Abstract
Objective
The aim of this study was to assess the impact of dose escalation on the proportion of patients requiring MR image-guided optimisation rather than standard Manchester-based CT-guided planning, and the level of escalation achievable.
Methods
30 patients with cervical cancer treated with external beam radiotherapy and image-guided brachytherapy (IGBT) had MR images acquired at the first fraction of IGBT. Gross tumour volume and high-risk clinical target volume (HR CTV) were contoured and treatment plans retrospectively produced for a range of total 2-Gy equivalent (EQD2) prescription doses from 66 Gyα/β=10 to 90 Gyα/β=10 (HR CTV D90). Standard Manchester system-style plans were produced, prescribed to point A and then optimised where necessary with the aim of delivering at least the prescription dose to the HR CTV D90 while respecting organ-at-risk (OAR) tolerances.
Results
Increasing the total EQD2 from 66 Gyα/β=10 to 90 Gyα/β=10 increased the number of plans requiring optimisation from 13.3% to 90%. After optimisation, the number of plans achieving the prescription dose ranged from 93.3% (66 Gyα/β=10) to 63.3% (90 Gyα/β=10) with the mean±standard deviation for HR CTV D90 EQD2 from 78.4±12.4 Gyα/β=10 (66 Gyα/β=10) to 94.1±19.9 Gyα/β=10 (90 Gyα/β=10).
Conclusion
As doses are escalated, the need for non-standard optimised planning increases, while benefits in terms of increased target doses actually achieved diminish. The maximum achievable target dose is ultimately limited by proximity of OARs.
Advances in knowledge
This work represents a guide for other centres in determining the highest practicable prescription doses while considering patient throughput and maintaining acceptable OAR doses.
It is well recognised that MRI is superior to CT for imaging of cancer of the cervix, enabling accurate delineation of the target volume. Recommendations from the GEC-ESTRO [formed in 1990 from the amalgamation of The Groupe Européen de Curiethérapie (GEC) and the European Society for Therapeutic Radiology and Oncology (ESTRO)] working group introduced MRI-based target concepts and three-dimensional (3D) dose–volume evaluation and reporting [1,2]. However, the number of centres using MRI for brachytherapy planning is very low owing to a number of difficulties and costs, such as availability of an MRI scanner at the time of insertion, increased time for imaging and planning, greater uncertainties in applicator definition and patient unsuitability for MRI. In 2008 only 4.3% (2 out of 46) of UK brachytherapy centres had access to MRI for high-dose-rate (HDR) brachytherapy planning [3].
It has been reported in a number of single-centre studies that dose escalation in brachytherapy, with total doses of up to D90=90 Gyα/β=10 (total biological equivalent dose in 2-Gy fractions), for locally advanced cervical cancer can lead to increased local control [4-10]. It is not always possible to simply deliver standard Manchester dosimetry-type plans to this dose level without overdosing the radiosensitive organs at risk (OARs); therefore, some form of 3D image guidance is required.
CT-based Manchester-type plans can be produced to satisfy OAR constraints by simply scaling down the prescription dose. However, MRI-based planning allows further optimisation to ensure that target volume doses are satisfied alongside OAR tolerances [11,12]. This function becomes increasingly important as prescription doses are increased; however, it is inherently more time and resource consuming and therefore has implications for staffing, patient throughput, and patient comfort and compliance. The potential benefits in both increased tumour control and decreased toxicity using an image-guided brachytherapy (IGBT) approach must be weighed against the increase in workload and delays between insertion and treatment.
