for all analyses. OR, odds ratio; CI, confidence interval; Other refers to any/all other distant/locoregional site(s).
Acknowledgments
Financial support: National Cancer Institute Specialized Program of Research Excellence in Breast Cancer (NIH P50 CA089393), the National Comprehensive Cancer Network, Breast Cancer Research Foundation, American Society of Clinical Oncology Cancer Foundation, Berry Junior Faculty Award, and the Karen Webster and David Evans Research Fund.
We thank Andrea Richardson, MD, PhD, Ann Partridge, MD, MPH, and Harold J. Burstein, MD, PhD for their critical input in the study design and analyses.
Footnotes
Previous Presentation: Results of this study have been presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, 2009, Orlando, FL.
Financial disclosures: Dr. Blayney reports that he has received salary support from the American Society of Clinical Oncology (as president).
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10963602. [DOI] [PubMed] [Google Scholar]
- 2.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9(5):R65. doi: 10.1186/bcr1771. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17910759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14(24):8010–8. doi: 10.1158/1078-0432.CCR-08-1208. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19088017. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16757721. [DOI] [PubMed] [Google Scholar]
- 5.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):439–43. doi: 10.1158/1055-9965.EPI-06-0806. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17372238. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17671126. [DOI] [PubMed] [Google Scholar]
- 7.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2−negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8. doi: 10.1002/cncr.22618. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17387718. [DOI] [PubMed] [Google Scholar]
- 8.Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2−overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:8–86. doi: 10.1158/1055-9965.EPI-08-0206. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18664548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39. doi: 10.1007/s10549-007-9632-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17578664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stead LA, Lash TL, Sobieraj JE, Chi DD, Westrup JL, Charlot M, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11(2):R18. doi: 10.1186/bcr2242. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19320967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troester MA, Swift-Scanlan T. Challenges in studying the etiology of breast cancer subtypes. Breast Cancer Res. 2009;11(3):104. doi: 10.1186/bcr2323. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19635173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE, Fulton RS, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. doi: 10.1186/bcr2261. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19463150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lara-Medina F, Perez-Sanchez V, Saavedra-Perez D, Blake-Cerda M, Arce C, Motola-Kuba D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117(16):3658–69. doi: 10.1002/cncr.25961. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21387260. [DOI] [PubMed] [Google Scholar]
- 14.Vona-Davis L, Rose DP, Hazard H, Howard-McNatt M, Adkins F, Partin J, et al. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3319–24. doi: 10.1158/1055-9965.EPI-08-0544. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19064545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic Behavior of Breast Cancer Subtypes. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.25.9820. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20498394. [DOI] [PubMed]
- 16.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–45. doi: 10.1002/cncr.23930. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18833576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punglia RS, Hughes ME, Edge SB, Theriault RL, Bookman MA, Wilson JL, et al. Factors associated with guideline-concordant use of radiotherapy after mastectomy in the national comprehensive cancer network. Int J Radiat Oncol Biol Phys. 2008;72(5):1434–40. doi: 10.1016/j.ijrobp.2008.03.020. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18472360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawood S, Broglio K, Kau SW, Green MC, Giordano SH, Meric-Bernstam F, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27(2):220–6. doi: 10.1200/JCO.2008.17.9952. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19047281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. Jama. 2006;295(14):1658–67. doi: 10.1001/jama.295.14.1658. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16609087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357(15):1496–506. doi: 10.1056/NEJMoa071167. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17928597. [DOI] [PubMed] [Google Scholar]
- 21.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–65. doi: 10.1056/NEJMoa0810266. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19439741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trivers KF, Lund MJ, Porter PL, Liff JM, Flagg EW, Coates RJ, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20(7):1071–82. doi: 10.1007/s10552-009-9331-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19343511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manders P, Pijpe A, Hooning MJ, Kluijt I, Vasen HF, Hoogerbrugge N, et al. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1120-8. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20730487. [DOI] [PubMed]
- 24.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010;121(2):479–83. doi: 10.1007/s10549-009-0591-y. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19851862. [DOI] [PubMed] [Google Scholar]
- 25.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60. doi: 10.1093/jnci/djn415. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19116382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vatten LJ, Holly JM, Gunnell D, Tretli S. Nested case-control study of the association of circulating levels of serum insulin-like growth factor I and insulin-like growth factor binding protein 3 with breast cancer in young women in Norway. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2097–100. doi: 10.1158/1055-9965.EPI-08-0212. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18708402. [DOI] [PubMed] [Google Scholar]
- 27.Harris LN, Broadwater G, Lin NU, Miron A, Schnitt SJ, Cowan D, et al. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342. Breast Cancer Res. 2006;8(6):R66. doi: 10.1186/bcr1622. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17129383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luck AA, Evans AJ, Green AR, Rakha EA, Paish C, Ellis IO. The influence of basal phenotype on the metastatic pattern of breast cancer. Clin Oncol (R Coll Radiol) 2008;20(1):40–5. doi: 10.1016/j.clon.2007.10.002. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17981444. [DOI] [PubMed] [Google Scholar]
- 29.Fulford LG, Reis-Filho JS, Ryder K, Jones C, Gillett CE, Hanby A, et al. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 2007;9(1):R4. doi: 10.1186/bcr1636. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17217540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–55. doi: 10.1158/1078-0432.CCR-06-2478. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17363517. [DOI] [PubMed] [Google Scholar]
- 31.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17(6):935–44. doi: 10.1093/annonc/mdl064. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16603601. [DOI] [PubMed] [Google Scholar]
- 32.Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112(11):2359–67. doi: 10.1002/cncr.23468. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18361426. [DOI] [PubMed] [Google Scholar]
- 33.Dawood S, Broglio K, Esteva FJ, Yang W, Kau SW, Islam R, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20(4):621–7. doi: 10.1093/annonc/mdn682. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19150943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123(1):236–40. doi: 10.1002/ijc.23518. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18398844. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–74. doi: 10.1158/1078-0432.CCR-04-0220. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15328174. [DOI] [PubMed] [Google Scholar]
- 36.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–76. doi: 10.1158/1078-0432.CCR-07-1658. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18316557. [DOI] [PubMed] [Google Scholar]
- 37.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer.gov/csr/1975_2006, based on November 2008 SEER data submission posted to the SEER web site. [Google Scholar]
- 38.Anderson WF, Luo S, Chatterjee N, Rosenberg PS, Matsuno RK, Goodman MT, et al. Human epidermal growth factor receptor-2 and estrogen receptor expression, a demonstration project using the residual tissue repository of the Surveillance, Epidemiology, and End Results (SEER) program. Breast Cancer Res Treat. 2009;113(1):189–96. doi: 10.1007/s10549-008-9918-3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18256926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–46. doi: 10.1200/JCO.1996.14.10.2738. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8874335. [DOI] [PubMed] [Google Scholar]



