Abstract
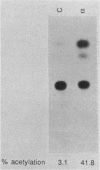
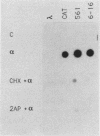
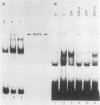
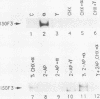
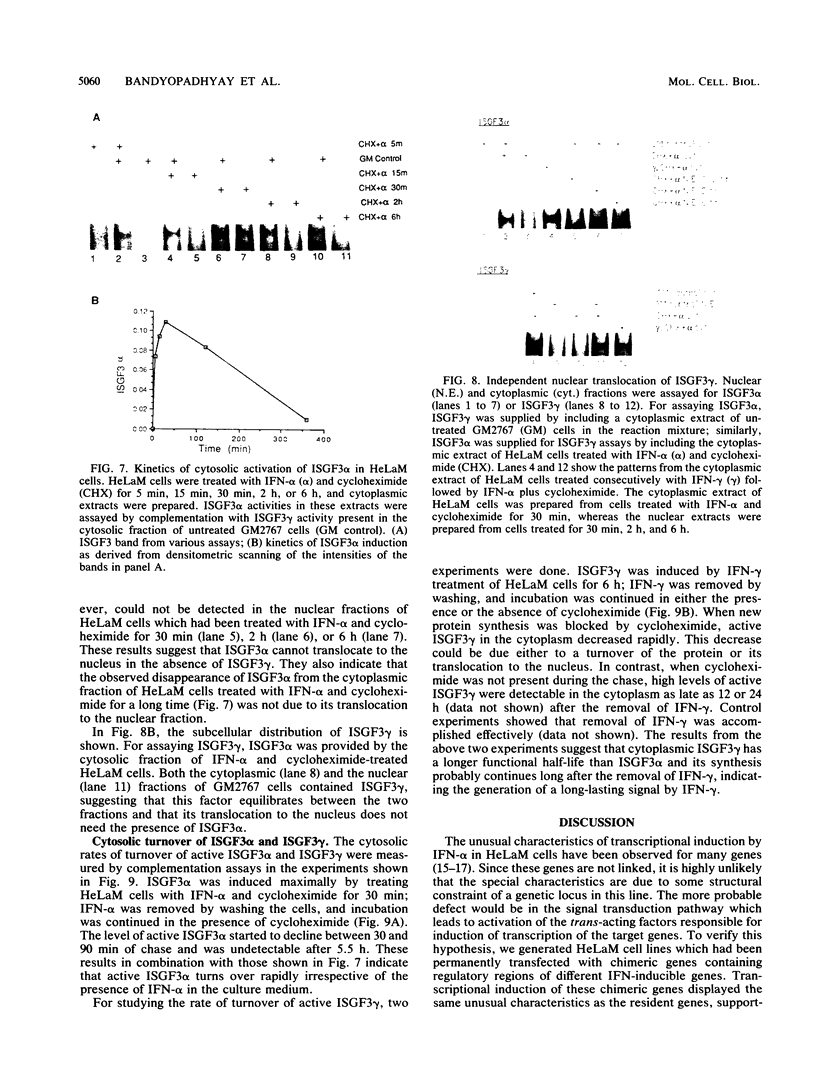
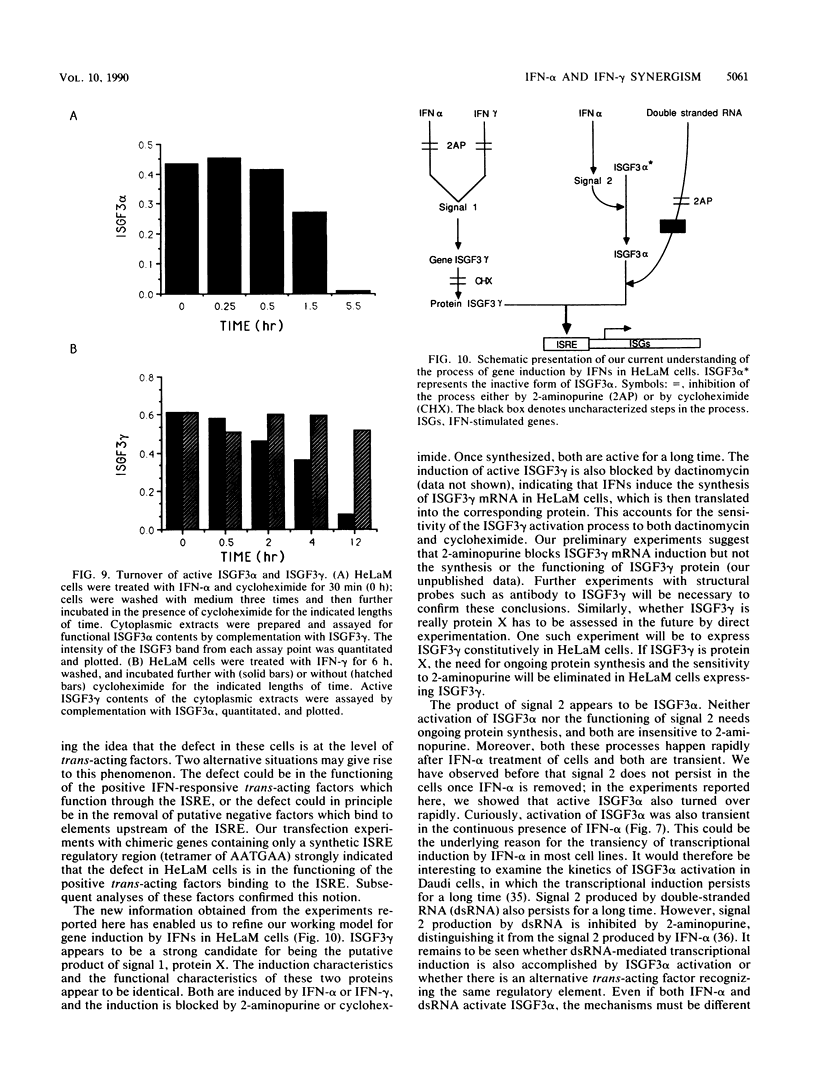
HeLaM is a variant cell line in which the transcriptional induction of many genes by alpha interferon has special characteristics (Tiwari et al., Mol. Cell. Biol. 8:4289-4294, 1988). The same characteristics were also displayed for induced transcription of a permanently transfected chimeric gene containing the interferon-stimulated response element of gene 561. For understanding the molecular basis of the special requirements of HeLaM cells, an analysis of the interferon-stimulated gene factors (ISGF) was undertaken. By using gel shift assays, it was shown that the activation of ISGF3 by alpha interferon treatment of HeLaM cells had characteristics identical to those of induced transcription: inhibition by 2-aminopurine and the need for ongoing protein synthesis which was obviated by pretreating the cells with gamma interferon. Upon mixing in vitro the cytoplasmic fraction of gamma interferon-treated HeLaM cells with that of cells treated with alpha interferon and cycloheximide, active ISGF3 was reconstituted, presumably through complementation of two components, ISGF3 gamma and ISGF3 alpha, present in the two respective fractions. Because, unlike other cells, untreated HeLaM cells did not contain detectable levels of either component, we could induce them individually and study their independent properties. Induction of ISGF3 gamma but not of ISGF3 alpha needed ongoing protein synthesis and was blocked by 2-aminopurine. Once induced, ISGF3 gamma was active for 24 h and was present in both the nuclear and cytoplasmic fractions. Activated ISGF3 alpha, on the other hand, did not translocate to the nucleus in the absence of ISGF3 gamma, and in the cytoplasm its activity decayed within 2 h of its activation. In reference to our working model, all of the above observations indicate that ISGF3 gamma is the product of signal 1 and ISGF3 alpha is the product of signal 2.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourrie B. J., Casellas P., Blythman H. E., Jansen F. K. Study of the plasma clearance of antibody--ricin-A-chain immunotoxins. Evidence for specific recognition sites on the A chain that mediate rapid clearance of the immunotoxin. Eur J Biochem. 1986 Feb 17;155(1):1–10. doi: 10.1111/j.1432-1033.1986.tb09451.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen B., Peretz D., Vaiman D., Benech P., Chebath J. Enhancer-like interferon responsive sequences of the human and murine (2'-5') oligoadenylate synthetase gene promoters. EMBO J. 1988 May;7(5):1411–1419. doi: 10.1002/j.1460-2075.1988.tb02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Vaiman D., Chebath J. Enhancer functions and in vitro protein binding of native and mutated interferon-responsive sequences. Nucleic Acids Res. 1989 Feb 25;17(4):1679–1695. doi: 10.1093/nar/17.