Abstract
Objectives
Our aim was to clinically commission an online seed matching image-guided radiotherapy (IGRT) protocol using modern hardware/software for patients undergoing prostate radiotherapy. An essential constraint was to achieve this within a busy centre without reducing patient throughput, which had been reported with other techniques.
Methods
45 patients had 3 fiducial markers inserted into the prostate and were imaged daily using kilovoltage orthogonal images with online correction applied before treatment. A total of 1612 image pairs were acquired and analysed to identify interfractional motion, seed migration and interobserver variability, and assess ease of use.
Results
This method of IGRT was implemented successfully in our centre with no impact on treatment times and patient throughput. Systematic (Σ) interfractional set-up errors were 2.2, 2.7 and 3.9 mm in right–left (RL), superoinferior (SI) and anteroposterior (AP) directions, respectively. Random (σ) interfractional set-up errors were 3.2 (RL), 3.7 (SI) and 5.7 mm (AP). There were significant differences between patients. Seed migration and interobserver variability were not significant issues.
Conclusions
The described technique is facilitated by the advanced imaging system, allowing a fast and effective method of correcting set-up errors before treatment. Extended implementation of this technique has improved treatment delivery to the majority of our prostate radiotherapy patients. The measurement of interfractional motion in this study is potentially valuable for margin reduction in intensity-modulated radiotherapy/volumetric arc therapy.
Advances in knowledge
This technique can be used within treatment time constraints, benefiting large numbers of patients by helping to avoid geographical miss and potentially reducing toxicity to organs at risk.
External beam radiotherapy is a well-established treatment modality in the radical treatment of prostate cancer [1]. To minimise biochemical failure rates, doses of >72 Gy are required [2]. However, dose escalation is associated with additional long-term toxicity such as proctitis, faecal urgency and rectal bleeding, which have a significant impact on quality of life [3,4]. To help maximise the conformality of the dose to the prostate and limit the dose to normal tissues, advanced planning methods such as intensity-modulated radiotherapy (IMRT) and volumetric arc therapy (VMAT) have been developed. These novel delivery systems create highly conformal radiation plans resulting in a reduced margin for geometric set-up error compared with the previous technique of three- or four-field conformal plans. When using new technologies, therefore, accurate treatment verification procedures are vital to prevent geographical miss and limit normal tissue toxicity [5].
Treatment verification has traditionally been performed using offline protocols. Megavoltage images created from the treatment beam [previously using X-ray film but currently with electronic portal imaging devices (EPIDs)] are reviewed retrospectively and the position of bony anatomy is compared with that on a reconstructed planning CT scan. Any large or consistent variation in position is managed with a shift in beam position for future radiation fractions, thus compensating for systematic errors [6,7].
This approach has two main limitations. All forms of geometric uncertainties must be incorporated into set-up margins, and offline review cannot correct for any random set-up errors. As a result, larger margins are required, giving larger planning target volumes (PTVs) and causing irradiation of larger volumes of adjacent organs at risk (OARs) [8]. Possibly of greater significance is the reliance on matching bony anatomy, as it has been demonstrated that the prostate moves independently of bone [9,10]. Interfractional motion of the prostate has been reported to be significant, especially in the anteroposterior (AP) direction where bladder and rectal filling can cause displacement [11].
It has been reported that clinical implementation of new and complex radiotherapy techniques in the UK has often been slow compared with other European countries. Jefferies et al [12] and Mayles [13] conducted audits between 2007 and 2009 and showed low implementation rates for IMRT, and to a lesser extent for image-guided radiotherapy (IGRT), which were primarily attributed to lack of equipment, staffing (all disciplines) and funding.
An existing linear accelerator (Varian Clinac® 21EX, Varian Medical Systems, Palo Alto, CA) was upgraded with the Varian® On-Board Imager® (OBI; Varian Medical Systems) at the end of 2008, and two new linear accelerators with OBIs were commissioned in 2009. We were keen to see this facility implemented in a timely manner, given that a significant group of patients stood to benefit. Patient workload constraints demanded that there should be no reduction in patient throughput.
We were aware of the use of fiducial markers (implanted radio-opaque seeds) for verification of prostate treatment set-up [14] which could overcome the problem associated with bony matching, which was our clinical standard at that time. Much of the early work with seeds relied on megavoltage imaging using EPIDs, but there are reports of problems with image quality and identification of individual seeds [14-16]. Incidence of seed migration or loss is generally low [10,15,17-20] and this may be especially so when seeds are implanted transperitoneally rather than transrectally.
Importantly, it was reported that additional time to perform the matching and make corrections to set-up can add significantly to the overall time required for patient treatment [15,16].
By utilising the Varian kilovoltage imaging, integrated matching software and remote application of couch corrections, we thought it would possible to apply this new technique without impacting on treatment times and patient throughput. As the fiducial matching technique was already clinically established elsewhere, it allowed implementation to be classified as a service development rather than an experimental or research study. This would facilitate faster implementation, as internal approval would suffice. We recognised the need for a robust system of staff training.
Our objective, therefore, was to establish a clinical system for fiducial matching for patients requiring radiotherapy to the prostate gland, with the aim of improving treatment quality for a large number of patients.
We describe our training and implementation techniques and present our results on interfractional motion, seed migration and interobserver variability relating to groups and individual patients.
