Abstract
Objective
The aim of this study was to assess clinical and pulmonary thin-section CT findings in patients with acute Pseudomonas aeruginosa (PA) pulmonary infection.
Methods
We retrospectively identified 44 patients with acute PA pneumonia who had undergone chest thin-section CT examinations between January 2004 and December 2010. We excluded nine patients with concurrent infections. The final study group comprised 35 patients (21 males, 14 females; age range 30–89 years, mean age 66.9 years) with PA pneumonia. The patients' clinical findings were assessed. Parenchymal abnormalities, enlarged lymph nodes and pleural effusion were evaluated on thin-section CT.
Results
Underlying diseases included malignancy (n=13), a smoking habit (n=11) and cardiac disease (n=8). CT scans of all patients revealed abnormal findings, including ground-glass opacity (n=34), bronchial wall thickening (n=31), consolidation (n=23) and cavities (n=5). Pleural effusion was found in 15 patients.
Conclusion
PA pulmonary infection was observed in patients with underlying diseases such as malignancy or a smoking habit. The CT findings in patients with PA consisted mainly of ground-glass attenuation and bronchial wall thickening.
Advances in knowledge
The CT findings consisted mainly of ground-glass attenuation, bronchial wall thickening and cavities. These findings in patients with an underlying disease such as malignancy or a smoking habit may be suggestive of pneumonia caused by PA infection.
Pseudomonas aeruginosa (PA) is an uncommon cause of community-acquired pneumonia but a common cause of nosocomial pneumonia. In critically ill, hospitalised patients, PA is an important pulmonary pathogen. In all, 25% of ventilator-associated pneumonias result from PA infection, with PA representing the most common isolate from the lower respiratory tract [1]. As nosocomial bronchopneumonia caused by PA is associated with a 60–80% mortality rate [2,3], it is crucial to identify the risk factors associated with this poor outcome and to evaluate the radiological findings as quickly as possible, thereby losing no time in initiating appropriate management [4,5].
Tillotson and Lerner [6] formulated the classic radiological description of PA pneumonia in 1968. They classified PA pneumonia as a diffuse bronchopneumonia with nodularity. Winer-Muram et al [7] identified confluent air space disease on chest radiographs in 97% of patients with ventilator-associated PA pneumonia, which was most commonly bilateral and multifocal. Shah et al [8] reported CT findings of nosocomial PA pneumonia, which commonly presents with multifocal air space consolidation followed by nodular features.
To the best of our knowledge, no other English-language studies of pulmonary thin-section CT findings in patients with acute PA pneumonia have been published. We describe thin-section CT findings in the largest series of patients who have undergone bronchoscopic sampling to establish the diagnosis.
Methods
Patients
Our institutional review board approved this retrospective study and waived the need for informed consent.
We retrospectively identified 53 patients with acute PA pneumonia between January 2004 and December 2010 at our institution. We excluded nine patients with concurrent infections diagnosed by isolating the organisms, serological tests and clinical findings. Of the 44 patients with PA alone, 35 underwent thin-section CT examinations of the chest.
The final study group comprised 35 patients with PA pneumonia (21 males, 14 females; age range 30–89 years, mean age 66.9 years). No patients with human immunodeficiency virus (HIV) infection, cystic fibrosis or diffuse panbronchiolitis were included in the study.
A diagnosis of pneumonia was established when all of the following features were present: symptoms of infection (cough with or without sputum, fever, leukocytosis or leukopenia, pulmonary infiltrates on chest radiography) coinciding with identification of PA isolated from a bronchoscopic specimen. Isolated PA pneumonia was diagnosed when specimens obtained at fibre-optic bronchoscopy yielded a sufficient concentration of organisms at quantitative culture, which was ≥105 colony-forming units (cfu) ml−1. Patients were considered to have community-acquired pneumonia if, at the time of hospital admission, they presented with respiratory symptoms and sputum and chest radiography revealed pulmonary infiltrates.
