Abstract
OBJECTIVE:
To identify the impact of supplemental zinc, vitamin A, and glutamine, alone or in combination, on long-term cognitive outcomes among Brazilian shantytown children with low median height-for-age z-scores.
METHODS:
A randomized, double-blind, placebo-controlled trial was conducted in children aged three months to nine years old from the urban shanty compound community of Fortaleza, Brazil. Demographic and anthropometric information was assessed. The random treatment groups available for cognitive testing (total of 167 children) were: (1) placebo, n = 25; (2) glutamine, n = 23; (3) zinc, n = 18; (4) vitamin A, n = 19; (5) glutamine+zinc, n = 20; (6) glutamine+vitamin A, n = 21; (7) zinc+vitamin A, n = 23; and (8) glutamine+zinc+vitamin A, n = 18. Neuropsychological tests were administered for the cognitive domains of non-verbal intelligence and abstraction, psychomotor speed, verbal memory and recall ability, and semantic and phonetic verbal fluency. Statistical analyses were performed using SPSS, version 16.0. ClinicalTrials.gov: NCT00133406.
RESULTS:
Girls receiving a combination of glutamine, zinc, and vitamin A had higher mean age-adjusted verbal learning scores than girls receiving only placebo (9.5 versus 6.4, p = 0.007) and girls receiving zinc+vitamin A (9.5 versus 6.5, p = 0.006). Similar group differences were not found between male study children.
CONCLUSIONS:
The findings suggest that combination therapy offers a sex-specific advantage on tests of verbal learning, similar to that seen among female patients following traumatic brain injury.
Keywords: Malnutrition, Cognition, Zinc, Vitamin A, Glutamine, Child
INTRODUCTION
The vicious cycle of diarrhea/malnutrition in early childhood acts synergistically to impair the child's full developmental potential. A series of cohort studies from our group, enrolling children under diarrhea and enteric disease surveillance in the first two years of life, has documented significant growth shortfalls (1), blighted cognitive potential (2), and subsequent poor school performance during the developmental childhood years (3). While these long-term negative outcomes have been well documented, our understanding of measures that could prevent or reverse these cognitive short falls is very limited. Micronutrients, such as zinc, vitamin A, and glutamine, hold promise to avert these deficits due to these compounds' potential role in protecting the brain and in intestinal development (4,5).
Numerous clinical zinc supplementation trials have revealed decreased diarrheal mortality and morbidity in children following supplementation (6). In addition, a pooled analysis of 37 supplementation trials regarding the impact of zinc supplementation on growth shortfalls revealed a highly significant impact on linear growth in prepubertal children (7). The mechanisms by which zinc acts to protect against diarrhea and growth deficits are not fully understood, but they are likely multi-factorial, including anti-bactericidal and antioxidant effects (8,9), with improved mucosal immune response and recovery (10). Zinc is also involved in neurogenesis, neuronal migration, and synaptogenesis (11,12), and oral zinc supplementation improved behavioral and hippocampal development in a murine model of under-nutrition early in life (13).
Vitamin A deficiency is a well-recognized cause of preventable blindness, but it now is also being recognized for its maintenance of intestinal epithelial integrity and the immune response (14). These effects could explain the reductions of the mortality and severity of diarrheal episodes seen following vitamin A supplementation in children.
Further, while the importance of vitamin A in embryogenesis and congenital abnormalities has been well documented, very little is known about the importance of post-natal vitamin A deficiency in the healthy functioning of the central nervous system. Studies of retinoid knockout mice and mice subjected to post-natal vitamin A deprivation have suggested that retinoid signaling pathways play critical roles in higher cognitive functioning in the central nervous system (15). No studies have been conducted regarding the impact of vitamin A supplementation alone on cognitive outcomes in humans.
Glutamine is a major energy source for the intestinal mucosa, and it plays a key role in nucleic acid biosynthesis (16). Glutamine supplementation has been associated with improved gastrointestinal function in a variety of clinical scenarios, including ameliorating mucosal atrophy after parenteral nutrition (17), improving gut mucosal healing following cancer therapy (18), and enhancing enteric immune responses (16). Glutamine is also found abundantly in the central nervous system, where it acts as a precursor of neurotransmitter amino acids, an oxidative energy source, and an ammonia detoxifier (19). To the best of our knowledge the impact of glutamine supplementation on cognition has never been investigated in children.
This prospective, randomized, double-blind, placebo-controlled trial investigated the impact of supplemental zinc, vitamin A, and glutamine, alone or in combination, on long-term cognitive outcomes in a group of high-risk children identified as below the median height-for-age z-score (HAZ) in a slum community in Fortaleza-Ceará, Brazil.
METHODS
Population
This study was undertaken in the urban shanty compound community of Parque Universitário, Fortaleza, the capital of the state of Ceará in northeast Brazil. All children (identified through a community census) between three months and nine years of age and below the median height-for-age z-score (HAZ, population median = -0.06) were eligible to participate. The latter eligibility criterion was selected because of the high predictive value of HAZ scores in identifying children with chronic, persistent diarrheal episodes in the first two years of life (20). The exclusion criteria included children being exclusively breast-fed, participants in any study over the previous two years, and children having illnesses with fevers greater than 38°C at the time of enrollment.
