Abstract
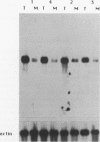
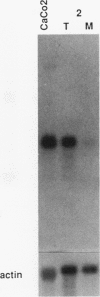
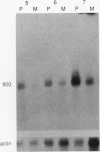
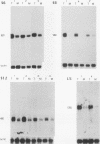
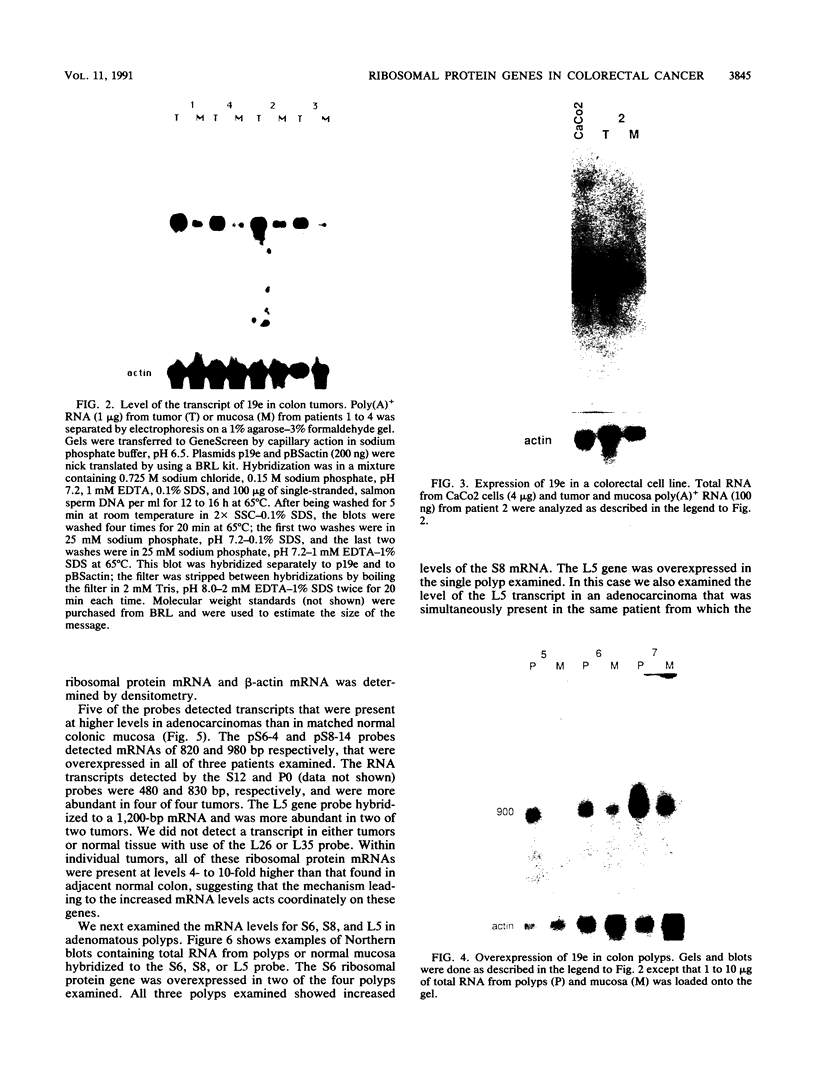
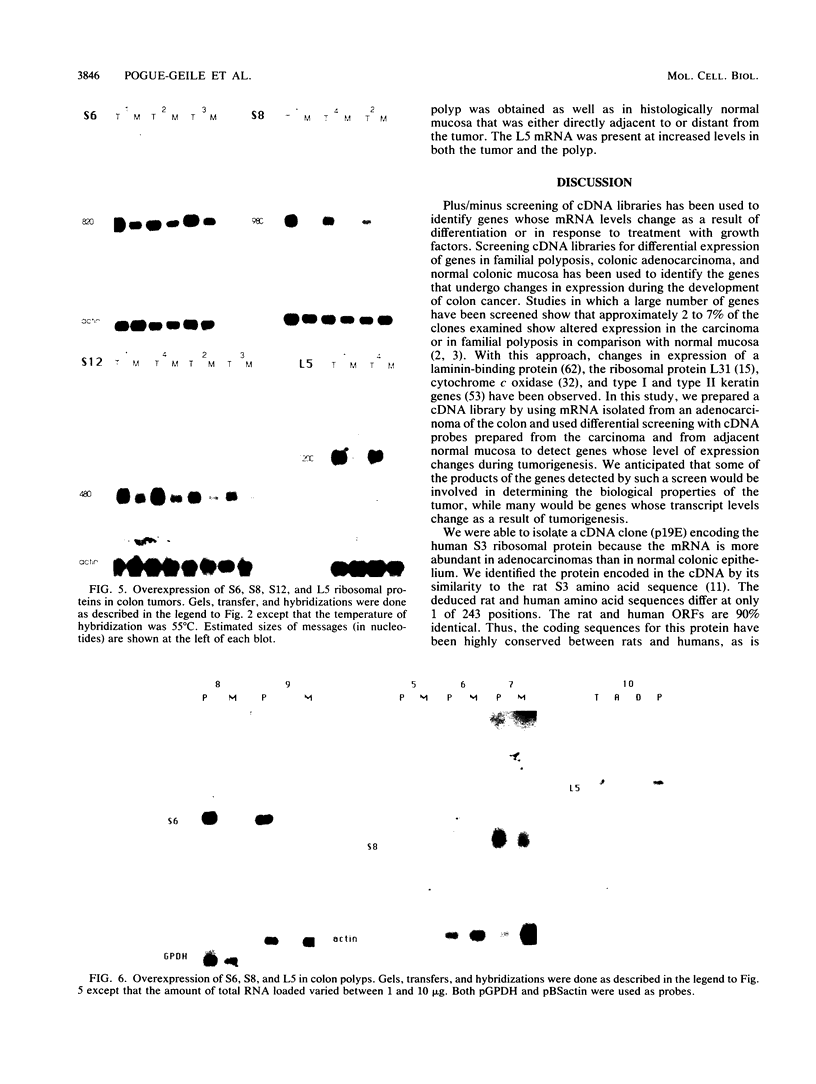
We have isolated a cDNA clone encoding the human S3 ribosomal protein from a normal human colon cDNA library. The clone was identified as one of many that detected genes whose level of expression was increased in adenocarcinoma of the colon relative to normal colonic mucosa. Increased levels of the S3 transcript were present in the tumors of all eight patients examined. Moreover, the S3 mRNA was also more abundant in 7 of 10 adenomatous polyps, the presumed precursor of carcinoma. Additional studies demonstrated that increased levels of mRNAs encoding several other ribosomal proteins, including S6, S8, S12, L5, and P0, were present in colorectal tumors and polyps. These results suggest that there is increased synthesis of ribosomes in colorectal tumors and that this increase is an early event in colon neoplasia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Beccari E., Bozzoni I., Luo Z. X., Pierandrei-Amaldi P. Nucleotide sequences of cloned cDNA fragments specific for six Xenopus laevis ribosomal proteins. Gene. 1982 Mar;17(3):311–316. doi: 10.1016/0378-1119(82)90147-0. [DOI] [PubMed] [Google Scholar]
- Augenlicht L. H., Wahrman M. Z., Halsey H., Anderson L., Taylor J., Lipkin M. Expression of cloned sequences in biopsies of human colonic tissue and in colonic carcinoma cells induced to differentiate in vitro. Cancer Res. 1987 Nov 15;47(22):6017–6021. [PubMed] [Google Scholar]
- Bartsch R. A., Joannou C., Talbot I. C., Bailey D. S. Cloning of mRNA sequences from the human colon: preliminary characterisation of defined mRNAs in normal and neoplastic tissues. Br J Cancer. 1986 Nov;54(5):791–798. doi: 10.1038/bjc.1986.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiberg H., Galand P. In vitro autoradiographic determination of cell kinetic parameters in adenocarcinomas and adjacent healthy mucosa of the human colon and rectum. Cancer Res. 1976 Feb;36(2 Pt 1):325–328. [PubMed] [Google Scholar]
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., DeSeau V., Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., Deseau V., Rosen N. Analysis of pp60c-src in human colon carcinoma and normal human colon mucosal cells. Oncogene Res. 1987 Jul;1(2):149–168. [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Kamps M. P., Meisler A. I., Pipas J. M., Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989 Jun;83(6):2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Meisler A. I., Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Devi K. R., Olvera J., Wool I. G. The primary structure of rat ribosomal protein S3. Arch Biochem Biophys. 