Among the 35 patients in this study, 6 had a community-acquired infection and 29 had a nosocomial infection (2 patients were undergoing mechanical ventilation at the time of CT).
The incidences of underlying diseases, alcoholism and smoking habit were recorded. An alcoholic was defined as having daily consumption of ≥80 g of alcohol over the past 2 years [9]. A heavy smoker was defined as having smoked for >10 pack-years.
CT examinations
Thin-section CT examinations were performed with 1-mm collimation at 10-mm intervals from the apex of the lung to the diaphragm (n=8) or volumetrically with a multidetector CT system with 1-mm reconstruction (n=27). CT examinations were performed with the patient supine at full inspiration and were reconstructed using a high-spatial-frequency algorithm. Images were captured at window settings that allowed us to view the lung parenchyma (window level −600 to −700 HU (Hounsfield units); window width 1200–1500 HU) and mediastinum (window level 20–40 HU; window width 400 HU).
The pulmonary CT examination was performed within 1–7 days (mean 4.0 days) after the onset of respiratory symptoms. Intravenously administered contrast material was used for nine of the examinations.
Image interpretation
Two radiologists (FO, YA), with 24 and 16 years of experience in chest CT image interpretation, respectively, evaluated the images, and made their interpretations independently. Conclusions were reached by consensus. An average of 2 sessions per week were reserved for reviewing the CT findings (about 60 sessions in total).
The CT images were assessed for radiological patterns: ground-glass attenuation (GGA), consolidation, nodules, centrilobular nodules, bronchial wall thickening, interlobular septal thickening, intralobular reticular opacity, bronchiectasis, enlarged hilar/mediastinal lymph node(s) (>1 cm diameter short axis), cavities and pleural effusion. GGA was defined as areas exhibiting hazy increases in attenuation without obscuring vascular markings [10,11]. Consolidation was defined as areas of increased attenuation that obscured normal lung markings [10,11].
Centrilobular nodules were defined as those present around the peripheral pulmonary arterial branches or 3–5 mm from the pleura, interlobular septa or pulmonary veins. The centrilobular nodules were divided into two patterns: (1) those with a tree-in-bud appearance and (2) those with ill-defined centrilobular nodules of GGA without a tree-in-bud appearance. The tree-in-bud appearance was characterised by well-defined centrilobular nodules of soft-tissue attenuation that were connected to linear and branching opacities. Interlobular septal thickening was defined as abnormal widening of the interlobular septa [11]. Intralobular reticular opacity was considered to be present when interlacing linear shadows were separated by a few millimetres [10,11].
The distribution of parenchymal disease was assessed as to whether the abnormal findings were unilateral or bilateral. If the main lesion was predominantly located in the inner third of the lung, it was classified as centrally distributed. If the lesion was predominantly located in the outer third of the lung, it was classified as peripherally distributed. If the lesions showed no predominant distribution, the disease was classified as randomly distributed. Zonal predominance was also classified as upper, lower or random. Upper lung zone predominance indicated that most abnormalities were seen at a level above the tracheal carina, whereas with lower zone predominance most abnormalities were located below the upper zone. When abnormalities showed no clear zonal predominance, the lung disease was classified as randomly distributed.
Follow-up CT examinations were performed 3 days to 2 months after antibiotic therapy in 13 patients. Follow-up chest radiography was performed 1 day to 1 month after antibiotic therapy in 35 patients. These follow-up CT images and radiographs were then assessed.
Results
Patients' background
The underlying conditions of all 35 patients are summarised in Table 1. Overall, 11 (31.4%) patients were chronic smokers, 7 (20.0%) were alcoholics and 6 (17.1%) were both alcoholics and chronic smokers. 8 (22.9%) patients had concomitant cardiac disease. Patients with post-operative malignancy (n=13; 37.1%), diabetes mellitus (n=7; 20.0%), pulmonary emphysema (n=6; 17.1%) or bronchial asthma (n=2; 5.7%) were included in the study.