Study Design and Baseline Evaluation
This study was a prospective, double-blind, randomized, placebo-controlled trial and was registered as a clinical trial (NCT00133406) with the US National Library of Medicine (http://www.clinicaltrials.gov). Enrollment/recruitment was conducted from June 2000 to August 2004. After parental consent was obtained, the parents and children were interviewed by an experienced field study team (one nurse and two community health workers) to collect demographic information, including age, sex, birth weight, level of maternal education, family income, and baseline anthropometric information. Weight was measured using a calibrated digital weighing scale with 100 g precision (Tanita Solar Scale, Tanita Corporation of American Inc., Arlington, IL, USA). Height was measured in the supine position for children younger than 24 months of age and standing for children aged 24 months old or older using an anthropometric rod with an accuracy of 0.1 cm. HAZ, weight-for-age z-scores (WAZ), and weight-for-height z-scores (WHZ) were calculated using the Epi-Info anthropometric software (Center for Disease Control, Atlanta, GA, USA) as markers of physical development and nutritional status. These anthropometric z-scores are the numbers of standard deviations greater or less than the median values for the National Center for Health Statistics (NCHS) and International Reference Population (21).
Sample Size Calculation
A sample size was calculated to allow for appropriate power to detect group differences in cognitive scores between children receiving zinc plus vitamin A compared with children receiving no vitamin A or zinc, averaging the difference in children receiving glutamine and children not receiving glutamine. Using a two-tailed test with type I error = 5%, 35 children per cell were calculated to be sufficient to have 80% power to detect a difference of 0.36 (small to medium effect size) and 90% power to detect a difference of 0.42 (medium effect size).
Nutritional Intervention and Surveillance
Three hundred and fourteen children were randomized with regard to receiving vitamin A (100,000 IU retinyl palmitate if <12 months old or 200,000 IU retinyl palmitate if ≥12 months old every four months), one dose at 0, four, and eight months of the study protocol; zinc (40 mg twice weekly); or both for one year. Half of each group received glutamine (16 g daily given over ten days started during the first month of the study protocol) (22), of whom one hundred and sixty-seven were available and eligible for cognitive testing. Only trained health care agents of the study surveillance team administered all of the micronutrients. The health workers administered the micronutrients during home visits with direct observation of intake. L-glutamine was obtained from Rexim (Courbevoie, France); L-glycine was obtained from Spectrum Chemicals (Gardena, CA, USA); zinc acetate was obtained from Spectrum Chemicals; and vitamin A (retinyl palmitate in vegetable oil with 40 IU of alpha-tochopherol as an antioxidant) was obtained from Hoffman-La Roche (Basel, Switzerland). Isomolar glycine (8.3 g/daily) was used as a placebo for glutamine. Tanjal juice was used as the placebo for zinc, and the same amounts of alpha-tocopherol and vegetable oil were used as the placebo for vitamin A. Tocopherol was chosen as the placebo for vitamin A because it is also a fat-soluble vitamin, and the tocopherol-dosing preparation is similar to vitamin A capsule dosing. The vitamin A and placebo capsules were the same color, size, and taste.
A computer-generated random number list assigned the children to one of eight treatment arms, including: 1) placebo; 2) glutamine; 3) zinc; 4) vitamin A; 5) glutamine+zinc; 6) glutamine+vitamin A; 7) zinc+vitamin A; or 8) glutamine+zinc+vitamin A. The treatment groups and oral treatment regimens, including dose, frequency, and duration of supplementation, are outlined in Table 1. A member of the field study team blinded to treatment group administered the supplementations and visited each child twice weekly to assess tolerance and any adverse effects of supplementation. No significant differences in the rates of adverse events were found among the treatment groups.
Table 1.
Treatment groups and oral treatment regimens of the study participants. A randomized, double-blind, and placebo-controlled clinical trial of zinc, vitamin A, and glutamine supplementation in Brazilian shanty-town children from Fortaleza, CE, northeastern Brazil, during June 2000 and August 2004, with follow-up between June 2000 and December 2007.
| Treatment Group | Oral Treatment Regimen |
| Placebo | Placebo*) (Placebo glutamine+Placebo zinc+Placebo vitamin A) |
| Glutamine | Glutamine**)+Placebo zinc+Placebo vitamin A |
| Zinc | Zinc†+Placebo glutamine+Placebo vitamin A |
| Vitamin A | Vitamin A‡)+Placebo zinc+Placebo glutamine |
| Glutamine+Zinc | Glutamine**)+Zinc†+Placebo vitamin A |
| Glutamine+Vitamin A | Glutamine**)+Vitamin A‡)+Placebo zinc |
| Zinc+Vitamin A | Zinc†+Vitamin A‡)+Placebo glutamine |
| Glutamine+Zinc+Vitamin A | Glutamine**)+Zinc†+Vitamin A‡) |
Isomolar glycine doses (8.3 g/day) over ten days started at one month of the study protocol; zinc vehicle (Tanjal juice) twice weekly for twelve months; placebo retinol = vitamin E (tocopherol 40 IU) each for four months at 0, four, and eight months.
