Abstract
We review the appearance of scleroma in the head and neck on imaging. Scleroma is a chronic granulomatous disease that primarily affects the nasal cavity, but the pharynx and larynx may also be involved. On imaging, nasal scleroma appears as bilateral or unilateral expanded homogeneous nasal masses that may exhibit hyperintense signal on T1 weighted images. Pharyngeal scleroma commonly narrows the pharyngeal lumen and may involve the soft and hard palate. Imaging is essential to detect the extent of subglottic stenosis in patients with laryngeal scleroma. Rarely, scleroma may involve the orbit or the middle ear. Imaging is essential for the early diagnosis of scleroma and for differentiating it from other granulomatous and neoplastic lesions. Also, imaging is important for treatment planning and follow-up of patients after therapy.
Scleroma is a chronic granulomatous disease affecting the upper respiratory airway. The causative organism is Klebsiella rhinoscleromatis, which is a Gram-negative bacterium. Rhinoscleroma is transmitted by means of direct inhalation of droplets or contaminated material. Scleroma usually begins at the nose and may progress to involve the larynx, pharynx or other regions of the neck. The term scleroma is preferred over the term rhinoscleroma because this disease affects not only the nose. Patients may present with non-specific rhinitis symptoms, signs of granulomatous proliferation or scarring. Scleroma tends to progress slowly over many years, and is characterised by periods of remission and relapse. When the disease progresses with proliferation, it may simulate a tumour. At a later stage, it may produce scarring of the respiratory tract. Early diagnosis of scleroma by differentiating it from other granulomatous or neoplastic lesions is critical to avoid recurrence and late sequelae [1-4].
Scleroma is commonly seen in tropical and temperate zones, such as Africa, Asia, eastern Europe, South America and Central America. Scleroma primarily affects the nasal cavity (95–100%), but the nasopharynx (18–43%), oropharynx (13–35%), larynx (15–40%), trachea (12%) and bronchi (2–7%) can also be involved [4]. Scleroma is rarely seen in the orbit, middle ear and cervical lymph nodes. Nasal and pharyngeal scleroma is more common in males (male-to-female ratio, 2:1), and laryngotracheal scleroma is more common in females (female-to-male ratio, 4:1). It is commonly seen in the second to fourth decade of life. Scleroma occurs at the junction regions where two types of epithelium meet. Nasal scleroma starts where squamous epithelium of the vestibule meets the ciliary epithelium of the nose. Pharyngeal scleroma is usually located in the transition between the respiratory and squamous epithelium at the naso-oropharyngeal junction. Laryngeal scleroma is located below the true vocal cord, where the transitional epithelium of the true vocal cord merges with the columnar epithelium of the subglottic area [2-6].
This article reviews the imaging of scleroma in the head and neck.
Stages of scleroma
The disease has three stages: the initial rhinitic (catarrhal, atrophic) stage; the granulomatous (hypertrophic) stage; and the sclerotic (fibrotic) stage. In the rhinitic stage, there is thickened mucosa with squamous metaplasia. Patients have non-specific rhinitis symptoms that progress to foetid odour, nasal crusting and nasal obstruction. The diagnostic features of rhinoscleroma are found in the granulomatous stage. In the granulomatous stage, there are masses of granulation tissue consisting of plasma cells, Russell bodies (elliptical structures thought to represent degenerated plasma cells) and Mikulicz cells (large vacuolated foamy histiocytes containing bacilli of K. rhinoscleromatis) (Figure 1). Patients present with nasal obstruction, deformity, epistaxis and anosmia, and anaesthesia of the soft palate may occur. In the fibrotic stage, there is scar tissue with foci of plasma and few or no Mikulicz cells or Russell bodies. This stage is characterised by nasal scarring, nasal vestibular stenosis with dysphonia and stridor [1-4].
Figure 1.

Pathology of scleroma. Microscopic examination of scleroma shows that many Mikulicz cells (long arrow) are present with scattered Russell bodies (short arrow) and many plasma cells. Haematoxylin and eosin, original magnification ×3400.
