Abstract
Takayasu arteritis is a chronic, idiopathic, inflammatory disease that primarily affects large vessels, such as the aorta and its major branches and the pulmonary and coronary arteries. The non-specific inflammation of involved vessels usually leads to concentric wall thickening, fibrosis and thrombus formation. Diseased arteries become stenotic or occluded, undergo vascular remodelling or develop aneurysms. According to the involvement of arteries, six types of Takayasu arteritis are documented. The purpose of this pictorial review is to illustrate the various multidetector CT angiography appearances of Takayasu arteritis and to discuss the differential diagnosis.
Takayasu arteritis (TA) is a chronic, idiopathic, inflammatory disease that primarily affects large vessels, such as the aorta and its major branches, pulmonary and coronary arteries. Because of considerable morbidity and mortality, accurate and early diagnosis plays a crucial role in improving the outcomes for patients with TA [1]. Unfortunately, the non-specific clinical presentations and laboratory test results frequently contribute to late diagnosis and delayed treatment [1]. Since large-artery biopsies cannot easily be done, imaging examination is essential for providing the diagnosis and differential diagnoses in patients with suspected TA.
Conventional angiography has been traditionally considered the gold standard for the diagnosis of TA [2]. However, multidetector CT angiography (CTA) is emerging as a reliable tool in non-invasively depicting both luminal and mural lesions in the aorta and its main branches, which may facilitate the detection of vasculitis during the early phase of TA.
In this article, we will review the CTA findings in TA and how this non-invasive method impacts patient care.
Epidemiology and pathology
TA has an annual incidence of 2.6 per million in North America [2,3]. The disease preferentially affects young women in the second and third decades of life, but can be observed in adults of any age [2,4]. The exact pathogenesis of TA is still not well established [3]. Histopathology reveals TA as granulomatous panarteritis characterized by T lymphocytes, B lymphocytes, macrophages and multinucleated giant cells infiltration of the arterial wall [1,3].
Classification
According to the vessels involved, the most recently proposed angiographic classification divides TA into six types (Figure 1) [5,6]:
Figure 1.
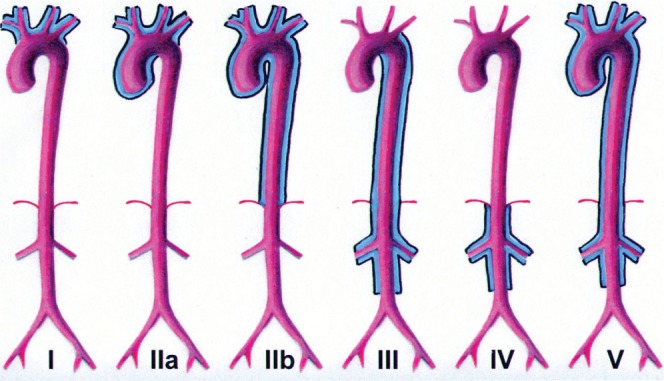
New angiographic classification of Takayasu arteritis according to vessels involved [5,6]. Reproduced with permission from RadioGraphics.
Type I involves only the branches of the aortic arch.
Type IIa involves ascending aorta, aortic arch and its branches.
Type IIb affects ascending aorta, aortic arch and its branches, and thoracic descending aorta.
Type III involves the descending thoracic aorta, the abdominal aorta and/or the renal arteries. The ascending aorta, the aortic arch and its branches are not affected.
Type IV involves only the abdominal aorta and/or renal arteries.
Type V has combined features of Type IIb and IV.
Additionally, involvement of the coronary and pulmonary arteries should be indicated as C (+) or P (+), respectively. Type V has been documented as the most common type [7-9].
Clinical features and diagnosis criteria
Manifestations of TA vary from asymptomatic disease to absent pulses to catastrophic cardiac failure [6,7,10]. The symptoms may be relieved by the development of collateral circulation. Cardiac and neurological complications are the most important cause of death [1,6,10,11].
The diagnosis of TA is still a challenge for clinicians, although several diagnosis criteria have been proposed to improve the early diagnosis [1,11]. Sharma criteria are the most commonly used (Table 1), with a higher sensitivity and specificity than Ishikawa's and the American College of Rheumatology criteria [12,13].
Table 1. Sharma criteria for diagnosis of Takayasu arteritis [12].
| Major criteria | Left mid-subclavian artery lesion |
| Right mid-subclavian artery lesion | |
| Characteristic signs and symptoms of at least one month durationa | |
| Minor criteria | High erythrocyte sedimentationb |
| Carotid artery tenderness | |
| Hypertensionc | |
| Aortic regurgitation or annuloaortic ectasis | |
| Pulmonary artery lesion | |
| Left mid-common carotid lesion | |
| Distal brachiocephalic trunk lesion | |
| Descending thoracic aorta lesion | |
| Abdominal aorta lesion | |
| Coronary artery lesion |
Presence of two major, or one major and two minor criteria, or four minor criteria suggests a high probability of Takayasu arteritis.
aIncluding limb claudication, pulselessness or pulse differences in limbs, an unobtainable or significant blood presence difference, fever, neck pain, transient amaurosis, blurred vision, syncope, dyspnea or palpitations.
bHigher than 20 mm h−1 (Westergren method).
cHigher than 140/90 mmHg brachial or 160/90 mmHg popliteal.