Oral glutamine (16.2 g/day) for ten days starting at one month of the study protocol. † Zinc (zinc acetate) 40 mg twice weekly for 12 months.
Vitamin A (retinyl palmitate) 100,000 IU for children <12 months or 200,000 IU for children at least 12 months old, given as one dose at 0, four, and eight months of the study protocol.
Neuropsychological Assessment
Children who completed the supplementation protocol and were at least five years of age (appropriate age for testing) underwent five cognitive tests at an average of four years later (range 1.4-6.6). The following tests with Brazilian adaptations were included: the Test of Non-Verbal Intelligence, 3rd Edition (TONI-3); the Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML-2); Verbal Learning and Delayed Recall; the Weschler Intelligence Scale for Children (WISC) Coding A (for children ages 5-7) and Coding B (for children ages 8-13); and the Developmental Neuropsychological Assessment (NEPSY) Verbal Fluency test. The cognitive assessment chosen covered the cognitive domains of non-verbal intelligence and abstraction, psychomotor speed, verbal memory and recall ability, and semantic and phonetic verbal fluency. These tests have been used in previous studies in this population (2) and in other non-English-speaking populations (23). All of the tests were administered in a standardized manner and scored by a Brazilian developmental psychologist who was blinded to treatment group. The tests were administered in a silent room located in a school facility within the community.
Ethical Approval
The study protocol and informed consent form were approved by the Comitê de Ética em Pesquisa e Complexo Hospitalar (COMEPE) at the Federal University of Ceará (UFC), Fortaleza, Ceará, Brazil; Conselho Nacional de Ética em Pesquisa (CONEP) at the Ministry of Health, Brasília, Brazil; and the University of Virginia IRB for Health Services Research. In addition, the study was followed and approved by the Data Safety and Management Board (DSMB) at the National Institutes of Health.
Statistical Analysis
The data were double-entered by independent persons and validated using Excel software, version 4.0. All of the data were analyzed using the SPSS statistical software package (SPSS, Inc. Chicago, IL, USA). A one-way analysis of variance with post-hoc least significant difference (LSD) tests for two-by-two group comparisons was used to identify simple main effect differences in age at study entry, age at cognitive testing, time between cognitive testing and study enrollment, sex, birth weight, maternal education, family income, and nutritional parameters at study entry and conclusion, including weight-for-age WAZ, HAZ, and WHZ z-scores, as well as performance on the neuropsychological assessments. The treatment groups were further categorized into four micronutrient exposure groups, including zinc, vitamin A, zinc+vitamin A, or glutamine, and compared with regard to these variables to identify the impact of simple single or combination micronutrient exposures on study variables. An analysis of covariance was used to adjust for the influence on cognitive performance in terms of sex and the time between study enrollment and cognitive assessment. Continuous variables in tables are presented as the means±standard deviations. Categorical variables are presented as percentages of the total population. A p-value <0.05 was considered statistically significant.
RESULTS
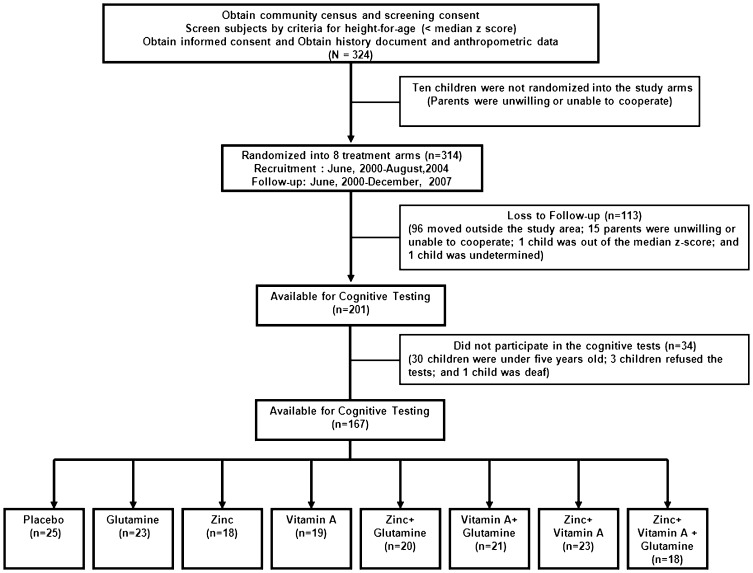
A flow chart of the study children is provided in Figure 1. A total of 324 children were identified and screened for study eligibility, of whom 201 (64%) completed nutrient supplementation during the 12-month study protocol. Among children completing the supplementation protocol, 167 (83%) children underwent the cognitive examinations. The most common reason for not undergoing the cognitive testing was that the child was younger than five years of age and thus too young to undergo the tests (n = 30). Children who completed the cognitive testing did not differ significantly from children who did not undergo cognitive testing with regard to sex, birth weight or length, or baseline WAZ or WHZ. However, the children who completed cognitive testing were significantly older (p<0.001), weighed more and were taller at baseline (both p-values <0.001), and had better baseline HAZ scores (p = 0.026) than those who did not complete the cognitive testing.