Methods
This technique was introduced with approval from our departmental Radiotherapy Management Group, following careful consideration to the resource implications it would have on all staff disciplines.
Patients
45 patients treated for low to intermediate risk prostate cancer with radical radiotherapy were included in this study. These patients required irradiation of the prostate gland alone or with seminal vesicles, delivered in two phases. Phase 1 was 56 Gy in 28 fractions, delivered to a PTV of the gross tumour volume plus a 5-mm margin posteriorly and 10 mm on the other axes; Phase 2 was 18 Gy in 9 fractions to a PTV with a reduced margin of 5 mm on all axes. Each patient was allocated to a treatment slot of 10 min, which is routine for this treatment in our department.
Marker insertion
Patients attended the outpatients clinic 2 weeks before radiotherapy planning for insertion of fiducial marker seeds. This allowed time for any oedema to resolve. A urine sample was checked for infection and an enema administered. Three gold markers measuring 1×5 mm (CyberMark™ Fiducial Marker Kit; CIVCO Medical Solutions, Kalona, IA) were inserted into the prostate using a transrectal ultrasound guidance approach. The intended positions of the seeds were left superior lobe, left apex and right mid-gland. Ultrasound images were acquired and a 5-day course of ciprofloxican antibiotics was given to reduce the risk of infection.
CT simulation and radiotherapy planning
Patients received a microenema before the CT simulation appointment. They emptied their bladders and drank 400 ml of water 30 min before scanning. Patients were scanned in the supine position with head support and knee rest in a GE Multislice LightSpeed™ 16 helical CT scanner (GE Medical Systems, Waukesha, WI). Slices were reconstructed as 2.5 mm in width. Orthogonal tattoos were aligned to the origin of the scan, to be used for daily set-up.
Radiotherapy treatment planning was performed using Eclipse v. 8.6 within the ARIA oncology information system (Varian Medical Systems). Seeds were outlined individually on the CT slices but stored as a single structure. AP and lateral “set-up” fields were created (i.e. for imaging purposes only) and the seed structure saved as an overlay on digitally reconstructed radiographs (DRRs). To achieve satisfactory precision, it was essential that this outlining was performed using a highly magnified view. All of the three-dimensional (3D) conformal radiotherapy treatment plans used four conformed fields (AP/posteroanterior and two direct laterals) with multileaf collimator (MLC), centred on the reference tattoos from scanning (i.e. using asymmetric fields).
Patients were treated using either 6-MV or 10-MV photons on a Varian Clinac 21EX series linear accelerator with an OBI.
Training
Because the use of an OBI for online correction to treatment set-up was a new facility in our department, we set up a formal training system. Implementation was conducted by a core group of five radiographers led by a research radiographer. Two members attended a 2-day off-site Varian course on IGRT and all five received 2 days on-site manufacturer training on the practical use of an OBI using anatomical phantoms. This was augmented by further practical sessions, using phantoms, at the end of the clinical working day.
The implementation group developed a training package with defined competencies so that skills could be cascaded to a much wider group of radiographers. Training comprised 1 day of practical sessions on a research linear accelerator (which had subsequently become available), private study of an in-house Microsoft PowerPoint training package (Microsoft Corporation, Albuquerque, NM) and practical experience of patient work given under supervision.
Competencies were met following satisfactory online acquisition, matching and shift application for 10 patients under supervision.
Quality assurance
A Varian phantom block was used for daily quality assurance (QA) and training. This block contained three seeds and orthogonal alignment markers. The block was scanned and two plans prepared with field centres which were offset from the alignment centre by 2 cm to the right, anterior and superior and by 2 cm to the left, posterior and inferior directions. Seeds were outlined as described above and DRRs created.
The block was set on the treatment couch and cross-lasers aligned to the reference markers. Anterior and lateral kilovoltage images were acquired. The Varian OBI v. 1.4 software was used to create a match between the outline of the seeds on the DRRs and the kilovoltage images. Requisite couch shifts were displayed—these should be 2 cm for each axis. Couch shifts were applied remotely and the radiographer entered the treatment room to verify the couch movement and field alignment. A tolerance of 1 mm was set.
These daily QA checks offered an additional element to staff training.
Treatment
For all 45 patients, we aimed to apply online correction for every treatment fraction using anterior and right lateral kilovoltage images. Rectal and bladder preparation was followed as for the CT planning appointment for the first nine treatment fractions and bladder-filling protocol alone thereafter. Matching did not require separate identification of the individual seeds. The OBI software displayed an overlay of the DRR on the kilovoltage image and the DRR image was moved under manual control until the single structure of the seed outline matched the position of the seeds on the kilovoltage image. This is shown in Figure 1 where the image blending tool has been used to fade out the DRR, showing only the kilovoltage image and the seed structure. The match was confirmed by a second radiographer. Couch shifts were displayed to the nearest millimetre and shifts were applied remotely for all axes whenever a correction on any single axis was ≥2 mm. Details of all mismatches and any applied shifts were automatically stored in the patient's electronic treatment file.
Figure 1.