Nasal and paranasal sinus scleroma
Nasal scleroma is commonly seen as either symmetrical or asymmetrical bilateral masses in two-thirds of patients, and may be unilateral in one-third of patients. In the catarrhal stage, imaging demonstrates nasal soft-tissue nodules and mucosal thickening simulating non-specific sinusitis. In the hypertrophic stage, scleroma appears as a well-defined mass of homogeneous density in the nasal cavity [5-9]. On MRI, the mass may show high signal intensity on T1 weighted images owing to the high protein content within Mikulicz cells and Russell bodies (Figure 2). There is slight hyperintensity on T2 weighted images because of the cellular component of scleroma; this is associated with hypointense foci within the lesion owing to fibrosis. After contrast administration, the lesion shows an inhomogeneous pattern of contrast enhancement [10]. At diffusion MRI, the lesions exhibit restricted diffusion with low apparent diffusion coefficients (ADCs) that simulate malignancy owing to the presence of fatty cells within the lesion and high cellularity (Figure 3) [11,12]. The middle and the inferior turbinates are always affected, with atrophy or complete destruction, and the nasal septum may be destroyed [13].
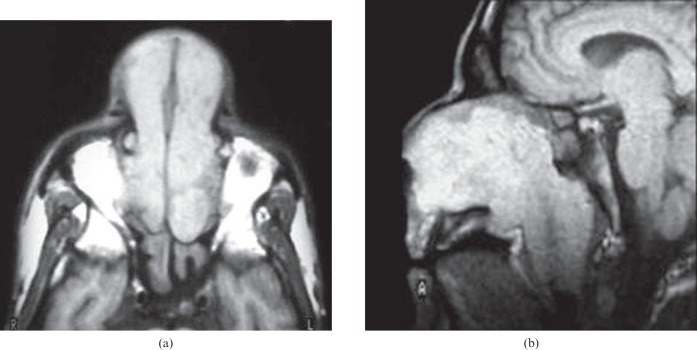
Figure 2.
Nasal scleroma. (a) Axial T1 weighted image shows a bilateral hyperintense nasal mass that extends through the anterior nares associated with retained secretions in the sphenoid sinus. (b) Sagittal T1 weighted image of another patient shows hyperintense signal intensity of the nasal mass that passes through the posterior choana and obliterates the nasopharyngeal airway.
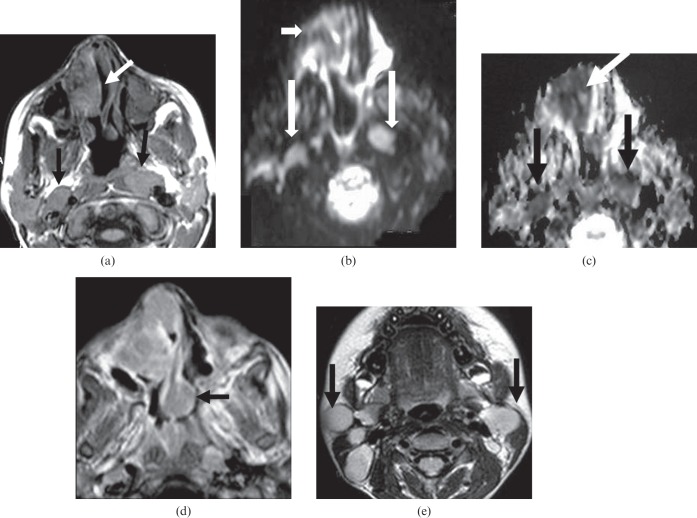
Figure 3.
Nasal and nodal scleroma. (a) Axial T1 weighted image shows right nasal mass with intermediate signal intensity similar to muscle with areas of increased signal intensity within the lesion (white arrow). It is associated with enlarged retropharyngeal lymph nodes (black arrows). (b) Axial diffusion-weighted MR image at B=1000 mm2 s−1 shows restricted diffusion of the nasal mass (short arrow) and retropharyngeal lymph nodes (long arrows). (c) Axial apparent diffusion coefficient (ADC) map shows low ADCs of the nasal mass (white arrow) and retropharyngeal lymph nodes (black arrows). (d) Axial contrast T1 weighted image at another level shows the nasal mass associated with infiltration and enlargement of the nasal septum (black arrow). (e) Axial T2 weighted image shows multiple enlarged upper deep cervical lymph nodes (black arrows).
Scleroma may extend into the maxillary sinus through the ostiomeatal unit in 40% of patients with nasal scleroma [14,15]. Also, scleroma has been reported in the sphenoid and ethmoid sinus with intracranial extensions [15]. It commonly has a scalloped edge, which lines the sinus wall, or it may absorb the sinus wall with extramaxillary sinus extensions. Involvement of the sinus wall is related to the chronic progress of this disease [16]. This finding helps to indicate a granulomatous lesion and to differentiate it from malignant lesions.