Table 1. Patient characteristics and underlying conditions.
| Characteristic/condition | Number | Percentage |
| Sex, male/female | 21/14 | |
| Age (years) | ||
| Range | 30–89 | |
| Mean | 66.9 | |
| Community-acquired | 6 | 17.1 |
| Nosocomial | 29 | 82.9 |
| Underlying conditions | ||
| Smoking habit | 11 | 31.4 |
| Cardiac disease | 8 | 22.9 |
| Alcoholic | 7 | 20.0 |
| Diabetes mellitus | 7 | 20.0 |
| Pulmonary emphysema | 6 | 17.1 |
| Collagen disease | 2 | 5.7 |
| Bronchial asthma | 2 | 5.7 |
| Malignancy | 13 | 37.1 |
| Presenting symptoms | ||
| Fever | 32 | 91.4 |
| Cough | 28 | 80.0 |
| Sputum | 28 | 80.0 |
| Dyspnoea | 25 | 71.4 |
| General weakness | 7 | 20.0 |
| Disturbance of consciousness | 5 | 14.3 |
| Chest pain | 4 | 11.4 |
Altogether, 1 of 6 patients with community-acquired infections and 9 of 29 patients with nosocomial infections were treated with antibiotic therapy prior to the CT examinations. Most of the patients in the present study, including those who had received antibiotic therapy prior to the CT examinations, showed progression of their respiratory symptoms. The most common presenting symptoms were fever (32 patients; 91.4%), cough (28 patients; 80.0%), sputum (28 patients; 80.0%) and dyspnoea (25 patients; 71.4%).
CT patterns
Chest CT scans revealed abnormalities in all patients with a PA pulmonary infection (Table 2). Among the 35 patients (Figures 1–3), GGA (n=34; 97.1%) was the most frequently observed abnormality, followed by bronchial wall thickening (n=31; 88.6%), consolidation (n=23; 65.7%) and intralobular reticular opacity (n=7; 20.0%). Cavities (n=5; 14.3%) (Figure 2) and ill-defined centrilobular nodules (n=3; 8.6%) were also observed. The tree-in-bud appearance characterised by well-defined centrilobular nodules was observed in 1 (2.9%) patient. The most frequently observed combination of abnormalities was GGA and bronchial wall thickening (n=31; 88.6%), followed by GGA and consolidation (n=23; 65.7%), and bronchial wall thickening and consolidation (n=22; 62.9%).
Figure 3.
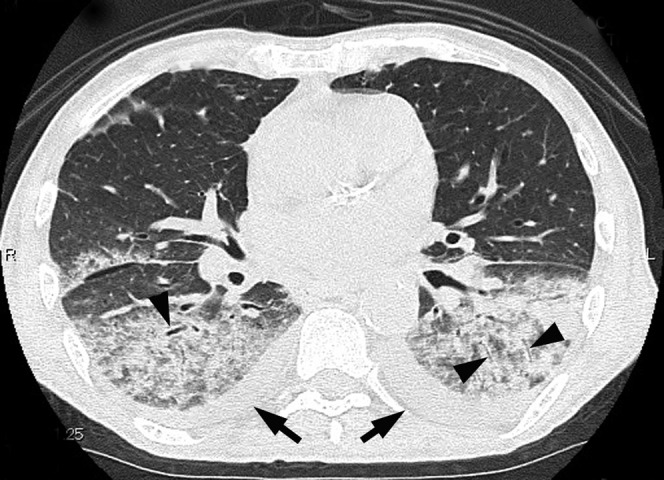
Acute pneumonia caused by Pseudomonas aeruginosa in a 67-year-old male with a smoking habit and diabetes mellitus 4 days after the onset of a sputum-producing cough and fever. Transverse thin-section CT image (1-mm thickness) of the lower lobes shows consolidation and bronchial wall thickening (arrowheads). Bilateral pleural effusions are present (arrows).
Figure 2.