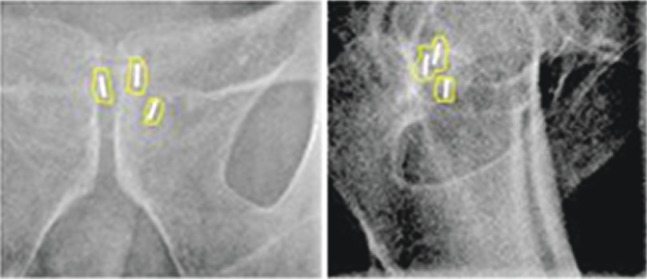
Example of seed matching showing overlay of seed structure from the digitally reconstructed radiographs onto the anteroposterior and lateral kilovoltage images.
Data assessment
Interfractional systematic (Σ) and random (σ) set-up errors were calculated for each patient throughout the course of treatment as described by McKenzie et al [21]. The appropriate set-up margins required without seed matching were identified using the Van Herk et al [8] formula. The frequency of application of shifts was compared for the first and last nine fractions.
Combining data from the AP and lateral views, the 3D separation between pairs of seeds was calculated. Seed migration was assessed by comparing data for the first and last treatment fractions.
The matching process was not automated and therefore might have been affected by interobserver variation. To assess this, independent matching was performed by five operators on an image set for each of three patients with evidence of seed migration and also on three image sets for each of another five randomly selected patients.
Data for all studies were entered into Microsoft Excel spreadsheets (Microsoft Corporation) where analysis was performed and charts created.
Results
Service implementation
The online matching process was implemented without change to the length of the patient appointment time. The quality assurance checks on the phantom showed correction and set-up accuracy to lie within the set tolerance of 1 mm. Overall, analysis was performed on 1612 pairs of images from 45 patients, which represented 97% of all treatment sessions. For the remaining fractions, pre-treatment imaging was not possible when patients were transferred to treatment units not fitted with the OBI system.
Population interfraction motion
The frequency and magnitude of recommended couch shifts for the three principal axes are presented in Table 1. Corrections were within tolerance, i.e. <2 mm for 665 (41%), 592 (37%) and 416 (26%) of the 1612 measurements for right–left (RL), superoinferior (SI) and AP axes, respectively; for all axes combined, 83 of 1612 sets (5.1%) of images were within tolerance and required no shift. In 844 cases (17.5%) mismatches were ≥5 mm, whereas in 38 cases (0.8%) much greater mismatches (14–23 mm) were observed. These larger corrections occurred most frequently in the AP axis.
Table 1. Frequency and magnitude of couch shifts (cm) for the three principal axes.
| Couch shift (cm) | RL | SI | AP |
| −2.3 to −2.5 | 0 | 0 | 1 |
| −2.0 to −2.2 | 0 | 0 | 0 |
| −1.7 to −1.9 | 0 | 0 | 1 |
| −1.4 to −1.6 | 0 | 0 | 22 |
| −1.1 to −1.3 | 2 | 6 | 66 |
| −0.8 to −1.0 | 11 | 22 | 111 |
| −0.5 to −0.7 | 112 | 91 | 222 |
| −0.2 to −0.4 | 430 | 276 | 316 |
| −0.1 to 0.1 | 665 | 536 | 416 |
| 0.2–0.4 | 351 | 424 | 263 |
| 0.5–0.7 | 61 | 162 | 112 |
| 0.8–1.0 | 13 | 35 | 53 |
| 1.1–1.3 | 5 | 2 | 20 |
| 1.4–1.6 | 1 | 2 | 6 |
| 1.7–1.9 | 1 | 0 | 2 |
| 2.0–2.2 | 1 | 0 | 1 |
| 1612 | 1612 | 1612 |
AP, anteroposterior; RL, right–left; SI, superoinferior.
Across all patients and all treatment fractions, the mean [standard deviation (SD)] recommended couch shifts were −0.3 (3.1) mm, 0.6 (3.5) mm and 1.3 (5.4) mm for RL, SI and AP directions, respectively. These represent the overall mean systematic error and are in good agreement with other reported values (Table 2), demonstrating no underlying system error.
Table 2. Calculated population systematic (Σ) and random (σ) set-up errors [21] and set-up margins required in the absence of daily online correction [8] for our study and as reported in the literature. All values are in millimetres.
| Systematic |
Random |
Margin |
|||||||
| Study author | RL | SI | AP | RL | SI | AP | RL | SI | AP |
| This study | 2.2 | 2.7 | 3.9 | 3.2 | 3.7 | 5.7 | 8 | 10 | 14 |
| Other studies | |||||||||
| Nederveen et al [9] | 2.4 | 3.7 | 4.4 | 2.1 | 2.7 | 3.4 | 7.5 | 11.1 | 13.4 |
| Dehnad et al [18] | 3.3 | 3.5 | 4.8 | 2.1 | 2.2 | 3.2 | 9.7 | 10.3 | 14.0 |
| Schallenkamp et al [11] | 2.0 | 1.9 | 2.5 | 1.6 | 2.0 | 3.5 | 5 | 5 | 7.3 |
| van der Heide et al [14] | 2.2 | 2.9 | 4.8 | 2.0 | 2.3 | 3.5 | 7.7 | 8.9 | 14.5 |
| McNair et al [10] | 1.8 | 2.4 | 3.6 | 2.1 | 2.2 | 2.8 | 6 | 7.5 | 11 |
| Rimmer et al [16] | 2.4 | 3.0 | 2.5 | 2.5 | 2.9 | 3.7 | 10 | 11.7 | 11.3 |
| Baker et al [23] | 2.3 | 2.3 | 3.3 | 1.8 | 2.0 | 2.5 | 7 | 7 | 10 |
| Button and Staffurth [5] | 5.3–9.2 | 7.0–14.6 | 8.7–11.0 | ||||||
| Overall range | 1.8–3.3 | 1.9–3.5 | 2.5–4.8 | 1.6–2.5 | 2.0–2.9 | 2.5–3.7 | 5.0–10.0 | 5.0–14.6 | 7.3–14.5 |
AP, anteroposterior; RL, right–left; SI, superoinferior.