Pharyngeal and palatal scleroma
Pharyngeal involvement is seen in about one-third of patients with scleroma. Pharyngeal scleroma develops as a result of contiguous extension along the palate, lateral wall of the nasopharynx and faucial pillars [17,18]. The bulky granuloma projecting from the nasal cavity may deform the nasopharyngeal lumen (Figure 2). Also, the granuloma may creep around or obstruct the cartilaginous part of the Eustachian tube with retained secretions in the middle ear cavity [5,17]. The oropharyngeal lumen may be narrowed as a result of associated fibrosis, with subsequent calcification along the wall of the oropharynx with abscesses in the tonsillar regions (Figure 4).
Figure 4.
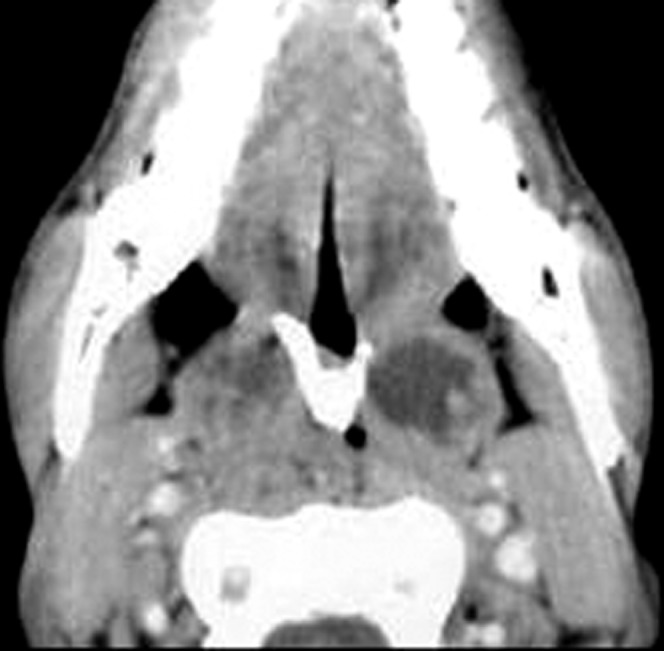
Pharyngeal scleroma. Axial contrast CT scan of the oropharynx shows marked narrowing of the oropharyngeal airway with abscesses in both tonsillar regions and V-shaped calcification along the soft palate.
The soft palate is more frequently involved than the hard palate. The soft palate is thickened at its attachment to the hard plate (Figure 3), which may taper off towards its free margin with a V-shaped deformity. When the hard palate is affected, there is bone destruction [19].
Laryngotracheal scleroma
Laryngeal scleroma usually follows the nasal infection and is commonly seen in the subglottic region at the fibrotic stage. It appears as symmetrical or asymmetrical circumferential subglottic stenosis with extension into the trachea and the bronchi (Figure 5). It may appear as a thickened epiglottis, aryepiglottic fold and vocal cords, and, rarely, there is concentric transglottic narrowing. There is concentric symmetrical or asymmetrical narrowing of the trachea, which may be associated with crypt-like irregularity. Tracheal scleroma is usually seen in continuity with laryngeal scleroma. Imaging is essential to determine the extent of scleroma for treatment planning [20-22].
Figure 5.
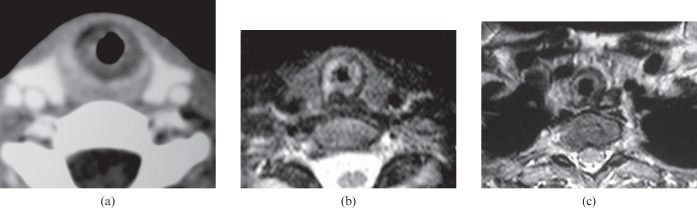
Laryngeal scleroma. (a) Axial CT scan of the larynx shows a circumferential narrowed subglottic region. (b) Axial T2 weighted image shows an asymmetrical narrowed subglottic region. (c) Axial T2 weighted image at the lower level shows extension of the lesion into the trachea.