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale T. C., Imam A. M., Kerr I. M., Stark G. R. Rapid activation by interferon alpha of a latent DNA-binding protein present in the cytoplasm of untreated cells. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1203–1207. doi: 10.1073/pnas.86.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale T. C., Rosen J. M., Guille M. J., Lewin A. R., Porter A. G., Kerr I. M., Stark G. R. Overlapping sites for constitutive and induced DNA binding factors involved in interferon-stimulated transcription. EMBO J. 1989 Mar;8(3):831–839. doi: 10.1002/j.1460-2075.1989.tb03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribaudo G., Toniato E., Engel D. A., Lengyel P. Interferons as gene activators. Characteristics of an interferon-activatable enhancer. J Biol Chem. 1987 Aug 25;262(24):11878–11883. [PubMed] [Google Scholar]
- Hug H., Costas M., Staeheli P., Aebi M., Weissmann C. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol Cell Biol. 1988 Aug;8(8):3065–3079. doi: 10.1128/mcb.8.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel A., Kimura A., Fournier A., Fellous M., Kourilsky P. Interferon response sequence potentiates activity of an enhancer in the promoter region of a mouse H-2 gene. Nature. 1986 Aug 21;322(6081):743–746. doi: 10.1038/322743a0. [DOI] [PubMed] [Google Scholar]
- Kessler D. S., Levy D. E., Darnell J. E., Jr Two interferon-induced nuclear factors bind a single promoter element in interferon-stimulated genes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8521–8525. doi: 10.1073/pnas.85.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Hood L., Stroynowski I. Regulation of murine class I genes by interferons is controlled by regions located both 5' and 3' to the transcription initiation site. Proc Natl Acad Sci U S A. 1987 May;84(10):3380–3384. doi: 10.1073/pnas.84.10.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J., Sen G. C. Regulation of synthesis and turnover of an interferon-inducible mRNA. Mol Cell Biol. 1986 Jun;6(6):2062–2067. doi: 10.1128/mcb.6.6.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J., Sen G. C. Transcriptional analyses of interferon-inducible mRNAs. Mol Cell Biol. 1987 Jan;7(1):528–531. doi: 10.1128/mcb.7.1.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Darnell J. E., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989 Sep;3(9):1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Lew D. J., Decker T., Kessler D. S., Darnell J. E., Jr Synergistic interaction between interferon-alpha and interferon-gamma through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J. 1990 Apr;9(4):1105–1111. doi: 10.1002/j.1460-2075.1990.tb08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Decker T., Darnell J. E., Jr Alpha interferon and gamma interferon stimulate transcription of a single gene through different signal transduction pathways. Mol Cell Biol. 1989 Dec;9(12):5404–5411. doi: 10.1128/mcb.9.12.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Huq A., Shan B. Beta and gamma interferons act synergistically to produce an antiviral state in cells resistant to both interferons individually. J Virol. 