Figure 1.
Flow of Study Children to Micronutrient Supplementation Groups and Cognitive Testing.
Table 2 outlines the demographic and other characteristics of the children who completed cognitive testing, overall and by treatment group. The study children ranged from five to 13 years of age at the time of cognitive testing. Slightly more than half of the study children were female (53%). Only 30 children (18%) had mothers with more than a primary school education. Data on family income were available for 123 children, of whom 59 (48%) were living in a household earning the Brazilian minimum wage of 415 Brazilian Reais per month (equivalent to 265 USD per month) or less. No significant group differences were identified regarding age at study entry, age at cognitive testing, time between cognitive testing and study entry, sex, birth weight, maternal education, or family income. Regarding nutritional parameters, no significant group differences were identified at study entry or conclusion regarding HAZ, WAZ, or WHZ scores, with the exception that children in the combination glutamine, zinc, and vitamin A group had higher WAZ scores at study entry compared with placebo children (p = 0.04). This placebo versus combination glutamine, zinc, and vitamin A group difference in WAZ scores almost reached significance at the end of the study (p = 0.06).
Table 2.
Demographics and other characteristics of the study participants. A randomized, double-blind, and placebo-controlled clinical trial of zinc, vitamin A, and glutamine supplementation in Brazilian shanty-town children from Fortaleza, CE, northeastern Brazil, during June 2000-August 2004, with follow-up between June 2000 and December 2007.
| Placebo (n = 25) | Glutamine (n = 23) | Zinc (n = 18) | Vitamin A (n = 19) | Glutamine+Zinc (n = 20) | Glutamine+Vitamin A (n = 21) | Zinc+Vitamin A (n = 23) | Glutamine+Zinc+Vitamin A (n = 18) | Total (n = 167) | ||
| Age at Study Entry (months) | 48.8±29.8 | 53.9±28.2 | 62.2±26.0 | 43.3±25.5 | 55.2±28.3 | 54.8±29.0 | 59.5±26.1 | 56.0±25.5 | 54.1±27.4 | |
| Age at Cognitive Testing (years) | 8.43±2.02 | 8.73±2.18 | 9.20±2.20 | 7.55±2.14 | 8.76±1.87 | 8.61±1.99 | 8.98±2.30 | 8.34±1.86 | 8.58±2.08 | |
| Time between Cognitive Testing and Study Entry (years) | 4.42±1.46 | 4.29±1.31 | 4.09±1.15 | 3.99±1.48 | 4.22±1.34 | 4.10±1.32 | 4.09±1.15 | 3.73±1.33 | 4.13±1.33 | |
| Female n (%) | 12 (48) | 9 (39) | 11(61) | 9 (47.4) | 9 (45) | 9 (43) | 16 (70) | 13 (72) | 88 (53) | |
| Birth weight | 2.93±0.63 | 3.18±0.45 | 3.13±0.68 | 3.10±0.52 | 3.19±0.41 | 3.13±0.54 | 3.22±0.37 | 3.2±0.42 | 3.13±0.51 | |
| Maternal Education (< primary school) n (%) | 5 (20) | 2 (9) | 6 (33) | 3 (16) | 2 (11) | 6 (29) | 3 (14) | 3 (18) | 30 (18) | |
| Family Income>than Minimum Wage n (%) | 9 (53) | 11 (65) | 6 (60) | 4 (31) | 9 (60) | 11(61) | 8 (50) | 6 (35) | 67 (55) | |
| Nutritional Parameters at Study Baseline | ||||||||||
| Weight-for-Age z score | -1.00±1.10 | -0.42±0.84 | -0.55±0.82 | -0.47±1.04 | -0.54±0.92 | -0.85±0.81 | -0.99±0.98 | -0.41±0.80*) | -0.67±0.94 | |
| Height-for-Age z score | -1.26±0.97 | -0.69±0.89 | -0.80±1.01 | -0.86±0.79 | -0.95±0.90 | -1.01±0.91 | -1.07±0.95 | -0.82±0.69 | -0.95±0.90 | |
| Weight-for-Height z score | -0.25±1.13 | 0.10±0.87 | -0.07±0.82 | 0.15±1.01 | 0.12±0.93 | -0.33±1.00 | -0.42±0.93 | 0.25±1.12 | -0.07+0.99 | |
Combination Zinc+Vitamin A+Glutamine versus Placebo, p = 0.04.