The changes in workflow required for individual plan optimisation compared with Manchester planning will be specific to the department in question. In this centre, patients receiving a standard fixed geometry insertion at fractions 1 and 2 proceed straight to treatment following a CT scan and verification of correct applicator placement. Treatment is given using a standard pre-calculated Manchester-type plan. The patient-specific plan and organ contouring are then completed retrospectively. If required, adjustments can be made at subsequent fractions, such as simple scaling of dwell times, or the use of different applicator sizes or packing. If MRI-guided optimised plans are created at these fractions the contouring and plan must be produced, checked and approved by physicists and the oncologist prior to treatment, thus delaying the patient's treatment by up to 3 h. This has many consequences, including increasing the chance of applicator movement, decreasing patient comfort and increasing staff workload in the time window between insertion and treatment. For a proportion of patients a Manchester-type plan will be adequate, deeming the delay unnecessary; for others, optimisation will be required leading to an improved plan for that patient.
A recent survey of international brachytherapy practice revealed that prescription doses vary significantly between centres, ranging from 56.8 to 115.4 Gy [biological equivalent dose in 2 Gy per fraction (EQD2)] [13]. For centres at the lower end of this range that are considering dose escalation it is important to consider the extra requirements for imaging and planning. This retrospective study aims to assess what proportion of patients would require MRI-optimised planning to achieve at least the prescription dose to the most exposed 90% of the high-risk clinical target volume (HR CTV D90) while respecting OAR tolerance for a range of dose levels, and what level of dose escalation can be achieved.
Methods and materials
Between April 2007 and February 2009, 30 patients treated with radical external beam radiotherapy (EBRT) and brachytherapy for cervical cancer were recruited to a study protocol at University Hospitals Bristol NHS Foundation Trust, Bristol, UK. Ethics approval was obtained from the UK Multi-site Research Ethics Committee prior to trial set-up and the study was run in compliance with the European Union Directive on good clinical practice for trials. Patients were enrolled with informed consent. All patients treated with brachytherapy for cervical cancer during the study period were offered entry into the trial, except those prevented from doing so by the exclusion criteria, which were inability to provide informed consent, contraindication to MRI scan and use of a non-standard or non-MRI-compatible intracavitary brachytherapy applicator. Patient demographics are shown in Table 1.
Table 1. Patient demographics.
| Patient characteristic | Number of patients |
| Age (years) | |
| Median | 52 |
| Minimum | 30 |
| Maximum | 83 |
| Histology | |
| Adenocarcinoma | 6 |
| Adenosquamous carcinoma | 1 |
| Squamous cell carcinoma | 22 |
| Not recorded | 1 |
| FIGO staging | |
| Ib2 | 2 |
| II | 14 |
| III | 10 |
| IVa | 4 |
FIGO, International Federation of Gynecology and Obstetrics.
EBRT consisting of 45.0–50.4 Gy in 25–28 fractions was followed by CT-guided HDR brachytherapy of 9.0–16.2 Gy in three fractions delivered over 1½ weeks based on standard Manchester plans, with doses simply scaled where OAR tolerance would otherwise be exceeded.
MRI-compatible titanium Fletcher-style brachytherapy applicator sets (Defined Geometry GM11006200; Varian Medical Systems, Palo Alto, CA) were inserted under general anaesthetic. The length of the single intra-uterine applicator was chosen to reach the cranial end of the uterine cavity and the two colpostats chosen to fill the upper vagina. Gauze packing was inserted around the upper vagina to move the bladder and rectum away from the high-dose area and to immobilise the applicators.
After brachytherapy applicator insertion for the first fraction, MRI scans (GE Sigma Ovation; GE Medical Systems, Milwaukee, WI) of the pelvis were obtained for each patient for the purposes of this study. These consisted of a sagittal view with 6-mm slices and either axial or para-axial (orthogonal to the intra-uterine tube) slices (4–7 mm). The slice thickness was chosen to achieve the best compromise between noise, resolution and scan time (minimising movement artefact). The accuracy of application definition was ensured via comparison with CT scans (Siemens SOMATOM® Plus 4; Siemens AG, Erlangen, Germany) obtained for treatment planning purposes immediately prior to the MRI scan. The acquisition of axial slices was changed to para-axial during the course of the study for ease of applicator definition. A distortion correction was applied to the MRI scan. The axial or para-axial MR images were subsequently imported into the brachytherapy planning system (BrachyVision; Varian Medical Systems). OAR (rectum, bladder and sigmoid colon) were retrospectively contoured by a radiation oncologist and target volumes [gross tumour volume (GTV), HR CTV], as defined in the GEC-ESTRO working group recommendations [1], contoured by a consultant radiologist.