CT angiography features
Mural thickening
The typical manifestation for TA on CT images is the concentric mural thickening of the involved arteries (Figure 2). The mural thickness can be several millimetres [14,15]. Previous works suggested that mural thickening may be the most important finding in the early phases of the disease [1,11]. Calcification in the thickened wall is another important sign of TA (Figure 3). The calcification is usually transmural and has been observed in 27% of patients [14]. Axial images allow accurate evaluation of the arterial wall thickness and calcification. On pre-contrast CT scanning, the mural thickening is of high attenuation compared with the lumen, while on the post-enhanced CTA images, it exhibits a double ring enhancement pattern, which is typically shown in venous phase [14,16]. Specifically, a poorly enhanced inside ring and an obviously enhanced outside ring is frequently observed (Figure 4). It has been proposed that the inside ring represents the swollen intima, while the outside ring indicates the active inflammation in the medial and adventitial layers [16-18]. Some studies also suggested that the double ring enhancement pattern is useful for evaluating treatment efficacy [17-19].
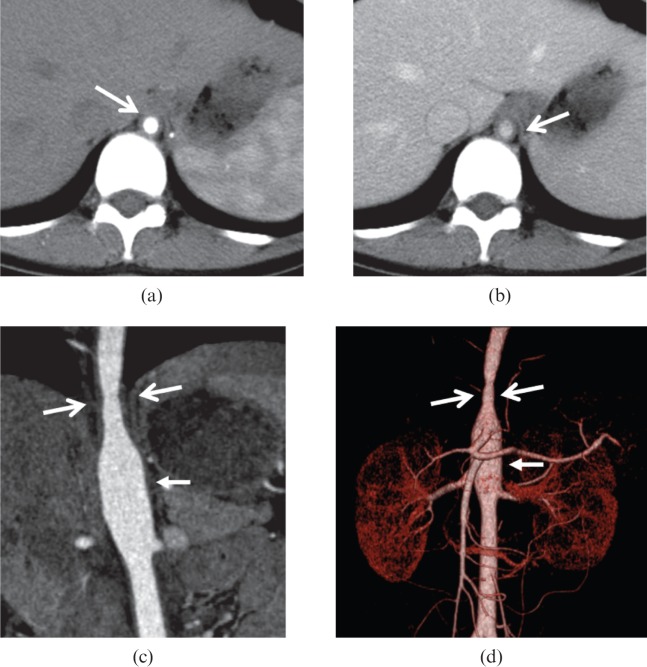
Figure 2.
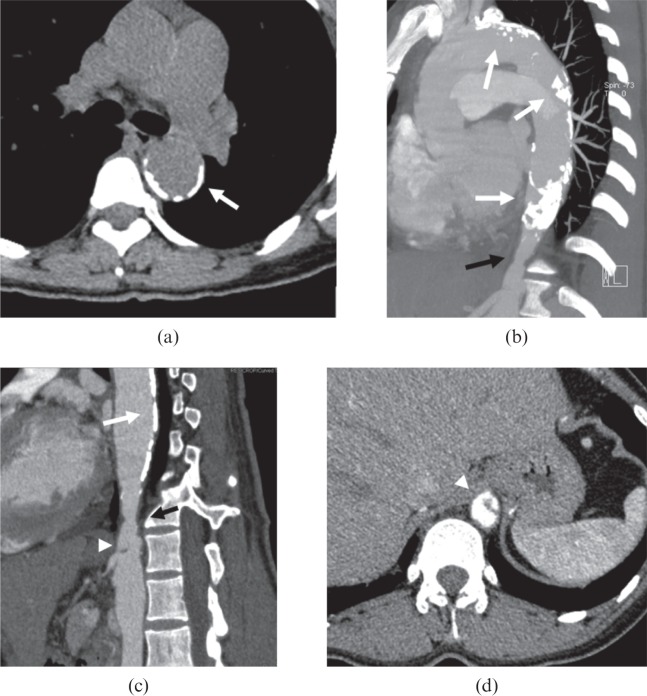
A 39-year-old female with Takayasu arteritis. (a, b) Axial pre-contrast CT image shows the concentric thickened high-attenuation wall [small arrows in (a)] in descending thoracic aorta; the wall appears low-attenuation compared with the lumen [arrow in (b)] on post-contrast images. (c) Innominate (arrowhead) and left common carotid arteries (large arrow) are involved. (d) Multiplanar reformatted image illustrates the concentric thickening wall of the left common carotid artery (small arrows); the lumen is narrowed.
Figure 3.
A 34-year-old female with Takayasu arteritis. (a–d) The transmural calcification in aortic arch and descending aorta (white arrows) can be observed, while a stenotic lesion (black arrows) is also depicted in maximum intensity projection (b) and curved planar reformation (c) images at the segment of diaphragmatic aortic hiatus, which is associated with a regional dissection [white arrowheads in (c, d)] at the level of ostium of coeliac trunk.
Figure 4.
A 39-year-old female diagnosed with Takayasu arteritis with a complaint of malaise and headache for 2 years. (a) The ring-shaped high attenuation (arrow) in pre-contrast axial CT image represents the thickened wall of descending thoracic aorta. (b, c) The thickened wall shows a double ring enhancement pattern (arrows) in arterial (b) and venous phases (c) images. The stenotic lumen is also visualized.