Population systematic (Σ) and random (σ) errors were calculated as defined by McKenzie et al [21] and are presented in Table 3. From these, set-up margins of 8 mm, 10 mm and 14 mm are required for RL , SI and AP directions, respectively, according to the formula of Van Herk [8], and assuming that online set-up correction is not applied. Values extracted from the literature are presented for comparison in Table 2.
Table 3. The number of shifts required in each direction (i.e. >2 mm) in the first Treatment sessions and last nine fractions.
| RL | SI | AP | |
| First nine | 249 (61%) | 260 (64%) | 299 (74%) |
| Last nine | 247 (61%) | 257 (63%) | 300 (74%) |
AP, anteroposterior; RL, right–left; SI, superoinferior.
For the full patient group, set-up consistency throughout treatment was assessed using the frequency of application of shifts across all patients for the first nine vs the last nine fractions (Table 3). The figures are almost identical and so no significant difference was detected (p>0.2, Wilcoxon signed rank test).
Individual patient interfraction motion
For a fuller picture of the variability of interfractional set-up it is important to examine individual patient data. The calculated mean and range of positional corrections for each of the patients is shown in Figure 2. Positive corrections mean that the beam is moved to right, anterior or inferior directions to achieve a match. There is considerable variation between patients and to a lesser extent according to axis. There is no clear association between the three axes, e.g. the patient with the largest range of motion in the AP axis does not have the largest motion in the other two axes.
Figure 2.
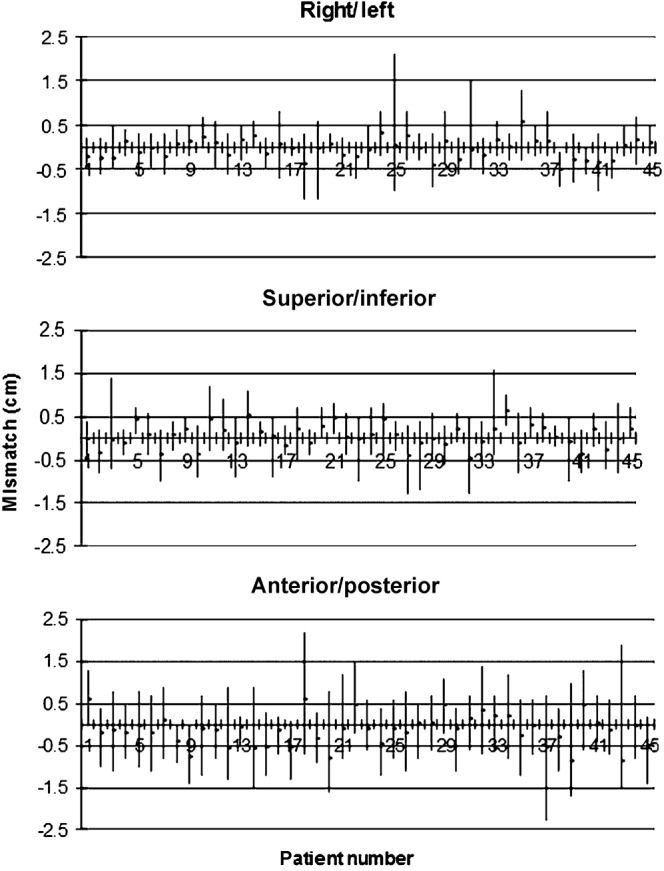
Set-up corrections (cm) for the three axes showing mean and range for the 45 individual patients.