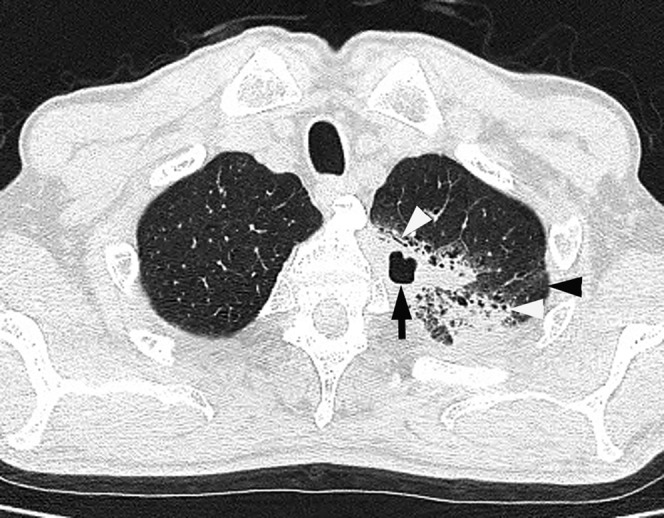
Acute pneumonia caused by Pseudomonas aeruginosa in a 74-year-old male alcoholic with a malignant lymphoma of the neck 4 days after the onset of sputum-producing cough and fever. Transverse thin-section CT image (1-mm thickness) of the upper lobes shows consolidation with cavities (black arrow), ground-glass attenuation (black arrowhead) and bronchial wall thickening (white arrowheads).
Table 2. Thoracic CT findings in 35 patients.
| Findings | Number of patients | Percentage |
| Ground-glass attenuation | 34 | 97.1 |
| Bronchial wall thickening | 31 | 88.6 |
| Consolidation | 23 | 65.7 |
| Intralobular reticular opacity | 7 | 20.0 |
| Cavity | 5 | 14.3 |
| Centrilobular nodules | 4 | 11.4 |
| Nodules | 3 | 8.6 |
| Interlobular septal thickening | 1 | 2.9 |
| Pleural effusion | 15 | 42.9 |
| Lymph node enlargement | 1 | 2.9 |
Disease distribution
Of the 35 patients with PA infection, abnormal findings were found bilaterally in 30 (85.7%) patients, unilaterally in 5 (14.3%) patients and peripherally in 17 (48.6%) patients (Figures 1 and 2). In all, 18 (51.4%) patients showed a random distribution, and no patients had a predominantly central distribution. The predominant zonal distribution of infection was in the upper zone in 6 (17.1%) patients (Figures 1 and 2), in the lower zone in 10 (28.6%) patients (Figure 3) and distributed randomly in 19 (54.3%) patients.
Figure 1.
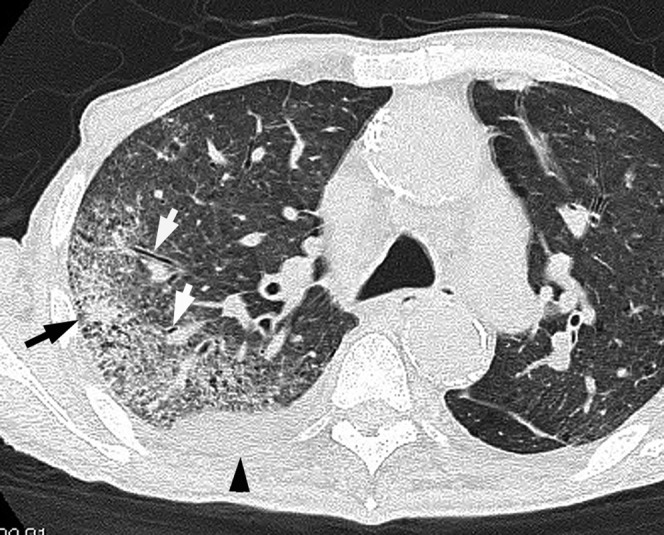
Acute pneumonia caused by Pseudomonas aeruginosa in a 78-year-old male with a smoking habit and cardiac disease 3 days after the onset of fever, cough and dyspnoea. Transverse thin-section CT image (1-mm thickness) at the level of the tracheal carina shows bronchial wall thickening (white arrows) and consolidation (black arrow) in the right upper lobe. Right pleural effusion is present (arrowhead).