Cognitive Performance
The mean age-adjusted cognitive test scores for all eight treatment arms are presented in Table 3. No significant group differences were found regarding the age-adjusted TONI-3 Intelligence Quotient (IQ), WISC-III Coding, WRAML-2 Delayed Verbal Learning, or NEPSY Verbal Fluency mean scores. z-scores for semantic and phonetic fluency among children aged 6-12 years old were also compared across treatment groups to identify any differential impact of supplementation group on verbal fluency categories. No significant group differences were identified based on verbal fluency subtest z-scores. However, children receiving combination glutamine, zinc, and vitamin A performed significantly better on WRAML-2 verbal learning than the placebo group, with mean age-adjusted scores of 9.28 (SD 3.41) and 7.28 (SD 3.34), respectively (p = 0.02). After controlling for time between study entry and cognitive assessment, sex stratification revealed that this improvement in verbal learning was highly significant among the girls (p = 0.007) but not among the boys (p = 0.43) who received combination glutamine, zinc, and vitamin A versus placebo (Table 4. In addition, female children receiving combination glutamine, zinc, and vitamin A performed significantly better on verbal learning than female children receiving zinc and vitamin A alone (p = 0.006). These group differences persisted after controlling for differences in baseline WAZ scores. However, within the placebo group, the girls had worse verbal learning scores than the boys (p<0.05).
Table 3.
Mean age-adjusted (scaled) performance on cognitive tests by treatment group. A randomized, double-blind, placebo-controlled clinical trial of zinc, vitamin A, and glutamine supplementation in Brazilian shanty-town children from Fortaleza, CE, northeastern Brazil, during June 2000-August 2004, with follow-up between June 2000 and December 2007.
| Cognitive Tests | Placebo (n = 25) | Glutamine (n = 23) | Zinc (n = 18) | Vitamin A (n = 19) | Glutamine+Zinc (n = 20) | Glutamine+Vitamin A (n = 21) | Zinc+Vitamin A (n = 23) | Glutamine+Zinc+Vitamin A (n = 18) |
| IQ | 79.1±9.12 | 81.8±7.76 | 79.9±6.92 | 82.2±5.87 | 80.4±8.05 | 81.91±7.12 | 82.5±9.94 | 82.1±9.78 |
| Coding | 9.45±3.51 | 10.4±3.13 | 9.67±3.66 | 9.84±3.15 | 9.75±3.17 | 9.71±3.78 | 9.78±4.17 | 10.8±3.38 |
| Verbal Learning | 7.28±3.34 | 8.43±2.41 | 7.61±2.40 | 8.74±2.47 | 8.15±2.83 | 7.9±2.77 | 7.57±2.81 | 9.28±3.41** |
| Delayed Verbal Learning | 8.20±2.69 | 7.78±1.57 | 8.00±1.88 | 7.79±2.22 | 8.35±2.08 | 8.71±2.43 | 8.52±2.64 | 8.11±3.08 |
| Verbal Fluency | 6.22±2.70 | 6.67±2.67 | 7.11±3.59 | 5.74±2.35 | 5.95±2.81 | 6.75±2.65 | 6.36±2.95 | 7.33±2.63 |
| NEPSY Verbal Fluency Subtest Z-scores*) | ||||||||
| Semantic Fluency | -1.17±0.84 | -0.63±0.78 | -0.43±0.82 | -1.15±0.49 | -0.63±0.86 | -0.36±0.94 | -0.82±0.73 | -1.24±1.38 |
| Phonetic Fluency | -1.38±0.67 | -1.48±0.71 | -1.04±1.19 | -1.56±0.80 | -1.49±0.70 | -1.18±0.88 | -1.54±0.87 | -1.14±0.83 |
Children aged 6-12 years old only; **Comparison of Combination Glutamine+Zinc+Vitamin A versus placebo, p = 0.02.
IQ = intelligence quotient.
NEPSY = A developmental neuropsychological assessment.
Table 4.
Mean and standard deviations of age-adjusted performance on cognitive tests by sex after adjusting for time between study enrollment and cognitive evaluation. A randomized, double-blind, placebo-controlled clinical trial of zinc, vitamin A, and glutamine supplementation in Brazilian shanty-town children from Fortaleza, CE, northeastern Brazil, during June 2000-August 2004, with follow-up between June 2000 and December 2007.