Luminal changes
Stenosis is the most commonly seen finding associated with mural thickening (Figure 5), and can be observed in approximately 90% of patients [1]. Luminal stenosis of the abdominal and thoracic descending aorta has been reported in more than 60% of patients [7-9]. With regard to the branches, stenotic lesions are most frequently founded in the subclavian and common carotid arteries, followed by the renal arteries (Figure 6) [1,7-9]. Occlusion, ectasis and aneurysm of the vessels can also be seen, but less commonly. Dilatation and aneurysms are usually seen in the ascending and abdominal aorta, respectively (Figure 7), which may lead to fatal consequence, such as aortic rupture [7,8].
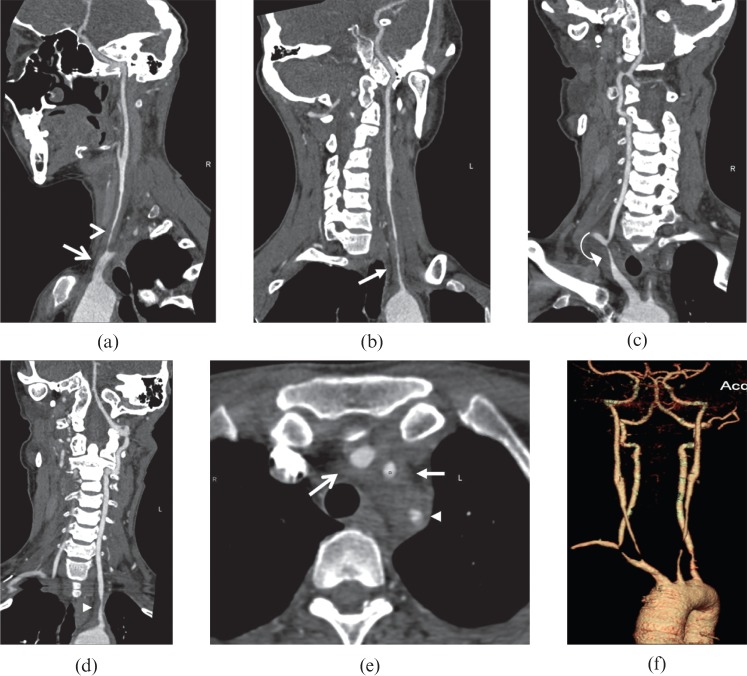
Figure 5.
A 34-year-old female Takayasu arteritis patient with malaise, dizziness, visual disturbance and arthralgia of the left limb. (a–d) Curved planar reformation and (e) axial images exhibit the involvement of innominate (open arrows), bilateral subclavian (arrowheads and curved arrow) and common carotid arteries (open arrowhead and arrows). (f) Volume-rendered reformatted image shows the luminal stenosis of the diseased arteries.
Figure 6.
A 41-year-old female patient diagnosed with Takayasu arteritis with a complaint of hypertension for 1 year. (a, b) Bilateral renal (open arrows) and superior mesenteric arteries (arrow) stenosis are seen on the maximum intensity projection images. (c) Volume-rendered reformatted image shows the stenotic lesion at the origin of bilateral renal arteries (open arrows).
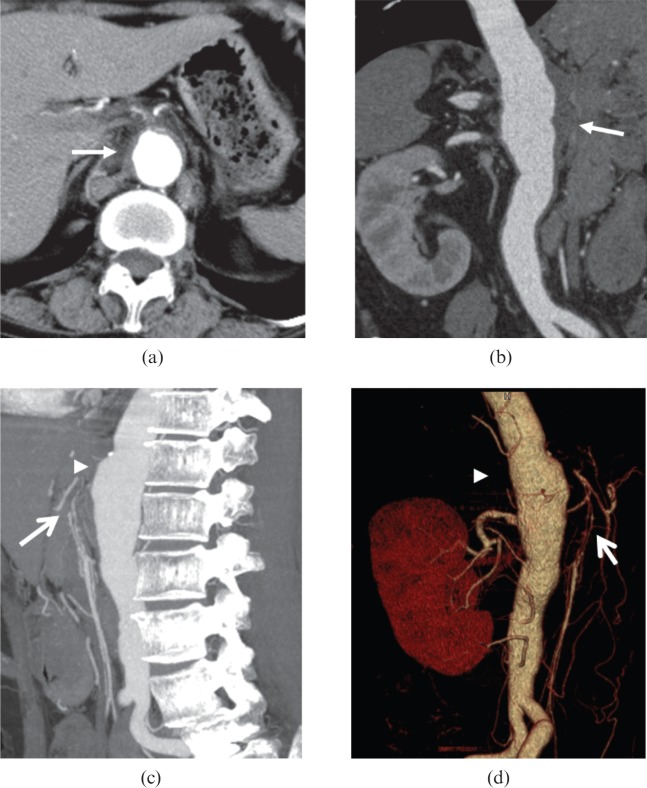
Figure 7.