1990 Dec;283(2):546–550. doi: 10.1016/0003-9861(90)90682-o. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Lin A., McNally J., Wool I. G. The primary structure of rat ribosomal protein L5. A comparison of the sequence of amino acids in the proteins that interact with 5 S rRNA. J Biol Chem. 1987 Sep 15;262(26):12879–12886. [PubMed] [Google Scholar]
- Chan Y. L., Lin A., Paz V., Wool I. G. The primary structure of rat ribosomal protein S8. Nucleic Acids Res. 1987 Nov 25;15(22):9451–9459. doi: 10.1093/nar/15.22.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Wool I. G. The primary structure of rat ribosomal protein S6. J Biol Chem. 1988 Feb 25;263(6):2891–2896. [PubMed] [Google Scholar]
- Chester K. A., Robson L., Begent R. H., Talbot I. C., Pringle J. H., Primrose L., Macpherson A. J., Boxer G., Southall P., Malcolm A. D. Identification of a human ribosomal protein mRNA with increased expression in colorectal tumours. Biochim Biophys Acta. 1989 Dec 22;1009(3):297–300. doi: 10.1016/0167-4781(89)90119-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- D'Emilia J., Bulovas K., D'Ercole K., Wolf B., Steele G., Jr, Summerhayes I. C. Expression of the c-erbB-2 gene product (p185) at different stages of neoplastic progression in the colon. Oncogene. 1989 Oct;4(10):1233–1239. [PubMed] [Google Scholar]
- DePhilip R. M., Rudert W. A., Lieberman I. Preferential stimulation of ribosomal protein synthesis by insulin and in the absence of ribosomal and messenger ribonucleic acid formation. Biochemistry. 1980 Apr 15;19(8):1662–1669. doi: 10.1021/bi00549a022. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman M. D., Rothberg P. G., Diehl R. E., Morse C. C., Spandorfer J. M., Astrin S. M. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol. 1985 Aug;5(8):1969–1976. doi: 10.1128/mcb.5.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Finley G. G., Schulz N. T., Hill S. A., Geiser J. R., Pipas J. M., Meisler A. I. Expression of the myc gene family in different stages of human colorectal cancer. Oncogene. 1989 Aug;4(8):963–971. [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Meyuhas O., Perry R. P., Johnson L. F. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982 Jun;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Heerdt B. G., Halsey H. K., Lipkin M., Augenlicht L. H. Expression of mitochondrial cytochrome c oxidase in human colonic cell differentiation, transformation, and risk for colonic cancer. Cancer Res. 1990 Mar 1;50(5):1596–1600. [PubMed] [Google Scholar]