Total doses to the target volume (from EBRT and brachytherapy) were converted to EQD2 by applying the linear quadratic model with α/β=10 Gy. Similarly, total OAR doses were converted using the linear quadratic model with α/β=3 Gy. Treatment plans were retrospectively produced on the MR images for total EQD2 prescription doses of 66 Gyα/β=10, 74 Gyα/β=10, 79 Gyα/β=10 and 90 Gyα/β=10. This consists of:
50.4 Gy (28 fractions of 1.8 Gy) EBRT, where the HR CTV and OARs are assumed to receive 100% of the prescription dose, according to the GEC-ESTRO recommendations [2]
three fractions of HDR brachytherapy with prescription doses of 4.5, 6, 7 or 8.7 Gy (three rather than four fractions are used at all levels to maintain consistency with current clinical practice).
The dosimetric parameters obtained for fraction 1 of the brachytherapy were assumed to be replicated for fractions 2 and 3 (since MRI was only obtained for the first fraction). However, it should be noted that OARs and target volumes may move between fractions so multiple imaging is required in clinical practice.
Standard Manchester system-type plans prescribed to point A were produced. If required, MRI-optimised plans were then produced with the aim of delivering at least the prescribed dose to the HR CTV D90 while limiting doses to OARs. The following EQD2 OAR tolerances were respected for each plan: 80 Gyα/β=3Gy for the bladder and 70 Gyα/β=3Gy for the rectum and rectosigmoid, following local protocol, with reference to the tolerances within the EMBRACE trial [14]. The relevant brachytherapy dose was the minimum dose to the most exposed 2 cm3 for each organ (D2 cc). Achieving OAR tolerance was mandatory for all optimised plans; however, plans were only considered acceptable if the minimum HR CTV D90 was also achieved.
When standard Manchester dose distributions did not achieve acceptable HR CTV D90 and/or OAR doses, the following plan optimisation procedure was followed, stopping at the step which achieved the planning criteria. This was repeated for each prescription dose level as required.
Point A doses were scaled until sufficient coverage was achieved, or until an OAR tolerance dose was reached. This results in symmetrically modified pear-shaped isodoses.
Relative weightings of each ovoid were altered to produce an asymmetrical dose distribution.
Dwell positions were shifted anteriorly or posteriorly to minimise rectum or bladder doses, respectively.
Individual dwell time weightings were adjusted for fine tuning of the optimisation if required. This was done manually when possible, although graphical optimisation tools were occasionally used.
HR CTV D90 doses greater than the prescription dose were not reduced unless required to achieve OAR tolerance. This technique was developed from the technique used by Kirisits et al [15] and ensures that practice does not deviate too far from the proven Manchester system. Interstitial needles were not considered in this study as they were not in local clinical use at this time.
GTV and HR CTV were recorded, and doses to target (HR CTV D90) and OARs (D2 cc) reported on both a per-fraction brachytherapy and total EQD2 basis, before and after optimisation.
Results
The GTV, HR CTV, bladder and rectosigmoid were contoured in all 30 patients. In one patient, owing to the angling of the MRI scan perpendicular to the intra-uterine tube, the rectum was not included in the scan; therefore, no rectal D2 cc was recorded for this patient. The mean GTV was 1.7 cm3 (0–9.7 cm3), and the mean HR CTV was 14.6 cm3 (1.5–30.2 cm3).