A 63-year-old female diagnosed with Takayasu arteritis with stomachache. (a) Axial and (b) curved planar reformation CT images uncovered an intramural haematoma (arrows) in abdominal aorta with aneurysm formation [arrowheads in (c, d)]. The original segment of superior mesenteric artery (open arrows) is severely narrowed in (c) maximum intensity projection and (d) volume-rendered reformatted images.
Curved planar reformation (CPR) allows tortuous vessels to be displayed along its long axis; multiplanar reconstruction (MPR) gives the anatomical information of arteries in the optimal planes; volume-rendered (VR) images can illustrate the extension of the luminal lesions and map the collaterals following artery occlusion. A combination of CPR, MPR, VR, and axial images permits optimal evaluation of luminal changes. The stenotic lesions usually appear as concentric narrowing of the arterial lumen (Figure 8). Sometimes, the normal or dilated proximal vessels associated with tapered narrowing of distal segments exhibit a characteristic “rat tail”-like configuration (Figure 9), especially in patients with both thoracic and abdominal aorta involved [16].
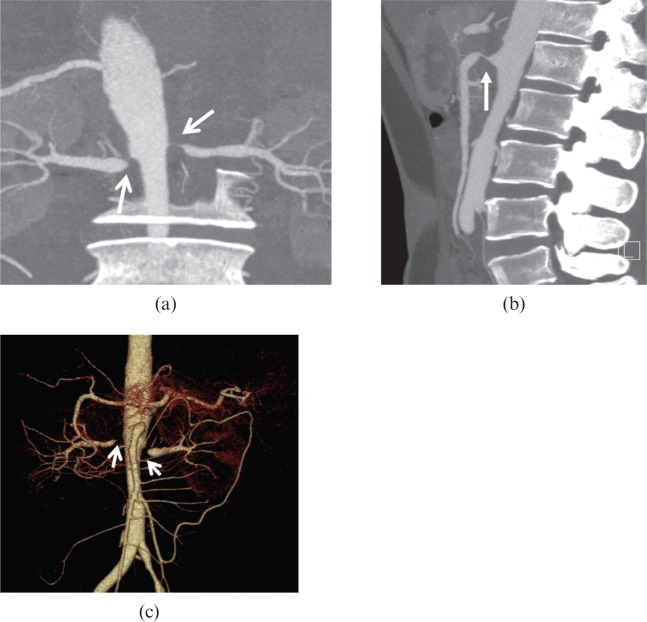
Figure 8.
A 28-year-old female with Takayasu arteritis. A concentric narrowing lesion (open arrows) at the level of diaphragmatic aortic hiatus is depicted in (a, b) axial, (c) curved planar reformatted and (d) volume-rendered images, with the distal segment mildly dilated [arrows in (c, d)].
Figure 9.
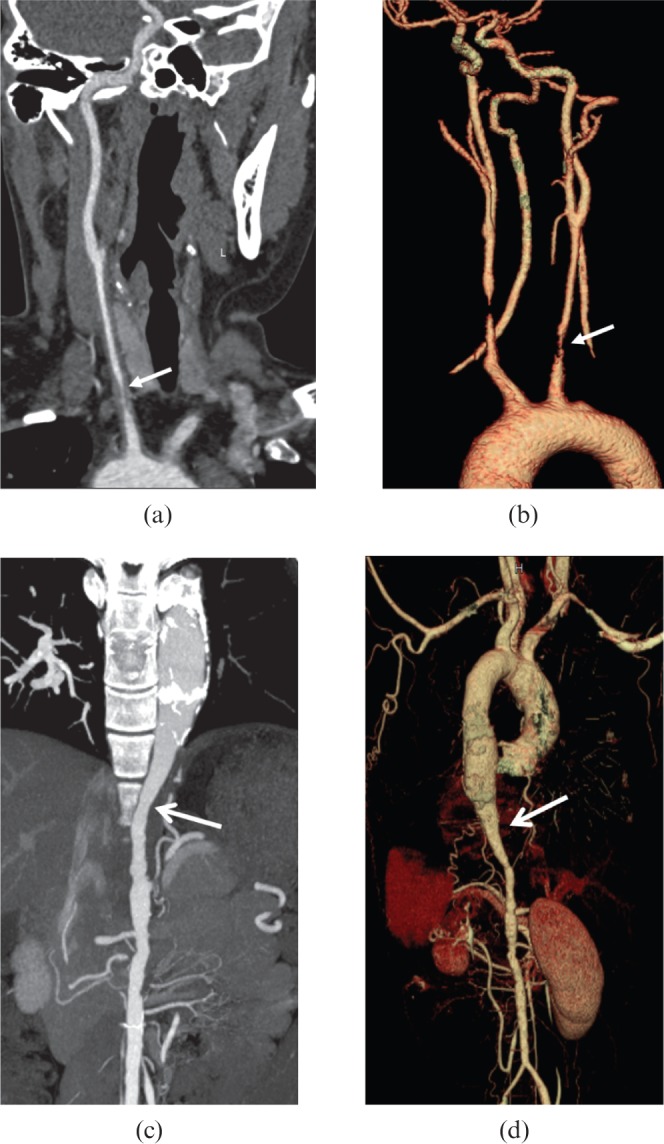
A 29-year-old female Takayasu arteritis patient with syncope, dyspnoea, and shortness of breath for 4 months, and left limb claudication for 3 months. (a, b) The mildly narrowed distal segment of left common carotid artery associated with a severely stenotic lesion (arrows) at the original segment makes a “rat-tail”-like configuration in (a) curved planar reformation and (b) volume-rendered images. (c) Maximum intensity projection and (d) volume-rendered reformatted images of the same patient illustrate the dilation of descending thoracic aorta associated with gradually narrowing distal segment (open arrows) and abdominal aorta, producing a characteristic “rat-tail” sign.