- Hoffman J., Post J. In vivo studies of DNA synthesis in human normal and tumor cells. Cancer Res. 1967 May;27(5):898–902. [PubMed] [Google Scholar]
- Ignotz G. G., Hokari S., DePhilip R. M., Tsukada K., Lieberman I. Lodish model and regulation of ribosomal protein synthesis by insulin-deficient chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2550–2558. doi: 10.1021/bi00512a029. [DOI] [PubMed] [Google Scholar]
- Kief D. R., Warner J. R. Coordinate control of syntheses of ribosomal ribonucleic acid and ribosomal proteins during nutritional shift-up in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Nov;1(11):1007–1015. doi: 10.1128/mcb.1.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Williamson C. L., Firgaira F. A., Yang-Feng T. L., Münke M., Francke U., Rosenberg L. E. Cloning and screening with nanogram amounts of immunopurified mRNAs: cDNA cloning and chromosomal mapping of cystathionine beta-synthase and the beta subunit of propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2047–2051. doi: 10.1073/pnas.83.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Dobbs M., Scambler P., O'Connell P., Nakamura Y., Stauffer D., Woodward S., Burt R., Hughes J., Gardner E. The gene for familial polyposis coli maps to the long arm of chromosome 5. Science. 1987 Dec 4;238(4832):1411–1413. doi: 10.1126/science.3479843. [DOI] [PubMed] [Google Scholar]
- Lin A., Chan Y. L., Jones R., Wool I. G. The primary structure of rat ribosomal protein S12. The relationship of rat S12 to other ribosomal proteins and a correlation of the amino acid sequences of rat and yeast ribosomal proteins. J Biol Chem. 1987 Oct 15;262(29):14343–14351. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Monpezat J. P., Delattre O., Bernard A., Grunwald D., Remvikos Y., Muleris M., Salmon R. J., Frelat G., Dutrillaux B., Thomas G. Loss of alleles on chromosome 18 and on the short arm of chromosome 17 in polyploid colorectal carcinomas. Int J Cancer. 1988 Mar 15;41(3):404–408. doi: 10.1002/ijc.2910410315. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Yen T. S., Wang Y. F., Kam W. K., Rutter W. J. Cloning and characterization of a human ribosomal protein gene with enhanced expression in fetal and neoplastic cells. Nucleic Acids Res. 1987 Nov 11;15(21):8919–8934. doi: 10.1093/nar/15.21.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich B. E., Steitz J. A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987 Nov;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfest C. W., Henderson K. W., Gu J. R., Kottaridis S. D., Besbeas S., Panotopoulou E., Papas T. S. Subtraction hybridization cDNA libraries from colon carcinoma and hepatic cancer. Genet Anal Tech Appl. 1990 May;7(3):64–70. doi: 10.1016/0735-0651(90)90042-e. [DOI] [PubMed] [Google Scholar]