Effusion and lymph nodes
Bilateral pleural effusions were found in 11 (31.4%) patients (Figure 3) and unilateral pleural effusion in 4 (11.4%) patients (Figure 1). 1 (2.9%) patient had mediastinal lymph node enlargement.
Follow-up study
All 35 patients underwent antibiotic therapy. In each of the six patients with community-acquired infections, the initial respiratory symptoms diminished, as did the abnormal findings on follow-up CT scans or chest radiographs. By comparison, in 25 (86.2%) of 29 patients with nosocomial infections, the initial respiratory symptoms diminished along with the abnormal findings on follow-up CT scans or radiographs. In the remaining 4 (13.8%) patients, the abnormal parenchymal findings and pleural effusions worsened, and the patients subsequently died.
Among these patients, one had pulmonary emphysema, cardiac disease, diabetes mellitus and malignancy; one was an alcoholic with a smoking habit and had diabetes mellitus; one was an alcoholic and had diabetes mellitus; and one had cardiac disease and malignancy.
Discussion
Ubiquitous in nature, PA is an opportunistic pathogen that causes a wide range of infections [12]. It is a particularly virulent pathogen that produces exotoxins and enzymes [13]. It also produces a biofilm that protects it from environmental elements and from host antibodies and phagocytes [14].
During the late 1980s, a US Centers for Disease Control and Prevention study noted a gradual increase in the incidence of PA as a nosocomial pathogen causing pneumonia. From 1975 to 2003, the incidence of nosocomial PA pneumonia almost doubled, from 9.6% to 18.1% [15]. In another study, it was the most frequent cause (20%) of 670 cases of ventilator-acquired pneumonia [16]. In a retrospective matched cohort study of 842 patients with ventilator-acquired pneumonia, PA was the most frequent pathogen isolated (9.3%) [17]. It was also the aetiological microbe in 0.9–1.9% of patients with community-acquired pneumonia requiring hospitalisation [18,19]. Risk factors for community-acquired pneumonia due to PA have been well elucidated [20,21].
In the present study, 6 patients had community-acquired pneumonia and 29 had a nosocomial infection, with 2 of the latter patients undergoing mechanical ventilation. Malignancy was the most commonly associated condition, followed by a smoking habit and cardiac disease. The mortality rate was 13.8%, which is lower than in previous reports [2,3]. This might be because the present study had only two intubated patients, and there were no patients with concurrent PA pneumonia, bacteraemia or HIV infection. Also, none was neutropaenic or highly immunosuppressed.
The diagnosis of pneumonia caused by PA can be challenging given the relative ease of colonisation in the upper and lower pulmonary tracts. In the intubated patient, PA is isolated consistently from endotracheal cultures for prolonged periods, often without signs of systemic infection. Many clinicians believe that an abrupt increase in the quantity and purulence of respiratory secretions, along with clinical signs of pneumonia and with a new pulmonary infiltrate, are circumstantial evidence that PA is the cause. This combination of changes forms the basis for initiating anti-pseudomonal antibiotic therapy, lowering the threshold for administering antibiotic treatment [22,23].
In a controlled trial of 413 French patients in 31 intensive care units, an invasive approach to diagnosing ventilator-acquired pneumonia minimised the broad-spectrum antibiotic use and reduce the mortality rate [24]. With the use of protected brush bronchoscopy and quantitative criteria to define colonisation (bacterial counts of <103 cfu ml−1) and infection (≥103 cfu ml−1), the mortality rate was significantly higher for PA-infected patients than for those simply colonised [25]. We diagnosed pneumonia when clinical features (symptoms of infection and pulmonary infiltrates on chest radiography) were present and fibre-optic bronchoscopy-cultured specimens (not sputum-cultured specimens) yielded a sufficient concentration of organisms (≥105 cfu ml−1).