Collateral vessels
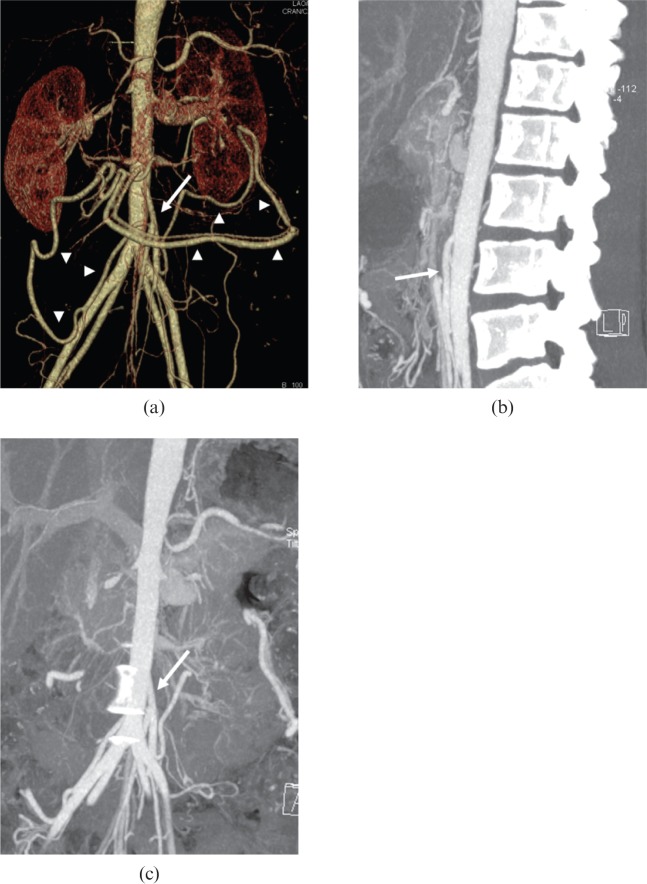
With luminal narrowing, collateral vessels may be observed in some cases. It may be helpful to evaluate these arteries in planning and modifying treatment [16]. Maximum intensity projection and VR are useful in demonstrating small vessel changes (Figure 10).
Figure 10.
A 29-year-old female Takayasu arteritis patient with fever, malaise and myalgia. (a) Volume-rendered image demonstrates occlusion of the superior mesenteric artery, and the collateral vessels (arrowheads) origins form a dilated inferior mesenteric artery (arrow). (b, c) Maximum intensity projection images show the dilated inferior mesenteric artery (arrows).
Other findings
Pulmonary and coronary artery involvement can be seen in 63.3% and 44.4% of patients, respectively [7-9,16,20]. It can also be demonstrated on CTA images (Figure 11). Additionally, CTA can provide the information associated with ischaemia of the end organs, such as decreased perfusion in the brain and lungs (Figure 12), which may be helpful in evaluating prognosis [21,22].
Figure 11.
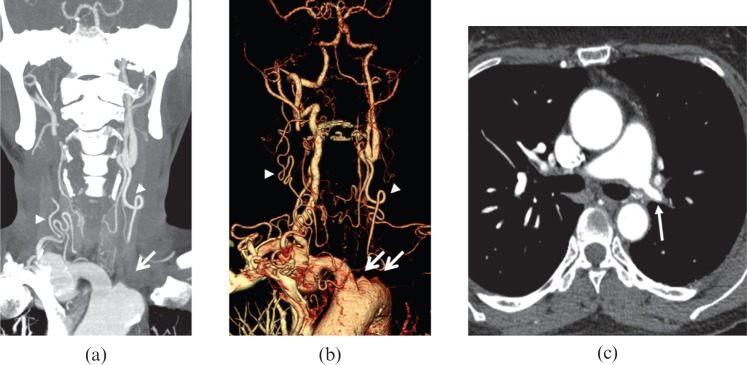
A 36-year-old female with Takayasu arteritis. (a) Maximum intensity projection and (b) volume-rendered reformatted images exhibit occlusion of left common carotid and subclavian arteries (arrows), with collateral vessel formation (arrowheads). (c) Axial CT image illustrates the involvement of left main pulmonary artery (arrow).