Orbital and skull base scleroma
Scleroma may extend into the orbit through the nasolacrimal duct and present variably as obstruction and/or infection of the lacrimal passages. Also, scleroma may erode and extend through the ethmoidal air cells or maxillary sinus into the orbit [9,23]. Rarely, scleroma may infiltrate the skull base with intracranial extension through the cribriform plate or lesser wing of sphenoid into the anterior cranial fossa and cavernous sinus. This progression may lead to cranial nerve palsies. It has been associated with septic infarction and occlusion of the internal carotid artery in one case [8].
Middle ear scleroma
Scleroma of the middle ear cavity may be isolated or may be associated with nasal scleroma. It may extend via the Eustachian tube into the mesotympanum. Scleroma may simulate cholesteatoma of the middle ear cavity and may be associated with some ossicular erosion without sclerosis [5,23].
Nodal scleroma
Scleroma may involve the cervical lymph nodes [24]. The affected enlarged lymph nodes show low signal intensity on T1 weighted images and high signal intensity on T2 weighted images with a homogeneous pattern of contrast enhancement. These nodes exhibit restricted diffusion on diffusion MRI with a low ADC on the ADC map (Figure 2), which may simulate malignant nodes. This is attributed to fatty tissue within the Mikulicz cells [11,12].
Differential diagnosis
Differentiation of scleroma from other granulomatous or neoplastic lesions is essential for an accurate diagnosis. The presence of specific clinical signs such as nasal crust, expanded nose and foetid odour with remission can indicate a diagnosis of scleroma. Imaging is suggestive but not definitive in differentiating all forms of scleroma from more sinister pathology. Imaging is essential and directs us towards the diagnosis, but not in all cases. Biopsy still has a role. Bilaterally, expansion of the nasal cavity, scalloping of the sinus wall, lack of bone destruction and hyperintensity of the nasal mass on T1 weighted images help to differentiate nasal scleroma from nasal malignancies. Pharyngeal scleroma can be differentiated from malignancy by a narrowed pharyngeal lumen with no definite mass along the pharyngeal wall detected. Palatal scleroma is differentiated from neoplastic lesions by a V-shaped deformity of the palate. Laryngeal scleroma can be differentiated from laryngeal tuberculosis, which is commonly seen in the supraglottic region, as well as from laryngeal cancer by its subglottic position, with tracheal invasion without a supraglottic or glottic mass, which is not seen with laryngeal cancer [1-6].
Post-treatment imaging
A great variety of broad-spectrum antibiotics, surgery and laser procedures have been used in the treatment of scleroma. Surgical removal of the nasal granuloma may be used in aggressive granulomatous nasal lesions. Surgery, laser or tracheotomy may be used in cases of laryngeal scarring with airway obstruction [2-4]. Imaging is important for pre-operative treatment planning to detect extension of the lesion as well as for follow-up of patients after treatment to detect residual or early recurrence to avoid complications.
Conclusion
We conclude that imaging is essential for the initial diagnosis, accurate localisation and determination of the extent of the scleroma, for differentiation from neoplastic and granulomatous lesions, and for monitoring patients with scleroma after therapy.
Footnotes
This review was presented as an educational exhibit at the 106th annual meeting of the American Roentgen Ray Society (ARRS) held 30 April–5 May, 2006, in Vancouver, BC, Canada.