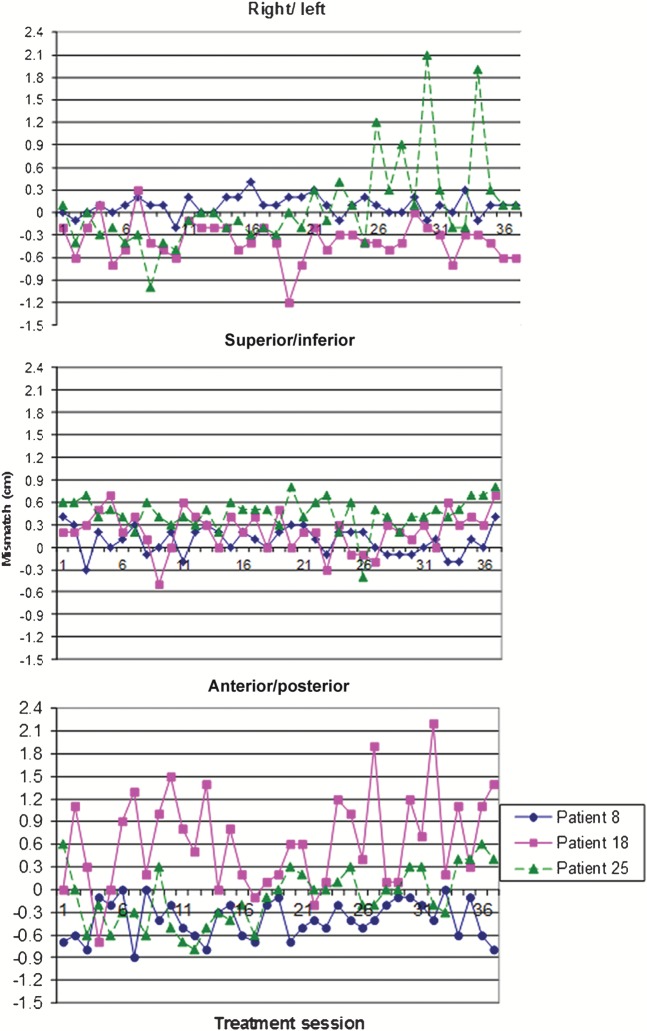
Set-up accuracy varied significantly between patients and throughout the course of treatment. This is illustrated in Figure 3 for three representative patients. For Patient 8, RL matches are good throughout treatment, with corrections ranging from −2 mm to 4 mm. A similar pattern is found for the SI axis, with corrections in the range −3 to 4 mm. The range of corrections for the AP axis is slightly larger (−9 to 0 mm) and the changes from one day to the next are more pronounced.
Figure 3.
Couch corrections (cm) throughout treatment for three representative patients.
For Patient 18, RL matches are mostly −7 to 3 mm, with quite large variations from one day to the next and a larger isolated mismatch (of −12 mm) part way through treatment. A more regular pattern is seen for SI, with a range of −5 to 7 mm. The pattern for AP is dramatically different, with large and unpredictable variations throughout treatment and corrections ranging from −7 to 22 mm. There are 13 mismatches of ≥10 mm occurring throughout treatment.
For Patient 25, the RL matches are generally within −5 mm to 4 mm for the first 25 fractions of treatment but there are larger isolated mismatches (of 12 mm, 9 mm, 21 mm and 19 mm) over the next 10 fractions. By contrast, the SI pattern is fairly regular in the range 2–7 mm with one isolated correction of −4 mm. The range of AP corrections is −8 to 6 mm but there seem to be mostly negative values for fractions 3–18 and a move towards positive values thereafter.
Seed migration
Change in seed separation was calculated for 137 pairs of seeds in 45 patients (Figure 4). The average change in seed separation (±SD) was 0.01 (±0.5) mm. Evidence of seed migration was seen in only seven measurements, with changes of >1 mm. The largest change was 2.5 mm (Figure 1) but it was judged that matching was still valid. For this patient, the recommended shifts were <1 mm regardless of whether the match was made using only the two seeds with no migration or whether a best fit was made using all three seeds.
Figure 4.
Changes in separation between pairs of seeds (first vs last fraction) showing evidence of seed migration.
In two patients a seed was lost between the time of insertion and the CT planning session. In these cases, matching was possible with the two residual seeds.
Interobserver variability
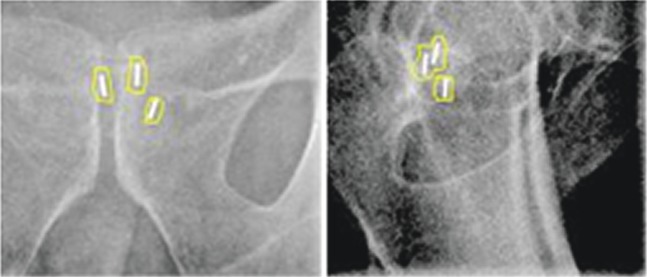
In all examples, and for all observers, the matches differed by a maximum of 1 mm. This included the case where a seed had migrated by 2.5 mm as shown in Figure 5.
Figure 5.
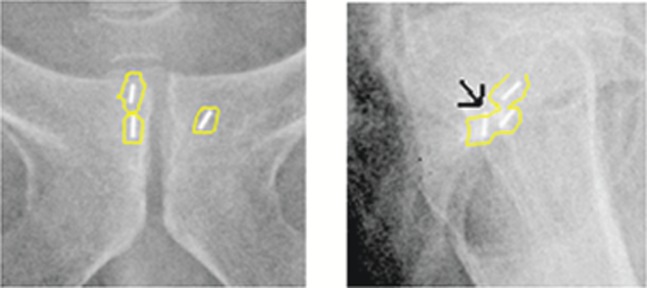
Anteroposterior and lateral kilovoltage images showing a matching process in an example with seed migration of 2.5 mm (arrow). The superior edge of the posterior seed overlaps with the seed structure outline based on the CT planning scan.
Discussion
Our prime aim of implementing an online set-up correction method for prostate treatment utilising implanted seeds, without impacting on treatment times and patient throughput, has been successfully accomplished. We did not gather quantitative data on the times required for the various elements of the process, but within a very busy department we were able to maintain our treatment slots to 10 min.