| Cognitive Tests | Placebo | Glutamine | Vitamin A | Zinc | Glutamine+Zinc | Glutamine+Vitamin A | Zinc +Vitamin A | Glutamine+Zinc+Vitamin A |
| Female | ||||||||
| IQ | 82.6 (2.4) | 83.2 (2.8) | 81.1 (2.8) | 80.0 (2.5) | 83.7 (2.8) | 83.3 (2.8) | 81.7 (2.1) | 81.1 (2.1) |
| Coding | 10.1 (1.1) | 11.0 (1.2) | 11.2 (1.2) | 10.7 (1.1) | 10.1 (1.2) | 10.4 (1.2) | 9.7 (0.9) | 11.4 (1.0) |
| Verbal Learning | 6.4 (0.8)*) | 7.6 (0.9) | 8.3 (0.9) | 8.3 (0.8) | 7.3 (0.9) | 7.5 (0.9) | 6.5 (0.7)**) | 9.5 (0.8) |
| Delayed Verbal Learning | 5.0 (8.5) | 7.5 (11.1) | 6.7 (8.8) | 6.6 (10.8) | 7.1 (10.9) | 7.8 (11.6) | 6.7 (10.7) | 6.2 (8.7) |
| Verbal Fluency | 6.2 (0.9) | 6.0 (1.0) | 6.0 (1.0) | 7.4 (0.9) | 6.3 (1.0) | 7.1 (1.0) | 6.2 (0.7) | 7.5 (0.8) |
| Male | ||||||||
| IQ | 75.9 (2.3) | 80.9 (2.2) | 82.1 (2.6) | 79.7 (3.1) | 77.7 (2.5) | 80.7 (2.4) | 84.4 (3.2) | 84.8 (3.7) |
| Coding | 8.8 (1.0) | 10.0 (0.9) | 8.6 (1.1) | 8.0 (1.3) | 9.4 (1.1) | 9.2 (1.0) | 10.0 (1.4) | 9.6 (1.6) |
| Verbal Learning | 8.0 (0.8) | 8.9 (0.7) | 9.2 (0.9) | 6.6 (1.1) | 8.8 (0.8) | 8.2 (0.8) | 9.9 (1.1) | 9.0 (1.2) |
| Delayed Verbal Learning | 6.7 (0.9) | 9.3 (0.9) | 7.8 (0.5) | 8.7 (1.1) | 9.0 (0.9) | 9.7 (1.0) | 8.7 (1.0) | 7.4 (0.6) |
| Verbal Fluency | 6.1 (0.8) | 7.1 (0.8) | 5.5 (0.9) | 6.5 (1.2) | 5.7 (0.9) | 6.5 (0.9) | 6.7 (1.1) | 7.0 (1.3) |
Glutamine+Zinc+Vitamin A versus Placebo, p = 0.007.
Glutamine+Zinc+Vitamin A versus Zinc+Vitamin A, p = 0.006.
IQ = intelligence quotient.
No significant between group differences were identified before or after sex stratification on the WRAML-2 delayed verbal learning score. This combination group effect also almost reached statistical significance compared with female children receiving glutamine and zinc alone (p = 0.08).
The treatment arms were also categorized into four groups based on micronutrient exposure (i.e., zinc, vitamin A, combination zinc+vitamin A, or glutamine) to assess the differential impact of exposure to a specific single micronutrient or combination of micronutrients on cognitive test performance. The results of cognitive testing revealed no significant between group differences based on micronutrient exposure categories.
DISCUSSION
This study found a superior impact of combination glutamine, zinc, and vitamin A supplementation on the WRAML-2 verbal learning in a group of children with low to median HAZ scores (and thus at high risk for the cognitive deficits seen in children with childhood diarrhea and malnutrition). These findings were statistically significant overall, but sex stratification identified highly significant differences only between female children receiving combination therapy and girls receiving zinc and vitamin A alone or placebo. Supplementation was found to be beneficial in girls compared with the placebo group. Thus, the combined intervention with glutamine, zinc, and vitamin A was more important to the female group because the girls were more affected than the boys.
No other differences were identified between supplementation treatment groups or following the categorization of treatment groups into one of four exposure groups. Previous studies from our group have shown improvements in intestinal barrier function after a ten day treatment regimen of glutamine-enriched formula (22). A recent meta-analysis showed that zinc supplementation reduces diarrhea morbidity in children, especially after six months of age (24); both of these effects could be helpful during the critical time window for brain development in the study children, who live in endemic areas of enteric infections and malnutrition.
This sex-specific benefit of combination glutamine, zinc, and vitamin A supplementation was an unexpected finding. Studies of recovery following traumatic brain injury (TBI) in children have shown a female recovery advantage in verbal list learning (25,26). In a study of 60 children (aged 6-16 years) with TBI identified through a private rehabilitation center in the midwestern U.S., female children performed significantly better than male children on the California Verbal Learning Test-Children's Version, even after controlling for the impact of the severity of head injury (25). In contrast with these findings, there have been studies reporting a sex-specific decrease in intelligence test results in children treated for acute lymphoblastic leukemia, with girls showing lower IQ scores than boys (27,28), in contrast with the specific deficits in verbal learning seen in our study and the verbal intelligence deficits seen in pediatric intracranial hemorrhage and TBI studies.
Our study, which included children at high risk for the long-term cognitive deficits seen with childhood diarrhea and malnutrition (based on low baseline HAZ scores), supports the findings of verbal list learning advantages following combination glutamine, zinc, and vitamin A supplementation.
There is accumulating evidence that β-estradiol (the primary biological form of estrogen) not only has a neuroprotective role but might also activate restorative processes, improving learning and memory, and promote the formation of synapses in the nervous system during aging and early development, as seen in animal models (29,30). However, estradiol-based hormonal treatment in post-menopausal women has been found to be detrimental, increasing the risk of dementia and stroke (30). Furthermore, sex differences during cognitive development remain controversial (31). One potential explanation for these contradictory findings is the requirement that β-estradiol interact with IGF-1 for its neuroprotective effects (32). This study did not evaluate IGF-1 serum levels in these children (a study that is under way by our group); however, early weight gain and subsequent linear growth were associated with increments in serum IGF-1 levels. The neuroprotective effects of the combination nutritional therapy seen in the girls in this study might be related to improved IGF-1 interactions. More studies are warranted to test this hypothesis.