The number of patients for whom the standard Manchester plan was acceptable decreased rapidly with increasing prescription dose as tolerance was reached for OARs, increasing the number of plans requiring optimisation. Table 2 summarises the proportion of patients requiring optimisation at each intended dose level, and for what reason. With a standard Manchester plan the minimum intended dose to the HR CTV D90 was achieved in 26 out of 30 patients for all dose levels. The proportion of patients who had at least one OAR exceeding tolerance was 6.7% (66 Gyα/β=10), 50.0% (74 Gyα/β=10), 73.3% (79 Gyα/β=10) and 90.0% (90 Gyα/β=10).
Table 2. Results with the standard Manchester-type plan.
| Intended minimum EQD2 to HR CTV D90 |
66 Gy |
74 Gy |
79 Gy |
90 Gy |
| (intended minimum HR CTV D90 per fraction brachytherapy) | (4.5 Gy) | (6 Gy) | (7 Gy) | (8.7 Gy) |
| Standard plan acceptable | 86.7% | 50.0% | 26.7% | 10.0% |
| HR CTV D90 <intended with standard plan | 13.3% | 13.3% | 13.3% | 13.3% |
| OAR exceeding tolerance | 6.7% | 50.0% | 70.0% | 83.35% |
| Bladder | 6.7% | 40.0% | 50.0% | 70.0% |
| Rectum (of 29) | 0.0% | 13.8% | 24.1% | 62.1% |
| Rectosigmoid | 0.0% | 10.0% | 30.0% | 56.7% |
D90, minimum dose to exposed 90%; EQD2, 2-Gy equivalent doses; HR CTV, high-risk clinical target volume; OAR, organ at risk.
Table 3 illustrates the results achieved for the whole cohort after optimisation where necessary and the type of optimisation technique used at each dose level. When an acceptable plan was not achieved, all OARs were in tolerance but the HR CTV D90 was less than intended. The optimisation process required just simple rescaling of the dose in 2 out of 4 patients (66 Gyα/β=10), 11 out of 15 (74 Gyα/β=10), 18 out of 22 (79 Gyα/β=10) and 17 out of 27 (90 Gyα/β=10), with the remaining patients requiring more complex adjustments.
Table 3. Results with optimisation when necessary.
| Intended minimum EQD2 to HR CTV D90 |
66 Gy |
74 Gy |
79 Gy |
90 Gy |
| (intended minimum HR CTV D90 per fraction brachytherapy) | (4.5 Gy) | (6 Gy) | (7 Gy) | (8.7 Gy) |
| Total number requiring optimisation | 4 | 15 | 21 | 27 |
| Rescaled | 2 | 11 | 17 | 17 |
| Weightings adjusted | 1 | 1 | 1 | 1 |
| Dwells shifted | 0 | 1 | 1 | 2 |
| Manual dwell time adjustments | 1 | 2 | 2 | 4 |
| Graphical optimisation tools | 0 | 0 | 0 | 3 |
| Proportion achieving acceptable plan | 93.3% | 86.7% | 83.3% | 63.3% |
| Mean (±SD) EQD2 for final plan (Gy) | 78.4 (12.4) | 87.8 (14.8) | 90.9 (16.2) | 94.1 (19.9) |
| Mean (±SD) HR CTV D90 per fraction brachytherapy for final plan (Gy) | 6.67 (2.02) | 8.15 (2.24) | 8.59 (2.37) | 9.00 (2.69) |
D90, minimum dose to most exposed 90%; EQD2, 2-Gy equivalent doses; HR CTV, high-risk clinical target volume; OAR, organ at risk; SD, standard deviation.
The mean D90 values are larger than the intended dose for all dose levels, since the dose was not reduced (from the standard Manchester plan) when it exceeded the intended value unless this benefited the OARs. The differences between the HR CTV D90s achieved for the different dose levels were statistically significant (p<0.0001) using analysis of variance repeated measures; however, a pairwise comparison showed that the largest differences were between 66 Gyα/β=10 and all the higher doses, with no significant difference between 74 and 79 Gyα/β=10 or 79 and 90 Gyα/β=10. Figure 1 (box and whisker plot) illustrates the distribution of D90 values achieved at each prescription level, clearly showing the biggest change from 66 to 74 Gyα/β=10.