1989 Nov;63(11):4569–4578. doi: 10.1128/jvi.63.11.4569-4578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Porter A. C., Chernajovsky Y., Dale T. C., Gilbert C. S., Stark G. R., Kerr I. M. Interferon response element of the human gene 6-16. EMBO J. 1988 Jan;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Engelhardt J., Au W. C., Levy D. E., Pitha P. M. Virus infection and interferon can activate gene expression through a single synthetic element, but endogenous genes show distinct regulation. J Biol Chem. 1989 Oct 5;264(28):16658–16666. [PubMed] [Google Scholar]
- Reich N., Evans B., Levy D., Fahey D., Knight E., Jr, Darnell J. E., Jr Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6394–6398. doi: 10.1073/pnas.84.18.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L. E., Brasnett A. H., Gilbert C. S., Porter A. C., Gewert D. R., Stark G. R., Kerr I. M. A single DNA response element can confer inducibility by both alpha- and gamma-interferons. Proc Natl Acad Sci U S A. 1989 Feb;86(3):840–844. doi: 10.1073/pnas.86.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford M. N., Hannigan G. E., Williams B. R. Interferon-induced binding of nuclear factors to promoter elements of the 2-5A synthetase gene. EMBO J. 1988 Mar;7(3):751–759. doi: 10.1002/j.1460-2075.1988.tb02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta H., Engel D. A., Chao H. M., Thakur A., García-Blanco M. A., Lengyel P. Interferons as gene activators. Cloning of the 5' terminus and the control segment of an interferon activated gene. J Biol Chem. 1986 Sep 5;261(25):11849–11858. [PubMed] [Google Scholar]
- Sen G. C. Biochemical pathways in interferon-action. Pharmacol Ther. 1984;24(2):235–257. doi: 10.1016/0163-7258(84)90036-6. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Priming: a nonantiviral function of interferon. J Virol. 1971 Jun;7(6):792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R. K., Kusari J., Kumar R., Sen G. C. Gene induction by interferons and double-stranded RNA: selective inhibition by 2-aminopurine. Mol Cell Biol. 1988 Oct;8(10):4289–4294. doi: 10.1128/mcb.8.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R. K., Kusari J., Sen G. C. Functional equivalents of interferon-mediated signals needed for induction of an mRNA can be generated by double-stranded RNA and growth factors. EMBO J. 1987 Nov;6(11):3373–3378. doi: 10.1002/j.1460-2075.1987.tb02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathelet M. G., Clauss I. M., Nols C. B., Content J., Huez G. A. New inducers revealed by the promoter sequence analysis of two interferon-activated human genes. Eur J Biochem. 1987 Dec 1;169(2):313–321. doi: 10.1111/j.1432-1033.1987.tb13614.x. [DOI] [PubMed] [Google Scholar]
- Wathelet M. G., Clauss I. M., Paillard F. C., Huez G. A. 2-Aminopurine selectively blocks the transcriptional activation of cellular genes by virus, double-stranded RNA and interferons in human cells. Eur J Biochem. 1989 Oct 1;184(3):503–509. doi: 10.1111/j.1432-1033.1989.tb15043.x. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]