Zinc supplementation trials in human children have not consistently shown improvement in cognitive outcomes. A randomized, controlled zinc supplementation trial in 740 Chinese children aged six to nine years attending three peri-urban schools found that children receiving micronutrient supplementation plus 20 mg of zinc six days/week for ten weeks had significantly improved fine and gross motor skills, attention, abstract concept formation, and abstract reasoning skills compared with children who received micronutrient supplementation without zinc or who received zinc supplementation alone (23). However, a study of 162 Guatemalan school children aged seven to eight years receiving 20 mg zinc/day for 25 weeks failed to find any significant differences after six months of follow-up in mental concentration or short-term memory compared with children receiving a placebo (24). Similarly, no significant differences in attention were found among 60 Canadian boys with low height percentiles attending first grade (aged 5-7 years) in 47 schools in southern Ontario who received 10 mg of zinc in a 1 mL solution of ZnSO4 added to apple/orange juice compared with children receiving a similar placebo solution (25). These differences might be related to the prevalence and severity of zinc deficiencies in the study population, the impact of other concurrent micronutrient deficiencies, the simultaneous use of other supplements, the dose and duration of the supplementation, and the sensitivity and appropriateness of the neuropsychological testing employed.
The finding of a verbal learning benefit among female children receiving combination glutamine, zinc, and vitamin A supplementation addresses previous concerns that multiple micronutrient deficiencies present in undernourished children could reduce or obscure the clinical benefits of supplementation with single micronutrients (33). However, it should be noted that the present study did not conduct serial micronutrient serum assessments, and thus, the reasons for the positive effect of the full combination of supplements compared with single supplements or other combinations of supplementation remain speculative.
Simultaneous supplementation with multiple micronutrients could act synergistically to improve long-term growth and cognitive outcomes in high-risk children by acting on multiple organ systems, including the gastrointestinal tract, systemic immune system, central nervous system, and neuroendocrine axes. Previous clinical studies of multiple micronutrient supplementation have produced divergent results, with some reporting positive benefits on attention, learning, and IQ, and others reporting no effects (34). Comparisons of these trials are difficult due to variations in population sampling, supplementation regimens (including some macronutrient and energy supplementation), routes of administration, durations of supplementation, and neuropsychological assessment instruments. No previous studies have applied supplementation with a combination of glutamine, zinc, and vitamin A.
It is noteworthy that the WAZ at the start of the study was higher in the zinc+vitamin A+glutamine groups compared with the placebo groups, which might have explained the improved verbal learning scores in that group; however, WAZ at start of the study was not a significant predictor of verbal learning scores and therefore cannot be used to explain that improvement.
Regarding limitations, we acknowledge that due to the small size of each interventional group at the end of follow-up, this study lost power and could have incorporated unexpected bias in the statistical analyses; therefore, our findings should be interpreted with caution and should be confirmed with larger study designs.
In conclusion, we documented sex-specific improvements in selected cognitive tests after combined nutrient supplementation in at-risk children living in impoverished settings in northeastern Brazil. More studies (now being planned by our group) are warranted to evaluate the potential mechanisms of nutrient-to-nutrient interactions underlying these findings and the potential associations with IGF-1 levels in children.
ACKNOWLEDGMENTS
The authors would like to thank Sayonara Bezerra de Alencar, Fátima Alves, Rosiana Maria de Paula Silva, and Maria Luzia F. Melo for all of their hard work and dedication to the Parque Universitário community and the Clinical Research Unit of the Federal University of Ceará. The study was supported by a Brazilian funding agency, CNPq, and an ICIDR program grant, #5 U01 AI026512, from the NIH, USA.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66(9):487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick PD, Oria RB, Madhavan V, Pinkerton RC, Lorntz B, Lima AA, et al. Limitations in verbal fluency following heavy burdens of early childhood diarrhea in Brazilian shantytown children. Child Neuropsychol. 2005;11(3):233–44. doi: 10.1080/092970490911252. [DOI] [PubMed] [Google Scholar]
- 3.Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, et al. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25(6):513–20. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 4.Benton D. Micronutrient status, cognition and behavioral problems in childhood. Eur J Nutr. 2008;47 Suppl 3:38–50. doi: 10.1007/s00394-008-3004-9. [DOI] [PubMed] [Google Scholar]
- 5.Suh SW, Won SJ, Hamby AM, Yoo BH, Fan Y, Sheline CT, et al. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J Cereb Blood Flow Metab. 2009;29(9):1579–88. doi: 10.1038/jcbfm.2009.80. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, Hidayat A, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr. 2000;72(6):1516–22. doi: 10.1093/ajcn/72.6.1516. [DOI] [PubMed] [Google Scholar]