Figure 1.
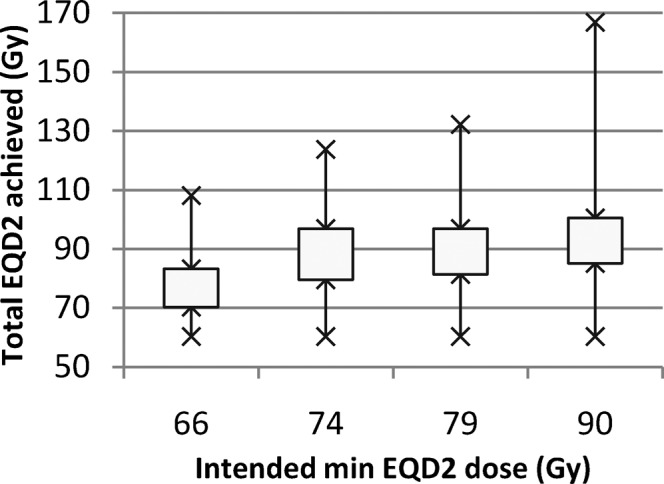
Box and whisker plot illustrating distribution of 2-Gy equivalent dose (EQD2) high-risk clinical target volume D90 values after optimisation. D90, minimum dose to most exposed 90%.
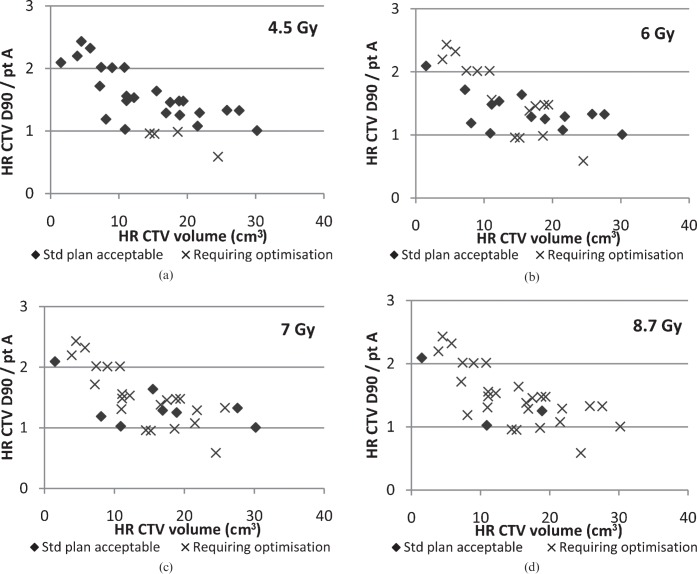
The relationship between HR CTV and dose is illustrated in Figure 2, which shows the ratio of HR CTV D90 to point A dose with HR CTV for the standard plan, with those requiring optimisation at each dose level highlighted. When the ratio of HR CTV D90 to point A has a value ≥1 this indicates that the HR CTV is receiving at least the intended dose with the standard plan; therefore, the need for optimisation in these cases is solely on the basis of OAR dose. These graphs show no correlation between HR CTV and the need for optimisation in order to achieve an acceptable plan. The maximum HR CTV in this study was 30.2 cm3. Similarly, no correlation was seen with GTV.
Figure 2.
(a–c) Requirement for optimisation at each dose level with ratio of point A (ptA) to high-risk clinical target volume D90 (HR CTV D90) doses. D90, minimum dose to most exposed 90%; HR CTV, high-risk clinical target volume; Std, standard.