Tillotson and Lerner [6] formulated the classic radiological description of PA pneumonia when they described 10 patients with PA pneumonia. They characterised PA pneumonia as diffuse bronchopneumonia with nodularity. Although the original report described these features in 100% of the study cases, two later studies showed nodularity in only 22% (5/23) [26] and 50% (14/28) [8]. The most consistent finding was bilateral distribution of infiltrates, which were present in 48–91% [7,8,26]. Radiological features for PA pneumonia appear in a non-specific pattern, and chest radiography cannot be used to make a specific diagnosis of pneumonia due to PA.
Shah et al [8] reported CT images in 28 patients with PA pneumonia (15 patients with PA alone; 13 patients with other isolates), including 20 with ventilator-acquired pneumonia. The diagnosis of PA pneumonia was based on isolating the organisms from respiratory cultures (sputum or tracheal aspirates in 16, transtracheal aspirates in 5, bronchoscopy in 7). Bacterial counts were not described. Among the 15 patients with PA pneumonia, CT findings included bilateral consolidation (100%) in 8 patients and nodules in 9; necrosis was present in 4 and pleural effusion in 9. CT findings in patients with and without other respiratory isolates did not differ regarding the distribution and frequency of consolidations, nodules or necrosis.
Winer-Muram et al [7] reported radiological features in 56 patients with ventilator-acquired pneumonia. Of the 56 patients, 53 had multifocal opacities with an air bronchogram on chest radiography. In 13 patients, cavities were detected on chest radiographs, CT or both. CT, performed in 8 of the 56 patients, provided important additional information (presence of cavities or effusions) in 4 of the 8 patients who underwent scanning.
To our knowledge, no other findings from studies of pulmonary thin-section CT in patients with acute PA pneumonia have appeared in the English-language literature. In the present study, the most common CT finding was GGA, followed by bronchial wall thickening and consolidation. These abnormalities were predominantly seen bilaterally and in random distribution.
PA is known to be an important pathogen causing bronchopneumonia, whereas Streptococcus pneumoniae, Chlamydia pneumoniae and Klebsiella pneumoniae cause air space pneumonia. Pathologically, bronchopneumonia is associated with inflammatory changes involving the bronchial and bronchiolar walls, with minimal exudation into adjacent alveoli [27]. In cases of PA infection, the frequency of bronchial wall thickening (88.6%) might be as high as that in patients harbouring other bronchopneumonia pathogens (Mycoplasma pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus: 88.1%, 85.8%, 78.0% and 68.9%, respectively) [28-31]. Also, the frequency of bronchial wall thickening was significantly higher in the patients with PA pneumonia than in the patients with air space pneumonia due to S. pneumoniae, C. pneumoniae or K. pneumoniae (25.6%, 35.0%, 26.3%, respectively) [28,32,33].
Cavities were found in 5 (14.3%) of our 35 patients with PA pneumonia. The frequency was significantly higher than in patients with other pathogens, namely M. pneumoniae, H. influenzae, M. catarrhalis, S. aureus, S. pneumoniae, C. pneumoniae and K. pneumoniae at 0/42, 0/211, 0/109, 5/151 (3.3%), 0/86, 0/40 and 1/198 (0.5%), respectively [27-33]. We compared thin-section CT findings in patients with S. pneumoniae alone and S. pneumoniae with PA, and in patients with K. pneumoniae alone and K. pneumoniae with PA [27,32]. The results showed that frequencies of cavities and bronchial wall thickening were significantly higher in patients with S. pneumoniae with PA and K. pneumoniae with PA than in those with S. pneumoniae alone or K. pneumoniae alone (p<0.001, all comparisons).
The pathological features of bacteraemic PA pneumonia were described as early as 1927 and were characterised by blood vessel invasion and necrosis [34]. PA pneumonia was characterised by microabscesses, haemorrhage and focal necrosis.