- 7.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002;75(6):1062–71. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 8.Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods. 2003;54(2):177–82. doi: 10.1016/s0167-7012(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 9.Soderberg TA, Sunzel B, Holm S, Elmros T, Hallmans G, Sjoberg S. Antibacterial effect of zinc oxide in vitro. Scand J Plast Reconstr Surg Hand Surg. 1990;24(3):193–7. doi: 10.3109/02844319009041278. [DOI] [PubMed] [Google Scholar]
- 10.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 11.Colvin RA, Davis N, Nipper RW, Carter PA. Zinc transport in the brain: routes of zinc influx and efflux in neurons. J Nutr. 2000;130(5S Suppl):1484S–7S. doi: 10.1093/jn/130.5.1484S. [DOI] [PubMed] [Google Scholar]
- 12.Frederickson CJ, Danscher G. Zinc-containing neurons in hippocampus and related CNS structures. Prog Brain Res. 1990;83:71–84. doi: 10.1016/s0079-6123(08)61242-x. [DOI] [PubMed] [Google Scholar]
- 13.Ladd FV, Ladd AA, Ribeiro AA, Costa SB, Coutinho BP, Feitosa GA, et al. Zinc and glutamine improve brain development in suckling mice subjected to early postnatal malnutrition. Nutrition. 2010;26(6):662–70. doi: 10.1016/j.nut.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima AA, Soares AM, Lima NL, Mota RM, Maciel BL, Kvalsund MP, et al. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr. 2010;50(3):309–15. doi: 10.1097/MPG.0b013e3181a96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etchamendy N, Enderlin V, Marighetto A, Pallet V, Higueret P, Jaffard R. Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signalling. Behav Brain Res. 2003;145(1-2):37–49. doi: 10.1016/s0166-4328(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 16.Carneiro-Filho BA, Bushen OY, Brito GA, Lima AA, Guerrant RL. Glutamine Analogues As Adjunctive Therapy for Infectious Diarrhea. Curr Infect Dis Rep. 2003;5(2):114–9. doi: 10.1007/s11908-003-0046-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu GH, Wang H, Zhang YW, Wu ZH, Wu ZG. Glutamine supplemented parenteral nutrition prevents intestinal ischemia- reperfusion injury in rats. World J Gastroenterol. 2004;10(17):2592–4. doi: 10.3748/wjg.v10.i17.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carneiro-Filho BA, Oria RB, Wood RK, Brito GA, Fujii J, Obrig T, et al. Alanyl-glutamine hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice. Nutrition. 2004;20(10):934–41. doi: 10.1016/j.nut.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht J, Sidoryk-Wegrzynowicz M, Zielinska M, Aschner M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010;6(4):263–76. doi: 10.1017/S1740925X11000093. [DOI] [PubMed] [Google Scholar]
- 20.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61(5):707–13. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 21.de Onis M, Onyango AW, Borghi E, Garza C, Yang H. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutr. 2006;9(7):942–947. doi: 10.1017/phn20062005. [DOI] [PubMed] [Google Scholar]
- 22.Lima AA, Brito LF, Ribeiro HB, Martins MC, Lustosa AP, Rocha EM, et al. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J Pediatr Gastroenterol Nutr. 2005;40(1):28–35. doi: 10.1097/00005176-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. 1997 3rd ed. Austin (TX): PRO-ED. [Google Scholar]
- 24.Lazzerini M, Ronfani L. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2012;6:CD005436. doi: 10.1002/14651858.CD005436.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Donders J, Hoffman NM. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16(4):491–9. doi: 10.1037//0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- 26.Donders J, Woodward HR. Gender as a moderator of memory after traumatic brain injury in children. J Head Trauma Rehabil. 2003;18(2):106–15. doi: 10.1097/00001199-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Robison LL, Nesbit ME, Jr, Sather HN, Meadows AT, Ortega JA, Hammond GD. Factors associated with IQ scores in long-term survivors of childhood acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1984;6(2):115–21. doi: 10.1097/00043426-198406020-00001. [DOI] [PubMed] [Google Scholar]
- 28.Waber DP, Urion DK, Tarbell NJ, Niemeyer C, Gelber R, Sallan SE. Late effects of central nervous system treatment of acute lymphoblastic leukemia in childhood are sex-dependent. Dev Med Child Neurol. 1990;32(3):238–48. doi: 10.1111/j.1469-8749.1990.tb16930.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood. Horm Behav. 2011;59(3):353–7. doi: 10.1016/j.yhbeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab. 2011;22(12):467–73. doi: 10.1016/j.tem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Ardila A, Rosselli M, Matute E, Inozemtseva O. Gender differences in cognitive development. Dev Psychol. 2011;47(4):984–90. doi: 10.1037/a0023819. [DOI] [PubMed] [Google Scholar]
- 32.Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30(20):6852–61. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black MM. Micronutrient deficiencies and cognitive functioning. J Nutr. 2003;133(11 Suppl 2):3927S–3931S. doi: 10.1093/jn/133.11.3927S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazir S, Nagalla B, Thangiah V, Kamasamudram V, Bhattiprolu S. Effect of micronutrient supplement on health and nutritional status of schoolchildren: mental function. Nutrition. 2006;22(1 Suppl):S26–S32. doi: 10.1016/j.nut.2004.07.021. [DOI] [PubMed] [Google Scholar]