A key aspect of this has been the use of the Varian OBI imaging and matching software with the outlining of the seeds as a single structure. The width of the CT slices (2.5 mm) sets a limit to the resolution of the seed position longitudinally, and there is some degree of flaring on the CT images. However, we did not find that this affected the overall accuracy of the matching process. The quality of the kilovoltage images was extremely good so that in no case were seeds undetectable. Matching involved the overlay of a single structure, avoiding the more labour-intensive identification of three individual seeds on each image, reducing the likelihood of error and increasing the speed of the process. In the matching process the AP and lateral kilovoltage images are linked, i.e. an SI movement on the AP image is replicated on the lateral image, which saves time and minimises the likelihood of error. Couch movement was applied remotely from the control desk, avoiding the need for staff to re-enter the treatment room.
By contrast, Herman et al [22] reported an average increase of 2 min in treatment delivery time and Chung et al [15] reported an additional 2.6 min to allow for couch shift and repeat imaging. In both of these studies, corrections were applied from within the treatment room. Rimmer et al [16] reported an increase in mean treatment time from 10 to 13.1 min, but much of this was associated with QA for a reticule required for their seed matching. Using a similar technique to us, Baker et al [23] reported an increase in “in-room” time from 12.5 to 14.5 min, although this was expected to reduce with further operator experience. The times required to implement the correction process clearly vary quite significantly. They depend upon the precise details of the matching process and the application of corrections. In smaller studies, there may be a disproportionate impact from staff familiarisation with a new process.
Our programme of staff training was relatively easy to fit into the departmental schedules, although we did utilise some time at the end of the treatment day and further time on a research accelerator that became available. We recommend our model of using a lead research radiographer with a small steering group followed by cascading to other staff. Initially, two patients per month received online matching with seeds, and we were able to train two radiographers every 2 weeks. Experience with the daily QA phantom tests helped to build staff confidence. To date, over 60 radiographers have achieved competency.
It is encouraging to find interobserver variations not exceeding 1 mm, which we again attribute to image quality and the one-step matching process.
Seed loss and seed migration occurred infrequently and did not inhibit matching for our group of patients. Retrospective investigation of the ultrasound images for the two patients with seed loss showed that the seeds had been inserted close to the peripheral edge of the prostate. Our findings are in agreement with other studies [10,15,17-20] which have shown low, but variable, incidences of seed migration or loss. Van der Heide et al [14] reported time trends in certain patients, with evidence of prostate shrinkage over the course of treatment. More recently, in a long-term study of 914 patients, Moman et al [24] reported seed loss in only one patient, and in only three patients was there seed migration of 3–4 mm. Seed implantation was transrectal for each of these four patients, but transperineal insertion is now recommended. They also reported no significant toxicity issues with transperineal insertion. Delouya et al [25] investigated the impact of seed migration on the ability to perform satisfactory matching and suggest the use of a 2-mm tolerance. 9% of their patient group had migration of >5 mm, but 23 of the 31 patients had the planning CT study on the same day as the insertion, which is advised against.
From our patient group as a whole, estimates were made of the population systematic (Σ) and random (σ) errors. These showed that clinical target volume to PTV set-up margins of 8, 10 and 14 mm are required for lateral, SI and AP directions, in the event of online set-up correction not being applied. Our systematic errors and set-up margins fall within the ranges reported in the literature [5,9-11,14,16,18,23]. By contrast, our random errors appear to be slightly high. Estimates of error can be influenced by the number of patients studied. They may also reflect different local practice for patient set-up and there is known to be variation in the use of bowel preparations and in instructions for bladder and/or bowel voiding. In our study we used a microenema for pre-treatment investigations and for the first nine fractions of treatment. We found no difference in the frequency of application of couch corrections when comparing the first nine and last nine fractions. This might suggest that early radiation bowel reaction has the same effect as an enema but might possibly indicate that other factors play a significant role.
Only 0.8% of our cases required large set-up corrections, i.e. in the range 14–23 mm. These were mostly, but not exclusively, in the AP direction. The largest single correction was 21 mm correction in the lateral axis. In hindsight, it would have been desirable for the radiographer to enter the room to investigate the cause of any significantly large correction. That was not part of our protocol (but is now recommended), as this may have added significantly to the time required to treat. We can only speculate that these patients may have moved significantly, perhaps owing to relaxation or some discomfort, after the radiographer had left the treatment room. We cannot discount the possibility of a misalignment, e.g. if tattoos are difficult to identify. A benefit of this online correction method is that such events are identified and corrected. They would be missed if they occurred on days when imaging was not performed with offline correction protocols.
It was very informative to study the results for individual patients, which are not always reported in detail. We found significant differences, e.g. there were patients for whom corrections were small in magnitude and fairly constant throughout treatment but others for whom there was an occasional large correction. Patients might exhibit periods of small correction and periods of larger corrections. Others showed larger corrections which seemed to occur quite randomly throughout treatment. This demonstrates the unpredictable nature of this type of treatment and supports the argument that offline correction protocols are unlikely to be adequate.
It has to be emphasised that the fiducial seed online correction protocol presented here can never offer a perfect solution to set-up correction. We chose to correct for translational errors alone and excluded rotational variations from our study. This was primarily to minimise complexity and time but was also done on the basis of reports that rotational errors are of lesser significance [9,14,26].