References
- 1.Zhong Q, Guo W, Chen X, Ni X, Fang J, Huang Z, et al. Rhinoscleroma: a retrospective study of pathologic and clinical features. J Otolaryngol Head Neck Surg 2011;40:167–74 [PubMed] [Google Scholar]
- 2.Fawaz S, Tiba M, Salman M, Othman H. Clinical, radiological and pathological study of 88 cases of typical and complicated scleroma. Clin Respir J 2011;5:112–21 [DOI] [PubMed] [Google Scholar]
- 3.Abdel Razek A, Castillo M. Imaging appearance of granulomatous lesions of head and neck. Eur J Radiol 2010;76:52–60 [DOI] [PubMed] [Google Scholar]
- 4.Bailhache A, Dehesdin D, François A, Marie J, Choussy O. Rhinoscleroma of the sinuses. Rhinology 2008;46:338–41 [PubMed] [Google Scholar]
- 5.Navazo Eguía AI, García Vicario F. Rhinoscleroma. Acta Otorrinolaringol Esp 2010;61:160–2 [PubMed] [Google Scholar]
- 6.Zhong Q, Huang Z, Guo W, Zhang S, Ge W. Rhinoscleroma: case report. Am J Otolaryngol 2010;31:381–3 [DOI] [PubMed] [Google Scholar]
- 7.Moraes M, Magalhães A, Marinho L, Azevedo A, Carneiro F, Raymundo I. Rhinoscleroma causing severe bilateral nasal obstruction. Braz J Infect Dis 2010;14:190–2 [DOI] [PubMed] [Google Scholar]
- 8.Becker T, Shum T, Waller T, Meyer P, Segall H, Gardner F, et al. Radiological aspects of rhinoscleroma. Radiology 1981;141:433–8 [DOI] [PubMed] [Google Scholar]
- 9.Le Hir P, Marsot-Dupuch K, Bigel P, Elbigourmie TM, Jacquier I, Brunereau L, et al. Rhinoscleroma with orbital extension: CT and MRI. Neuroradiology 1996;38:175–8 [DOI] [PubMed] [Google Scholar]
- 10.Abdel Razek A, Elasfour A. MRI appearance of rhinoscleroma. AJNR Am J Neuroradiol 1999;20:575–8 [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel Razek A, Gaballa G, Elhawarey G, Megahed A, Hafez M, Nada N. Characterization of pediatric head and neck masses with diffusion weighted MR imaging. Eur Radiol 2009;19:201–8 [DOI] [PubMed] [Google Scholar]
- 12.Abdel Razek A. Diffusion-weighted magnetic resonance imaging of head and neck. J Comput Assist Tomogr 2010;34:808–15 [DOI] [PubMed] [Google Scholar]
- 13.Shoeib MA. Septal rhinoscleroma. Indian J Plast Surg 2010;43:219–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsot-Dupuch K, Le Hir P. Erdheim-Chester disease: a sinonasal lesion mimicking rhinoscleroma. Neuroradiology 2000;42:625. [DOI] [PubMed] [Google Scholar]
- 15.Evrard I, Gruyer X, Desse P, Francois A, Marie JP, Dehesdin D, et al. Spheno-ethmoidal rhinoscleroma. Report of a case and review of the literature. Ann Otolaryngol Chir Cervicofac 1998;115:85–8 [PubMed] [Google Scholar]
- 16.Bhowate RR, Degwekar S, Rawlani S, Dangore S. Rhinoscleroma with involvement of the maxillary sinus, orbital floor, and temporomandibular joint: a case report. J Oral Maxillofac Surg 2012;70:135–40 [DOI] [PubMed] [Google Scholar]
- 17.Dawlatly EE, Anim JT, Baraka ME. Local iatrogenic complications in nasopharyngeal rhinoscleroma. J Laryngol Otol 1988;102:1115–18 [DOI] [PubMed] [Google Scholar]
- 18.Castillo M. CT findings in a case of pharyngeal rhinoscleroma. AJNR Am J Neuroradiol 1993;14:770. [PMC free article] [PubMed] [Google Scholar]
- 19.Hasson O, Levi G, Huszar M. Scleroma of the soft and hard palate. J Oral Maxillofac Surg 2005;63:1536–8 [DOI] [PubMed] [Google Scholar]
- 20.Muñoz-Saavedra D, Olavarría-Leiva C. Laryngeal stenosis as late manifestation of rhinoscleroma. Case report. Acta Otorrinolaringol Esp 2010;61:241–3 [DOI] [PubMed] [Google Scholar]
- 21.Fajardo-Dolci G, Chavolla R, Lamadrid-Bautista E, Rizo-Alvarez J. Laryngeal scleroma. J Otolaryngol 1999;28:229–31 [PubMed] [Google Scholar]
- 22.Abou-Seif SG, Baky FA, El-Ebrashy F, Gaafar HA. Scleroma of the upper respiratory passage: a CT study. J Laryngol Otol 1991;105:198–202 [DOI] [PubMed] [Google Scholar]
- 23.Yassin A, Safwat F. Unusual features of rhinoscleroma. J Laryngol Otol 1996;80:524–32 [DOI] [PubMed] [Google Scholar]
- 24.Kasper H, Hegenbarth V, Buhtz P. Rhinoscleroma associated with Rosai-Dorfman reaction of regional lymph nodes. Pathol Int 2004;54:101–4 [DOI] [PubMed] [Google Scholar]