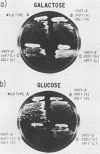
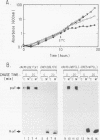
Abstract
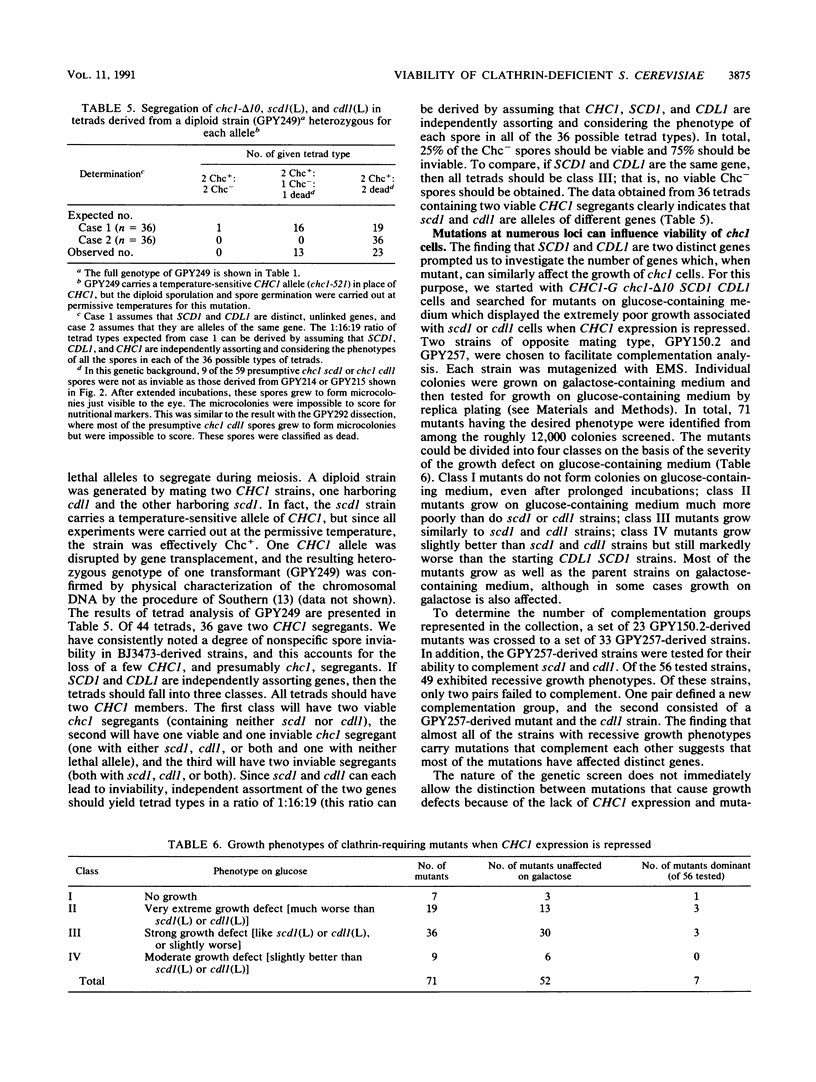
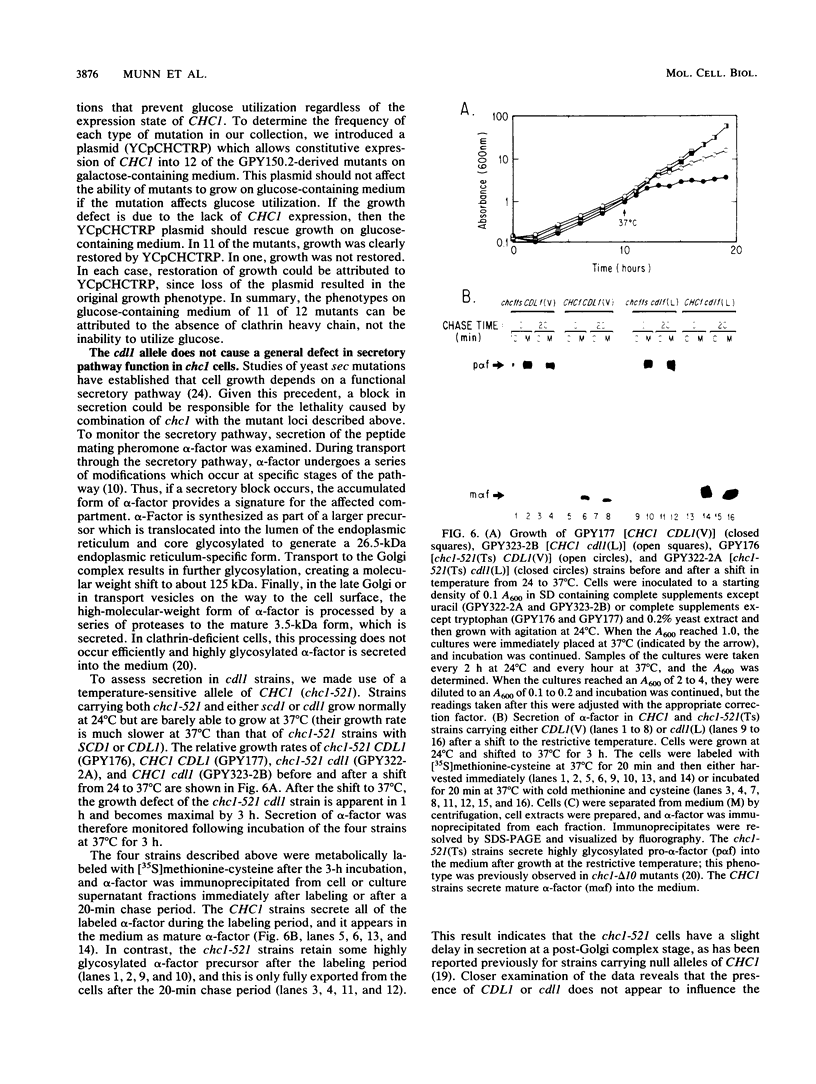
The gene encoding clathrin heavy chain in Saccharomyces cerevisiae (CHC1) is not essential for growth in most laboratory strains tested. However, in certain genetic backgrounds, a deletion of CHC1 (chc1) results in cell death. Lethality in these chc1 strains is determined by a locus designated SCD1 (suppressor of clathrin deficiency) which is unlinked to CHC1 (S. K. Lemmon and E. W. Jones, Science 238:504-509, 1987). The lethal allele of SCD1 has no effect on cell growth when the wild-type version of CHC1 is present. This result led to the proposal that most yeast strains are viable in the absence of clathrin heavy chain because they possess the SCD1 suppressor. Discovery of another yeast strain that cannot grow without clathrin heavy chain has allowed us to perform a genetic test of the suppressor hypothesis. Genetic crosses show that clathrin-deficient lethality in the latter strain is conferred by a single genetic locus (termed CDL1, for clathrin-deficient lethality). By constructing strains in which CHC1 expression is regulated by the GAL10 promoter, we demonstrate that the lethal alleles of SCD1 and CDL1 are recessive. In both cases, very low expression of CHC1 can allow cells to escape from lethality. Genetic complementation and segregation analyses indicate that CDL1 and SCD1 are distinct genes. The lethal CDL1 allele does not cause a defect in the secretory pathway of either wild-type or clathrin heavy-chain-deficient yeast. A systematic screen to identify mutants unable to grow in the absence of clathrin heavy chain uncovered numerous genes similar to SCD1 and CDL1. These findings argue against the idea that viability of chc1 cells is due to genetic suppression, since this hypothesis would require the existence of a large number of unlinked genes, all of which are required for suppression. Instead, lethality appears to be a common, nonspecific occurrence when a second-site mutation arises in a strain whose cell growth is already severely compromised by the lack of clathrin heavy chain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldari C., Murray J. A., Ghiara P., Cesareni G., Galeotti C. L. A novel leader peptide which allows efficient secretion of a fragment of human interleukin 1 beta in Saccharomyces cerevisiae. EMBO J. 1987 Jan;6(1):229–234. doi: 10.1002/j.1460-2075.1987.tb04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M. Living with clathrin: its role in intracellular membrane traffic. Science. 