- Sikora K., Chan S., Evan G., Gabra H., Markham N., Stewart J., Watson J. c-myc oncogene expression in colorectal cancer. Cancer. 1987 Apr 1;59(7):1289–1295. doi: 10.1002/1097-0142(19870401)59:7<1289::aid-cncr2820590710>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Solomon E. Molecular genetics. Colorectal cancer genes. Nature. 1990 Feb 1;343(6257):412–414. doi: 10.1038/343412a0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Adams M. E., Harkins J. L., Pollard J. W. Transformed cells have lost control of ribosome number through their growth cycle. J Cell Physiol. 1979 Jul;100(1):127–138. doi: 10.1002/jcp.1041000113. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Olvera J., Wool I. G. The primary structure of rat ribosomal protein L35. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1377–1382. doi: 10.1016/0006-291x(90)90675-d. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Wakasugi K., Kuwano Y., Ishikawa K., Ogata K. Nucleotide sequence of cloned cDNA specific for rat ribosomal protein L35a. Eur J Biochem. 1986 Feb 3;154(3):523–527. doi: 10.1111/j.1432-1033.1986.tb09429.x. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Warner J. R. Ribosomal proteins are synthesized preferentially in cells commencing growth. J Cell Physiol. 1982 Jul;112(1):128–135. doi: 10.1002/jcp.1041120119. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Yow H. K., Wong J. M., Chen H. S., Lee C. G., Davis S., Steele G. D., Jr, Chen L. B. Increased mRNA expression of a laminin-binding protein in human colon carcinoma: complete sequence of a full-length cDNA encoding the protein. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6394–6398. doi: 10.1073/pnas.85.17.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. T., Tan Y. M., Tan Y. H. Isolation of a cDNA encoding human 40S ribosomal protein s3. Nucleic Acids Res. 1990 Nov 25;18(22):6689–6689. doi: 10.1093/nar/18.22.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Müller R. G., Fu X. C., Hynes N. E., Kozma S. Oncogenic activation of the human trk proto-oncogene by recombination with the ribosomal large subunit protein L7a. EMBO J. 1990 Jan;9(1):191–196. doi: 10.1002/j.1460-2075.1990.tb08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]