A more complete picture of prostate motion is available with on-treatment cone beam CT (CBCT) imaging. This requires matching of the actual organ outline instead of just three seeds. Moseley et al [20] and Logadóttir et al [27] concluded that CBCT gave similar results to fiducial seeds. By contrast, Barney et al [28] found significant differences in dose coverage for 28% of patients but elected to continue with seed matching because CBCT took longer, had problems with image quality and gave extra dose to the patients.
Our method addresses only interfractional set-up error. Button and Staffurth [5] commented on additional sources of error including delineation errors, intrafractional motion, observer error and delivery errors. Intrafractional error is particularly important, and investigations using various methods [23,29-32] indicated that it is affected by bladder and rectal filling as well as patient movement. Minimising treatment time is important but residual margins of a few millimetres are necessary.
Reduction of set-up margins on the basis of online fiducial seed matching should be applied with caution. The CHHiP (Conventional or hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer) clinical trial investigated conventional vs hypofractionated high-dose IMRT for prostate cancer. This was extended—CHHiP-IGRT (ISRCTN 97182923)—to examine the clinical effect of reduced margins with daily online IGRT, and will give important data on the safety and effectiveness of this technique.
In the UK, participation in multicentre clinical trials [such as CHART (Continuous hyperfractionated accelerated radiotherapy), RTO1: a randomised controlled trial of high-dose vs standard-dose conformal radiotherapy for localised prostate cancer, PARSPORT (Parotid-sparing intensity-modulated vs conventional radiotherapy in head and neck cancer), and CHHiP] has been a mechanism for centres to introduce new technology and techniques into clinical practice. This, however, should not prevent service development. In this case, we felt that we could accomplish benefit for a greater number of patients more quickly by working with the constraints of an organised trial. The technique is offered to all suitable prostate patients and has been applied to IMRT treatments with dynamic MLC and VMAT.
Conclusion
The use of two-dimensional IGRT has been successfully implemented to a large patient group within our large cancer centre. This has been done with no impact on the length of treatment time. The technique is facilitated by the advanced imaging system allowing a fast and effective method of correcting for errors prior to treatment. Implementation of this technique has improved treatment delivery to a test group of radically treated prostate patients and is now routinely offered to the majority of such patients (approximately 600 patients per annum), many of whom are currently being treated with IMRT/VMAT. The measurements of interfractional motion in this study is potentially valuable for setting reduced margins when using these advanced treatment techniques but it is also essential to quantify other potential causes of set-up error.
Acknowledgments
The authors acknowledge the work of the radiography staff who so willingly participated in this study and also to members of the physics staff who assisted with planning/contouring aspects.
References
- 1.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet 2011;378:2104–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy ≥72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:25–33 [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer 1999;85:2460–8 [DOI] [PubMed] [Google Scholar]
- 4.Wachter S, Gerstner N, Goldner G, Pötzi R, Wambersie A, Pötter R. Rectal sequelae after conformal radiotherapy of prostate cancer: Dose-volume histograms as predictive factors. Radiother Oncol 2001;59:65–70 [DOI] [PubMed] [Google Scholar]
- 5.Button MR, Staffurth JN. Clinical application of image-guided radiotherapy in bladder and prostate cancer. Clin Oncol 2010;22:698–706 [DOI] [PubMed] [Google Scholar]
- 6.de Boer HCJ, Heijmen BJM. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys 2001;50:1350–65 (review) [DOI] [PubMed] [Google Scholar]
- 7.Baum C, Alber M, Birkner M, Nüsslin F. Robust treatment planning for intensity modulated radiotherapy of prostate cancer based on coverage probabilities. Radiother Oncol 2006;78:27–35 [DOI] [PubMed] [Google Scholar]
- 8.van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1121–35 [DOI] [PubMed] [Google Scholar]
- 9.Nederveen AJ, Dehnad H, van derHeide UA, van Moorselaar RJA, Hofman P, Lagendijk JJW. Comparison of megavoltage position verification for prostate irradiation based on bony anatomy and implanted fiducials. Radiother Oncol 2003;68:81–8 [DOI] [PubMed] [Google Scholar]
- 10.McNair HA, Hansen VN, Parker CC, Evans PM, Norman A, Miles E, et al. A comparison of the use of bony anatomy and internal markers for offline verification and an evaluation of the potential benefit of online and offline verification protocols for prostate radiotherapy. Int J Radiat Oncol Biol Phys 2008;71:41–50 [DOI] [PubMed] [Google Scholar]