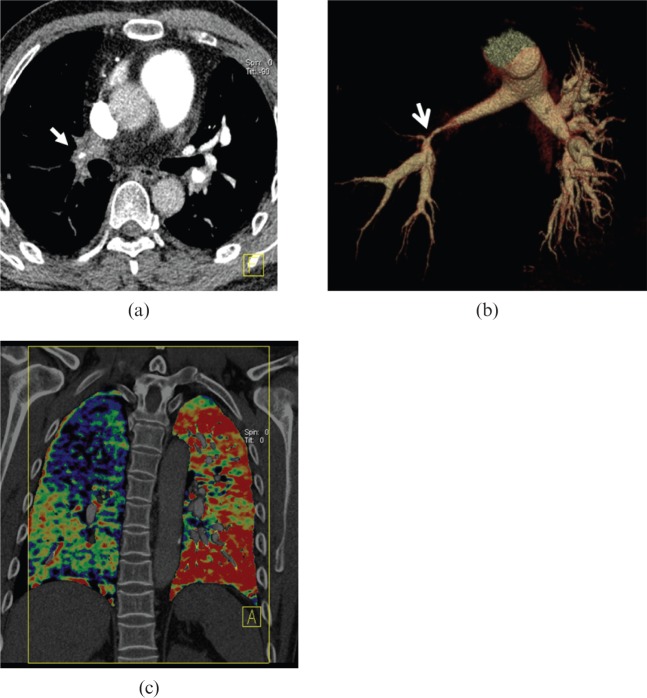
Figure 12.
A 41-year-old male with Takayasu arteritis. (a) Axial post-enhanced CT image shows the right lower lobe pulmonary artery wall is significantly thickened with a severely narrowed lumen [arrows in (a) and (b)]. (b) Volume-rendered image exhibits the significantly stenotic lesion in the right pulmonary artery trunk with decrease in blood perfusion of the right lung, compared with the left one in the dual energy CT iodine mapping image (c).
Differential diagnosis
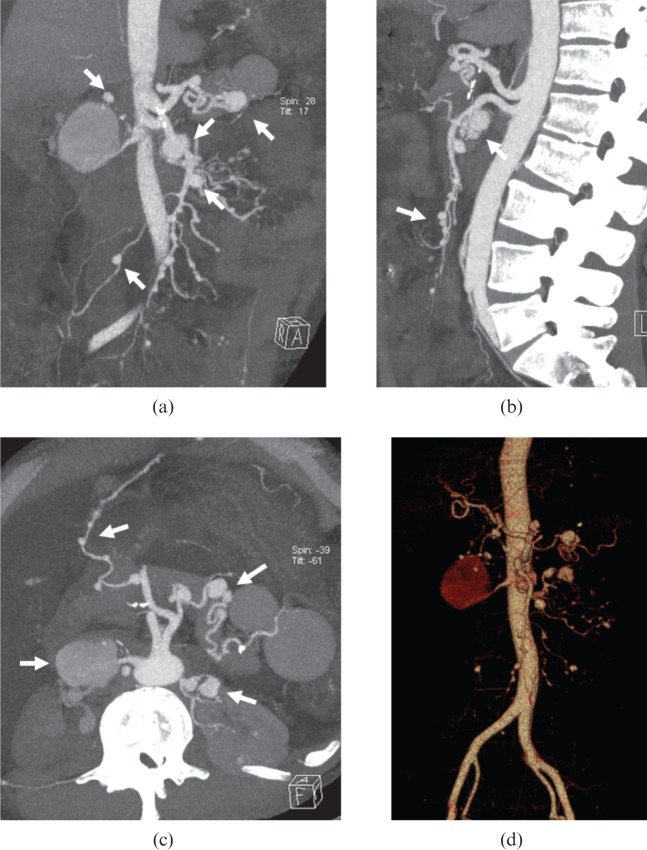
The differential diagnoses should include common diseases such as atherosclerosis, giant cell arteritis and polyarteritis nodosa. It is not an easy task to differentiate aortic calcification in TA from that in atherosclerosis. Atherosclerotic plaques are more common in patients aged 45 years and above, and not usually associated with long segment luminal stenosis [14,23]. Calcification in ascending aorta can be observed in some TA patients, but it is rare in atherosclerosis. Giant cell arteritis shares similar pathogenesis and imaging features with TA; however, giant cell arteritis commonly affects patients older than 50 years. In giant cell arteritis, branches of the external and internal carotid arteries are most frequently diseased [2,3,11]. Polyarteritis nodosa frequently occurs in adults who are 30–50 years old, affecting males more than females, and it also more commonly affects patients with hepatitis B. Gastrointestinal and renal arteries are the primary sites diseased. Multiple small aneurysm formation in the involved artery is the characteristic manifestation on CTA images (Figure 13) [24].
Figure 13.
A 36-year-old male with polyarteritis nodosa. (a–d) CT angiography images show the splenetic, superior mesenteric and bilateral renal arteries are all affected, while the aorta is disease-free. Multiple aneurysm formation (arrows) in the involved vessels can be observed.
Other non-invasive imaging modalities
Nuclear medicine, ultrasonography and magnetic resonance angiography (MRA) are all potentially useful for diagnosis and evaluation of TA. Nuclear medicine is limited by a lack of standardized technique and high cost, while ultrasonography suffers from operator-dependent artefacts from overlying structures and bowel gas. These limitations prevent these two modalities from being the ideal methods in the serial evaluation of TA patients [10,19]. Taking the advantages of freedom from iodinated contrast material and ionizing radiation, MRA shares the similar features of TA with CTA images, and can be used not only in the diagnosis of TA but also follow-up of patients undergoing treatment, especially those who are young. The disadvantages of MRA are the contraindications associated with electronic devices, intraluminal stent implantation and claustrophobia [6,10].