High levels of chemotactic factors, such as interleukin-8 and neutrophils, have been associated with chronic PA infection. Neutrophils that enter the infection site secrete multiple mediators (e.g. reactive oxygen species, elastases). Although the environment is filled with these caustic agents, PA tends to resist their effects. The exact mechanism is unknown, although it is most probably due partly to the bacterial growth in protective biofilms [14]. PA also produces catalases and superoxide dismutases, which are able to neutralise the deleterious effects of hydrogen peroxide and oxidative stress [35]. Thus, instead of clearing the bacterial infection, the neutrophil mediator release results in tissue destruction and abscess formation (PA quorum-sensing system). Therefore, among the causative pathogens for community-acquired pneumonia and nosocomial pneumonia, cavity formation on thin-section CT images indicates pneumonia caused by PA infection alone or combined PA infection.
There were some limitations to our study. First, it was retrospective, and CT image interpretation was performed by consensus. Second, thin-section CT images were obtained using different protocols. Third, comparison of CT findings between patients with PA alone and those with concurrent PA was not carried out.
Pulmonary infection caused by PA was observed in patients with underlying diseases such as malignancy or a smoking habit. The CT findings in patients with PA consisted mainly of GGA and bronchial wall thickening, and cavities were found significantly more frequently than with other common pathogens.
References
- 1.Iannini PB, Claffey T, Quintiliani R. Bacteremic pseudomonas pneumonia. JAMA 1974;230:558–61 [DOI] [PubMed] [Google Scholar]
- 2.Pennington JE, Reynolds HY, Carbone PP. Pseudomonas pneumonia. A retrospective study of 36 cases. Am J Med 1973;55:155–60 [DOI] [PubMed] [Google Scholar]
- 3.Lipchil RJ, Kuzo RS. Nosocomial pneumonia. Radiol Clin North Am 1996;34:47–58 [PubMed] [Google Scholar]
- 4.Blot S, Vandewoude K. Early detection of systemic infections. Acta Clin Belg 2004;59:20–3 [DOI] [PubMed] [Google Scholar]
- 5.Spapen HD, Hachimi-Idrissi S, Corne L, Huyghens LP. Diagnostic markers of sepsis in the emergency department. Acta Clin Belg 2006;61:138–42 [DOI] [PubMed] [Google Scholar]
- 6.Tillotson JR, Lerner AM. Characteristics of nonbacteremic Pseudomonas pneumonia. Ann Intern Med 1968;68:295–307 [DOI] [PubMed] [Google Scholar]
- 7.Winer-Muram HT, Jennings SG, Wunderink RG, Jones CB, Leeper KV., Jr Ventilator-associated Pseudomonas aeruginosa pneumonia: radiologic findings. Radiology 1995;195:247–52 [DOI] [PubMed] [Google Scholar]
- 8.Shah RM, Wechsler R, Salazar AM, Spirn PW. Spectrum of CT findings in nosocomial Pseudomonas aeruginosa pneumonia. J Thorac Imaging 2002;17:53–7 [DOI] [PubMed] [Google Scholar]
- 9.Torres A, Serra-Batlles J, Ferrer A, Jimenez P, Celis R, Cobo E, et al. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312–18 [DOI] [PubMed] [Google Scholar]
- 10.Webb WR, Muller NL, Naidich DP. High-resolution computed tomography findings of lung disease. In: Webb WR, Muller NL, Naidich DP, eds. High-resolution CT of the lung. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp 71–192 [Google Scholar]
- 11.Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327–31 [DOI] [PubMed] [Google Scholar]
- 12.Richards MJ, Edward JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 2000;21:510–15 [DOI] [PubMed] [Google Scholar]
- 13.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005;171:1209–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000;407:762–4 [DOI] [PubMed] [Google Scholar]
- 15.Gaynes R, Edwards JR. National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005;41:848–54 [DOI] [PubMed] [Google Scholar]
- 16.Fujitani S, Bhat S, Linden PK, Gunn S, Capitano B, Potoski B, et al. Causes and effects of inadequate empiric therapy for serious Pseudomonas aeruginosa infection. Washington, DC: American Society of Microbiology; 2004 [Google Scholar]