1988 Dec 9;242(4884):1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Hanspal M., Luna E., Branton D. The association of clathrin fragments with coated vesicle membranes. J Biol Chem. 1984 Sep 10;259(17):11075–11082. [PubMed] [Google Scholar]
- Harrison S. C., Kirchhausen T. Clathrin, cages, and coated vesicles. Cell. 1983 Jul;33(3):650–652. doi: 10.1016/0092-8674(83)90007-7. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Hoyt M. A., Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemmon S. K., Jones E. W. Clathrin requirement for normal growth of yeast. Science. 1987 Oct 23;238(4826):504–509. doi: 10.1126/science.3116672. [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Branton D. Identification of coated vesicles in Saccharomyces cerevisiae. J Cell Biol. 1984 Jan;98(1):341–346. doi: 10.1083/jcb.98.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Payne G. S. Genetic analysis of clathrin function in yeast. J Membr Biol. 1990 Jun;116(2):93–105. doi: 10.1007/BF01868668. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Hasson T. B., Hasson M. S., Schekman R. Genetic and biochemical characterization of clathrin-deficient Saccharomyces cerevisiae. Mol Cell Biol. 1987 Nov;7(11):3888–3898. doi: 10.1128/mcb.7.11.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985 Nov 29;230(4729):1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. Clathrin: a role in the intracellular retention of a Golgi membrane protein. Science. 1989 Sep 22;245(4924):1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schekman R. Protein localization and membrane traffic in yeast. Annu Rev Cell Biol. 1985;1:115–143. doi: 10.1146/annurev.cb.01.110185.000555. [DOI] [PubMed] [Google Scholar]
- Sherman F., Wakem P. Mapping yeast genes. Methods Enzymol. 1991;194:38–57. doi: 10.1016/0076-6879(91)94006-x. [DOI] [PubMed] [Google Scholar]
- Silveira L. A., Wong D. H., Masiarz F. R., Schekman R. Yeast clathrin has a distinctive light chain that is important for cell growth. J Cell Biol. 1990 Oct;111(4):1437–1449. doi: 10.1083/jcb.111.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]