Discussion
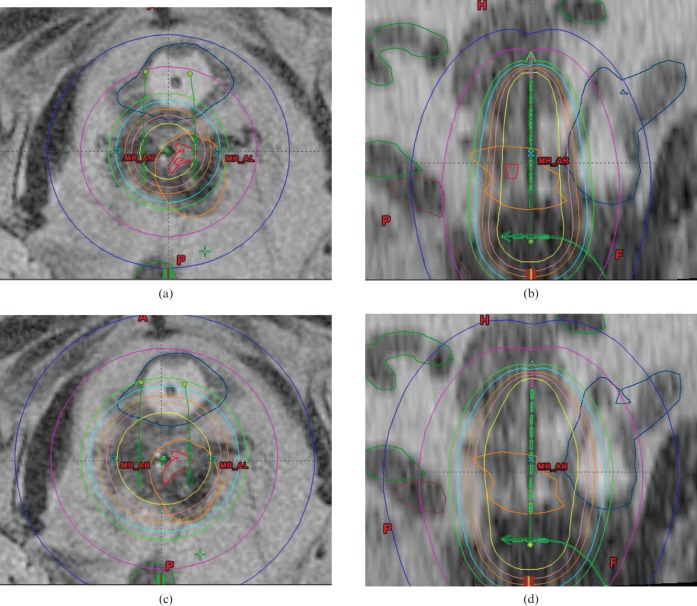
The requirement for optimisation increases as the prescribed dose increases because the absolute maximum D2 cc doses to the OARs remain constant. The OARs in close proximity to the treatment volume will always limit the maximum dose deliverable because of the fixed geometry of the applicators and the limited number of ways in which the dose distribution can be modified without introduction of interstitial needles. Hence, choosing a high intended prescription dose will not deliver a high prescription dose in all patients. See Figure 3 for an example when HR-CTV D90 is less than the prescription dose using the standard Manchester plan. Simply scaling up the dwell times resulted in an overdose to the bladder at higher prescription doses; therefore, a graphical optimisation tool was used to produce an acceptable plan.
Figure 3.
Example of high-risk clinical target volume dose not achieved with the standard plan, compromised by bladder proximity at D90 ≥74 Gy, optimised using graphical optimisation tools. (a) Standard plan (axial). (b) Standard plan (sagittal). (c) Optimised plan (axial). (d) Optimised plan (sagittal). Cyan, prescription isodose; D90, minimum dose to most exposed 90%.
For an intended minimum total EQD2 dose of 74 Gyα/β=10 to the HR CTV D90, the mean dose achieved was 87.8 Gyα/β=10 with 50% of patients requiring optimisation, whereas increasing the intended minimum dose to 90 Gyα/β=10 produced only a small increase in the mean dose achieved to 94.1 Gyα/β=10 with 90% of patients requiring optimisation. This represents a large potential delay between insertion and treatment for a limited improvement in target coverage to a decreasing number of patients. For instance, in 47% of cases the optimised plan produced for 74 Gyα/β=10 could not be improved upon at higher dose levels. At 74 Gy, 86.7% achieved an acceptable plan, agreeing well with the results of Tan et al [9], who found that 85.7% achieved the intended target dose using CT to plan to the cervix. That study only limited dose to the rectum, finding 61% to be in tolerance with a standard plan, compared with our 50%, which in addition limited dose to the bladder and rectosigmoid.
Non-standard planning has implications for patient comfort and throughput, as a single consultant cannot be contouring and discussing individual plans at the same time as performing insertions or treating other patients, whereas standard plans can be treated quickly and planned retrospectively at least for initial fractions.
Several single-centre studies have shown that patients receiving a higher dose to the HR CTV achieve higher rates of local control [4-10]. With a fixed geometry, the patients in whom those high doses can be achieved are those with small target volumes not compromised by proximity to OARs, who would tend to have a better prognosis overall, so the causality of the relationship between target dose and local control is not clear. A randomised controlled trial investigating local control for different prescribed doses would be required to determine the optimal dose for these patients [16].