- 17.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2001;122:2115–21 [DOI] [PubMed] [Google Scholar]
- 18.Blanquer J, Blanquer R, Borras R, Nauffal D, Morales P, Menendez R, et al. Aetiology of community acquired pneumonia in Valencia, Spain: a multicenter prospective study. Thorax 1991;46:508–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neill AM, Martin IR, Weir R, Anderson R, Chereshsky A, Epton MJ, et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax 1996;51:1010–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rello J, Rodriguez A, Torres A, Roig J, Sole-Violan J, Garnacho-Montero J, et al. Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J 2006;27:1210–16 [DOI] [PubMed] [Google Scholar]
- 21.von Baum H, Welte T, Marre R, Suttorp N, Ewig S; CAPNETZ study group Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J 2010;35:598–605 [DOI] [PubMed] [Google Scholar]
- 22.Pennington JE. Pseudomonas aeruginosa pneumonia and other respiratory tract infections. In: Baltch AL, Smith RP, eds. Pseudomonas aeruginosa: infection and treatment. New York, NY: Marcel Dekker; 1994. pp 159–73 [Google Scholar]
- 23.Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 1991;143:1121–9 [DOI] [PubMed] [Google Scholar]
- 24.Fagon JY, Chastre J. Management of suspected ventilator-associated pneumonia. Ann Intern Med 2000;133:1009. [DOI] [PubMed] [Google Scholar]
- 25.Fagon JY, Chastre J, Domart Y, Trouillet JL, Gibert C. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: assessment by quantitative culture of samples obtained by a protected specimen brush. Clin Infect Dis 1996;23:538–42 [DOI] [PubMed] [Google Scholar]
- 26.Renner RR, Coccaro AP, Heitzman ER, Dailey ET, Markarian B. Pseudomonas pneumonia: a prototype of hospital-based infection. Radiology 1972;105:555–62 [DOI] [PubMed] [Google Scholar]
- 27.Okada F, Ando Y, Honda K, Nakayama T, Ono A, Tanoue S, et al. Acute Klebsiella pneumoniae pneumonia alone and with concurrent infection: comparison of clinical and thin-section CT findings. Br J Radiol 2010;83:854–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada F, Ando Y, Wakisaka M, Matsumoto S, Mori H. Chlamydia pneumoniae pneumonia and Mycoplasma pneumoniae pneumonia: comparison of clinical findings and CT findings. J Comput Assist Tomogr 2005;29:626–32 [DOI] [PubMed] [Google Scholar]
- 29.Okada F, Ando Y, Tanoue S, Ishii R, Matsushita S, Ono A, et al. Radiological findings in acute Haemophilus influenzae pulmonary infection. Br J Radiol 2012;85:121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada F, Ando Y, Nakayama T, Tanoue S, Ishii R, Ono A, et al. Pulmonary thin-section CT findings in acute Moraxella catarrhalis pulmonary infection. Br J Radiol 2011;84:1109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morikawa K, Okada F, Ando Y, Ishii R, Matsushita S, Ono A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus pneumonia: comparison of clinical and thin-section CT findings. Br J Radiol 2012;85:e168–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada F, Ando Y, Matsushita S, Ishii R, Nakayama T, Morikawa K, et al. Thin-section computed tomography findings of patients with acute Streptococcus pneumoniae pneumonia with and without concurrent infection. Br J Radiol 2012;85:e357–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada F, Ando Y, Honda K, Nakayama T, Kiyonaga M, Ono A, et al. Clinical and pulmonary thin-section CT findings in acute Klebsiella pneumoniae pneumonia. Eur Radiol 2009;19:809–15 [DOI] [PubMed] [Google Scholar]
- 34.Stanly MM. Bacillus pyocyaneus infections; a review, report of cases and discussion of newer therapy including streptomycin. Am J Med 1947;2:347–67 [DOI] [PubMed] [Google Scholar]
- 35.Hassett DJ, Ma JF, Elkins JG, McDermott TR, Ochsner UA, West SE, et al. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol Microbiol 1999;34:1082–93 [DOI] [PubMed] [Google Scholar]