Treatment
Corticosteroids have been considered to be the mainstay of treatment for TA, with a remission rate up to 60% [1,2,11]. In patients with symptomatic stenotic or occlusive lesions, percutaneous transluminal angioplasty and stenting or bypass surgery is the most common palliative treatment [1,2]. Indications for surgical management include aneurismal enlargement with risk of rupture, stenotic or occlusive lesions leading to critical organ ischaemia, and uncontrolled hypertension resulting from renal artery stenosis. Generally, interventional management is not recommended until clinical remission is achieved, due to the high rate of restenosis. It has been believed that short focal stenostic lesions without activated inflammation tend to yield good results after interventional management [1]. Following surgery or percutaneous angioplasty with or without endovascular stenting, long-term follow-up should be initiated with CTA or MRA to monitor the patency and complications of diseased arteries, stent (Figure 14) or bypass vessels (Figure 15) [1].
Figure 14.
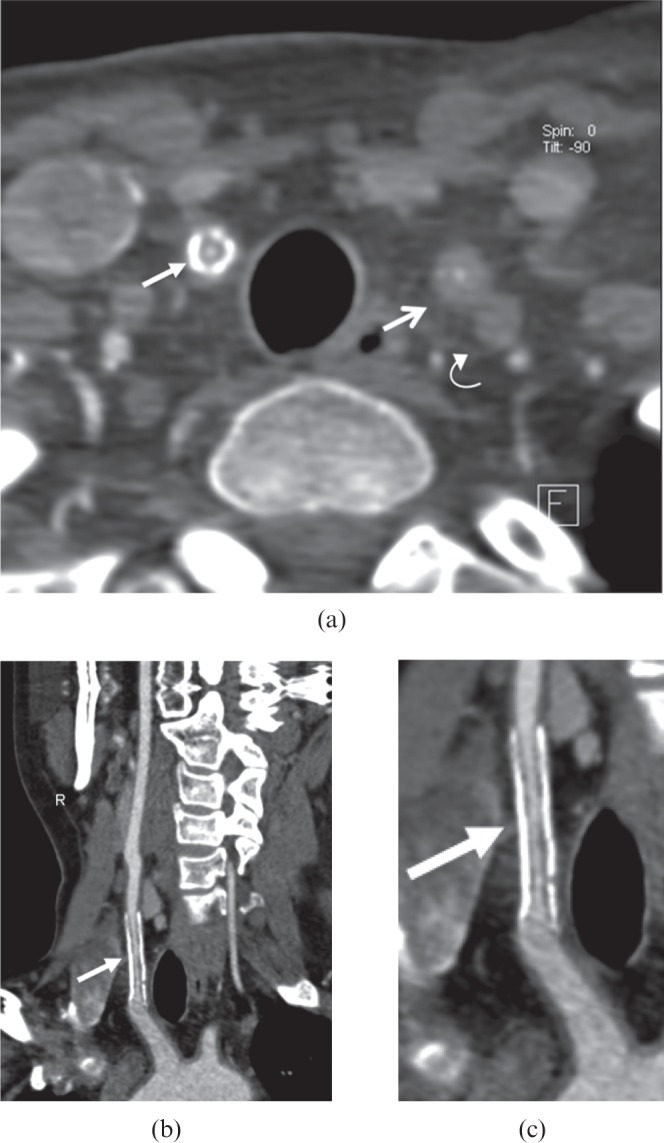
A 37-year-old female with Takayasu arteritis. A stent was placed in her right common carotid artery with 10-month follow-up. (a) Axial post-enhanced CT image demonstrates stent restenosis (arrow). A severely narrowed lesion in the left common carotid artery (open arrow) and occlusion in the left subclavian artery (curved arrow) are also depicted. (b) Coronal multiplanar reformatted image confirms in-stent restenosis (arrow) in the right common carotid artery stent. (c) The focally magnified image (b) clearly visualizes the stent restenosis (arrow).
Figure 15.
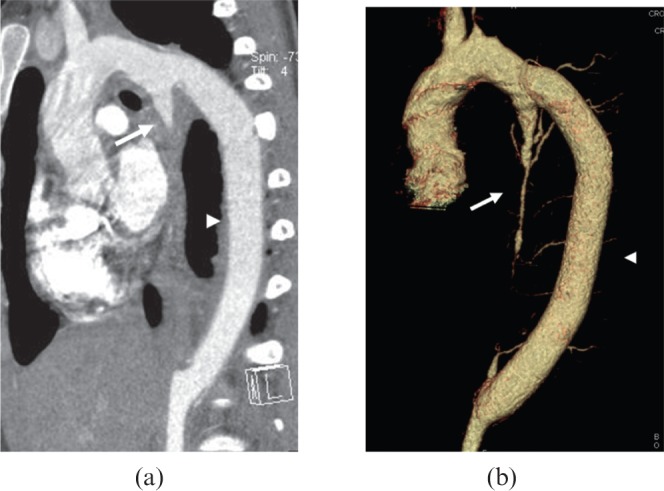
A 16-year-old female Takayasu arteritis patient with severely narrowed lesion in the thoracic descending aorta was treated with bypass graft surgery and followed up for 40 months. (a) Multiplanar reformatted and (b) volume-rendered images show the patent bypass vessel (arrowheads) from aorta arch to distal segment of thoracic descending aorta. Note the occluded native thoracic descending aorta (arrows).