According to Tanderup et al [12] patients with volumes greater than 30 cm3 are more likely to require optimisation as the ratio of HR CTV to point A dose decreases, but also that standard plans give larger doses to OARs in patients with smaller tumours owing to OARs being in closer proximity to the target and therefore to the applicators. The HR CTVs in this study are small in comparison, with a maximum of just 30.2 cm3; however, as doses were escalated optimisation was required to treat even very small targets because of high OAR doses.
The small HR CTVs found in this study could be due to a difference in the stage at which patients present, how patients are selected, or how EBRT and HDR brachytherapy are scheduled. At University Hospitals Bristol NHS Foundation Trust patients receive a full course of EBRT prior to starting HDR brachytherapy, allowing the maximum tumour shrinkage prior to commencing HDR brachytherapy and without creating gaps in the EBRT delivery. All treatment is given within 56 days.
The availability of MRI for all fractions will have an impact on whether target doses can be accurately assessed for all fractions, or whether assumptions must be made for subsequent fractions based on information from fraction 1. A limitation of this study is that it assumes three identical fractions of brachytherapy; although it can be argued that if the same applicator and plan are used then the target doses from fraction 1 should be representative of subsequent fractions, OAR doses will differ from fraction to fraction because of internal organ movement and filling. In practice, the OAR doses from fraction 1 can be used to influence subsequent fractions in terms of applicator choice, packing/rectal retraction and prescription dose, all of which can help manipulate the final dose while still using standard plans. Best clinical practice requires imaging at each fraction [17].
It is important to recognise that choice of OAR tolerances has the largest impact on the need for optimised planning. Late effects in the bladder and rectum follow a dose–response curve [18], so increasing tolerance levels in order to increase target doses or avoid optimisation will result in increased normal tissue toxicity. The results of Potter et al [10] show HR CTV D90 doses of 93±17 Gyα/β=3Gy but with a corresponding bladder EQD2 of 86±17 Gyα/β=3Gy (D2 cc), which would put most patients outside our chosen tolerance. The tolerances used in this study are conservative with respect to recent published guidelines [3,15,19] but are similar to those used by Tanderup et al [12]. Caution is advised when making any increases in tolerance doses in order that changes in toxicity rates can be monitored.
Conclusion
The potential benefits in both increased tumour control and decreased toxicity using an IGBT approach with individual patient-optimised planning must be weighed against the increase in workload and delays between insertion and treatment. Dose escalation in brachytherapy for cervical cancer will increase the number of patients requiring optimisation in order to limit doses to OARs, and it is doses to OARs that will ultimately limit the maximum achievable dose to the HR CTV. The requirement for optimisation is therefore highly sensitive to the choice of prescription dose and normal tissue tolerances. Optimisation has an impact on patient throughput and increases the treatment planning workload. As doses are escalated, the returns, in terms of increased target doses achieved, diminish.
Acknowledgments
John Hughes, Consultant Radiologist, Department of Radiology; Christopher Herbert, Consultant Oncologist, Department of Oncology; Pauline Humphrey, Consultant Radiographer, Department of Oncology; and Paul Cornes, Consultant Oncologist, Department of Oncology, University Hospitals Bristol NHS Foundation Trust, Bristol, UK.