- 11.Schallenkamp JM, Herman MG, Kruse JJ, Pisansky TM. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys 2005;63:800–11 [DOI] [PubMed] [Google Scholar]
- 12.Jefferies S, Taylor A, Reznek R. Results of a national survey of radiotherapy planning and delivery in the UK in 2007. Clin Oncol 2009;21:204–17 [DOI] [PubMed] [Google Scholar]
- 13.Mayles P. Survey of the availability and use of advanced radiotherapy technology in the UK. Clin Oncol 2010;22:636–42 [DOI] [PubMed] [Google Scholar]
- 14.van derHeide UA, Kotte ANTJ, Dehnad H, Hofman P, Lagenijk JJW, van Vulpen M. Analysis of fiducial marker-based position verification in the external beam radiotherapy of patients with prostate cancer. Radiother Oncol 2007;82:38–45 [DOI] [PubMed] [Google Scholar]
- 15.Chung PWM, Haycocks T, Brown T, Cambridge Z, Kelly V, Alasti H, et al. On-line aSi portal imaging of implanted fiducial markers for the reduction of interfraction error during conformal radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 2004;60:329–34 [DOI] [PubMed] [Google Scholar]
- 16.Rimmer YL, Burnet NG, Routsis DS, Twyman N, Hoole ACF, Treeby J, et al. Practical issues in the implementation of image-guided radiotherapy for the treatment of prostate cancer within a UK department. Clin Oncol 2008;20:22–30 [DOI] [PubMed] [Google Scholar]
- 17.Poggi MM, Gant DA, Sewchand W, Warlick WB. Marker seed migration in prostate localization. Int J Radiat Oncol Biol Phys 2003;56:1248–51 [DOI] [PubMed] [Google Scholar]
- 18.Dehnad H, Nederveen AJ, van derHeide UA, van Moorselaar RJA, Hofman P, Lagendijk JJW. Clinical feasibility study for the use of implanted gold seeds in the prostate as reliable positioning markers during megavoltage irradiation. Radiother Oncol 2003;67:295–302 [DOI] [PubMed] [Google Scholar]
- 19.Kupelian PA, Willoughby TR, Meeks SL, Forbes A, Wagner T, Maach M, et al. Intraprostatic fiducials for localization of the prostate gland: Monitoring intermarker distances during radiation therapy to test for marker stability. Int J Radiat Oncol Biol Phys 2005;62:1291–6 [DOI] [PubMed] [Google Scholar]
- 20.Moseley DJ, White EA, Wiltshire KL, Rosewall T, Sharpe MB, Siewerdsen JH, et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 2007;67:942–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie A, Coffey M, Greener T, Hall C, van Herk M, Mijnheer B, et al. Geometric uncertainties in radiotherapy. London, UK: British Institute of Radiology Working Party; 2003 [Google Scholar]
- 22.Herman MG, Pisansky TM, Kruse JJ, Prisciandaro JI, Davis BJ, King BF. Technical aspects of daily online positioning of the prostate for three-dimensional conformal radiotherapy using an electronic portal imaging device. Int J Radiat Oncol Biol Phys 2003;57:1131–40 [DOI] [PubMed] [Google Scholar]
- 23.Baker A, Fenwick J, Mayles W, Syndikus I, Wong H. A comparison of imaging schedules for prostate radiotherapy including online tracking techniques. J Radiother Pract 2011;10:239 [Google Scholar]
- 24.Moman MR, van derHeide UA, Kotte ANTJ, van Moorselaar RJA, Bol GH, Franken SPG, et al. Long-term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiother Oncol 2010;96:38–42 [DOI] [PubMed] [Google Scholar]
- 25.Delouya G, Carrier J, Béliveau-Nadeau D, Donath D, Taussky D. Migration of intraprostatic fiducial markers and its influence on the matching quality in external beam radiation therapy for prostate cancer. Radiother Oncol 2010;96:43–7 [DOI] [PubMed] [Google Scholar]
- 26.Redpath AT, Wright P, Muren LP. The contribution of on-line correction for rotational organ motion in image-guided radiotherapy of the bladder and prostate. Acta Oncol 2008;47:1367–72 [DOI] [PubMed] [Google Scholar]
- 27.Logadóttir Á, Korreman S, Petersen PM. Comparison of the accuracy and precision of prostate localization with 2D–2D and 3D images. Radiother Oncol 2011;98:175–80 [DOI] [PubMed] [Google Scholar]
- 28.Barney BM, Lee RJ, Handrahan D, Welsh KT, Cook JT, Sause WT. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys 2011;80:301–5 [DOI] [PubMed] [Google Scholar]
- 29.Polat B, Guenther I, Wilbert J, Goebel J, Sweeney RA, Flentje M, et al. Intra-fractional uncertainties in image-guided intensity-modulated radiotherapy (IMRT) of prostate cancer. Strahlenther Onkol 2008;184:668–73 [DOI] [PubMed] [Google Scholar]
- 30.Kotte ANTJ, Hofman P, Lagendijk JJW, van Vulpen M, van derHeide UA. Intrafraction motion of the prostate during external-beam radiation therapy: Analysis of 427 patients with implanted fiducial markers. Int J Radiat Oncol Biol Phys 2007;69:419–25 [DOI] [PubMed] [Google Scholar]
- 31.Budiharto T, Slagmolen P, Haustermans K, Maes F, Junius S, Verstraete J, et al. Intrafractional prostate motion during online image guided intensity-modulated radiotherapy for prostate cancer. Radiother Oncol 2011;98:181–6 [DOI] [PubMed] [Google Scholar]
- 32.Reggiori G, Mancosu P, Tozzi A, Cantone M, Castiglioni S, Lattuada P, et al. Cone beam CT pre- and post-daily treatment for assessing geometrical and dosimetric intrafraction variability during radiotherapy of prostate cancer. J Appl Clin Med Phys 2010;12:3371. [DOI] [PMC free article] [PubMed] [Google Scholar]