Conclusions
TA is a rare entity with poor prognosis. Familiarity with its CT appearances can aid the radiologist to make the appropriate diagnosis.
Footnotes
F P Zhu and S Luo contributed equally to this work.
References
- 1.Mason JC. Takayasu arteritis—advances in diagnosis and management. Nat Rev Rheumatol 2010;6:406–15 [DOI] [PubMed] [Google Scholar]
- 2.Brunner J, Feldman BM, Tyrrell PN, Kuemmerle-Deschner JB, Zimmerhackl LB, Gassner I, et al. Takayasu arteritis in children and adolescents. Rheumatology 2010;49:1806–14 [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 2003;349:160–9 [DOI] [PubMed] [Google Scholar]
- 4.Watts R, Al-Taiar A, Mooney J, Scott D, Macgregor A. The epidemiology of Takayasu arteritis in the UK. Rheumatology 2009;48:1008–11 [DOI] [PubMed] [Google Scholar]
- 5.Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol 1996;54Suppl.:S155–63 [DOI] [PubMed] [Google Scholar]
- 6.Nastri MV, Baptista LP, Baroni RH, Blasbalg R, de Avila LF, Leite CC, et al. Gadolinium-enhanced three-dimensional MR angiography of Takayasu arteritis. Radiographics 2004;24:773–86 [DOI] [PubMed] [Google Scholar]
- 7.Mwipatayi BP, Jeffery PC, Beningfield SJ, Matley PJ, Naidoo NG, Kalla AA, et al. Takayasu arteritis: clinical features and management: report of 272 cases. ANZ J Surg 2005;75:110–17 [DOI] [PubMed] [Google Scholar]
- 8.Lee GY, Jang SY, Ko SM, Kim EK, Lee SH, Han H, et al. Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: analysis of 204 Korean patients at a single center. Int J Cardiol 2012;159:14–20 [DOI] [PubMed] [Google Scholar]
- 9.Cong XL, Dai SM, Feng X, Wang ZW, Lu QS, Yuan LX, et al. Takayasu's arteritis: clinical features and outcomes of 125 patients in China. Clin Rheumatol 2010;29:973–81 [DOI] [PubMed] [Google Scholar]
- 10.Canyigit M, Peynircioglu B, Hazirolan T, Dagoglu MG, Cil BE, Haliloglu M, et al. Imaging characteristics of Takayasu arteritis. Cardiovasc Intervent Radiol 2007;30:711–18 [DOI] [PubMed] [Google Scholar]
- 11.Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol 2002;55:481–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol 1996;54(Suppl.):S141–7 [DOI] [PubMed] [Google Scholar]
- 13.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu's arteritis. Arthritis Rheum 1990;33:1129–34 [DOI] [PubMed] [Google Scholar]
- 14.Khandelwal N, Kalra N, Garg MK, Kang M, Lal A, Jain S, et al. Multidetector CT angiography in Takayasu arteritis. Eur J Radiol 2011;77:369–74 [DOI] [PubMed] [Google Scholar]
- 15.Gotway MB, Araoz PA, Macedo TA, Stanson AW, Higgins CB, Ring EJ, et al. Imaging findings in Takayasu's arteritis. AJR Am J Roentgenol 2005;184:1945–50 [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga N, Hayashi K, Sakamoto I, Ogawa Y, Matsumoto T. Takayasu arteritis: protean radiologic manifestations and diagnosis. Radiographics 1997;17:579–94 [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Chung JW, Im JG, Kim SK, Park YB, Han MC. Takayasu arteritis: evaluation of mural changes in the aorta and pulmonary artery with CT angiography. Radiology 1995;196:89–93 [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Park JH, Chung JW, Kim HC, Lee W, So YH, et al. Follow-up CT evaluation of the mural changes in active Takayasu arteritis. Korean J Radiol 2007;8:286–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kissin EY, Merkel PA. Diagnostic imaging in Takayasu arteritis. Curr Opin Rheumatol 2004;16:31–7 [DOI] [PubMed] [Google Scholar]
- 20.Soto ME, Meléndez-Ramírez G, Kimura-Hayama E, Meave-Gonzalez A, Achenbach S, Herrera MC, et al. Coronary CT angiography in Takayasu arteritis. JACC Cardiovasc Imaging 2011;4:958–66 [DOI] [PubMed] [Google Scholar]
- 21.Chang GY. Perfusion alterna in Takayasu arteritis. Neurology 2008;71:614. [DOI] [PubMed] [Google Scholar]
- 22.Zhang LJ, Lu GM. Takayasu's arteritis involving the pulmonary arteries: evaluation by quantitative dual-energy computed tomographic pulmonary angiography. Eur Heart J 2012;33:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seyahi E, Ugurlu S, Cumali R, Balci H, Seyahi N, Yurdakul S, et al. Atherosclerosis in Takayasu arteritis. Ann Rheum Dis 2006;65:1202–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mnif N, Chaker M, Oueslati S, Ellouze T, Tenzakhti F, Turki S, et al. Abdominal polyarteritis nodosa: angiographic features. J Radiol 2004;85:635–8 [DOI] [PubMed] [Google Scholar]