References
- 1.Haie-Meder C, Potter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45 [DOI] [PubMed] [Google Scholar]
- 2.Potter R, Haie-Meder C, Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77 [DOI] [PubMed] [Google Scholar]
- 3.Tan L, Blake P, Hoskin P, Davidson S, Rathmell A, Bidmead M, et al. Implementing image-guided brachytherapy for cervix cancer in the UK. London, UK: Royal College of Radiologists; 2009 [Google Scholar]
- 4.Choy D, Wong LC, Sham J, Ngan HY, Ma HK. Dose-tumor response for carcinoma of cervix: an analysis of 594 patients treated by radiotherapy. Gynecol Oncol 1993;49:311–17 [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Kutuki S, Nishiguchi I, Shigematsu N, Kuribayashi T, Uematsu M, et al. Radiotherapy for cervical cancer with high-dose rate brachytherapy: correlation between tumour size, dose and failure. Radiother Oncol 1994;31:240–7 [DOI] [PubMed] [Google Scholar]
- 6.Perez C, Grigsby P, Clifford Chao K, Mutch D, Lockett M. Tumour size, irradiation dose and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys 1998;41:307–17 [DOI] [PubMed] [Google Scholar]
- 7.Potter R, Dimopoulos J, Georg P, Lang S, Waldhausl C, Wachter-Gerstner N, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007;83:148–55 [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos J, Lang S, Kirisits C, Fidarova E, Beger D, Georg P, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2009;75:56–63 [DOI] [PubMed] [Google Scholar]
- 9.Tan L, Coles C, Hart C, Tait E. Clinical impact of computed tomography-based image-guided brachytherapy of cervix cancer using the tandem-ring applicator: the Addenbrookes experience. Clin Oncol 2009;21:175–82 [DOI] [PubMed] [Google Scholar]
- 10.Potter R, Georg P, Dimopoulos J, Grimm M, Berger D, Nesvacil N, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks S, Bownes P, Lowe G, Bryant L, Hoskin P. Cervical brachytherapy utilizing ring applicator: comparison of standard and conformal loading. Int J Radiat Oncol Biol Phys 2005;63:934–9 [DOI] [PubMed] [Google Scholar]
- 12.Tanderup K, Nielsen S, Nyvang G, Pedersen E, Rohl L, Aagaard T, et al. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol 2010;94:173–80 [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan A, Creutzberg C, Craighead P, McCormack M, Toita T, Narayan K, et al. International brachytherapy practice patterns: a survey of the Gynecologic Cancer Intergroup (GCIG). Int J Radiat Oncol Biol Phys 2012;82:250–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimopoulos J, Stepien A, Pötter R, Lindegaard J, Kirisits C, Tanderup K, et al. A European study on MRI-guided brachytherapy in locally advanced cervical cancer (EMBRACE). Available from: www.embracestudy.dk/UserUpload/PublicDocuments/EmbraceProtocol.pdf. [Google Scholar]
- 15.Kirisits C, Potter R, Lang S, Dimopoulos J, Wachter-Gerstner N, Georg D. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2005;62:901–11 [DOI] [PubMed] [Google Scholar]
- 16.Perez C, Fox S, Lockett M, Grigsby P, Camel H, Galakatos A, et al. Impact of dose in outcome of irradiation alone in carcinoma of the uterine cervix: analysis of two different methods. Int J Radiat Oncol Biol Phys 1991;21:885–98 [DOI] [PubMed] [Google Scholar]
- 17.Kirisits C, Lang S, Dimopoulos J, Oechs K, Georg D, Potter R. Uncertainties when using only one MRI-based treatment plan for subsequent high-dose-rate tandem and ring applications in brachytherapy of cervix cancer. Radiother Oncol 2006;81:269–75 [DOI] [PubMed] [Google Scholar]
- 18.Georg P, Potter R, Georg D, Lang S, Dimopoulos J, Sturdza A, et al. Dose effect relationship for late side effects of the rectum and urinary bladder in magnetic resonance image-guided adaptive cervix cancer brachytherapy. Int J Radiat Oncol Biol Phys 2012;82:653–7 [DOI] [PubMed] [Google Scholar]
- 19.Viswanthan A, Beriwal S, De LosSantos J, Demanes D, Gaffney D, Hansen J, et al. American brachytherapy society consensus guidelines for locally advanced carcinoma of the cervix. Part II. High-dose-rate brachytherapy. Brachytherapy 2012;11:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]