Abstract
OBJECTIVE:
Ischemic stroke may result from transient or permanent reductions of regional cerebral blood flow. Polymorphonuclear neutrophils have been described as the earliest inflammatory cells to arrive in ischemic tissue. CXCR1/2 receptors are involved in the recruitment of these cells. However, the contribution of these chemokine receptors during transient brain ischemia in mice remains poorly understood. In this work, we investigated the effects of reparixin, an allosteric antagonist of CXCR1/2 receptors, in a model of middle cerebral artery occlusion and reperfusion in mice.
METHODS:
C57BL/6J male mice treated with reparixin or vehicle were subjected to a middle cerebral artery occlusion procedure 1 h after the treatment. Ninety minutes after ischemia induction, the monofilament that prevented blood flow was removed. Twenty-four hours after the reperfusion procedure, behavioral changes, including motor signs, were analyzed with the SmithKline/Harwell/Imperial College/Royal Hospital/Phenotype Assessment (SHIRPA) battery. The animals were sacrificed, and brain tissue was removed for histological and biochemical analyses. Histological sections were stained with hematoxylin and eosin, neutrophil infiltration was estimated by myeloperoxidase activity and the inflammatory cytokine IL-1β was measured by ELISA.
RESULTS:
Pre-treatment with reparixin reduced the motor deficits observed in this model of ischemia and reperfusion. Myeloperoxidase activity and IL-1β were reduced in the reparixin-treated group. Histological analysis revealed that ischemic injury was also attenuated by reparixin pre-treatment.
CONCLUSIONS:
Our results suggest that the blockade of the CXCR1/2 receptors by reparixin promotes neuroprotective effects by reducing the levels of polymorphonuclear infiltration in the brain and the tissue damage associated with middle cerebral artery occlusion and reperfusion.
Keywords: Cerebral Ischemia, Neutrophils, Reparixin, CXCR1/CXCR2 Receptors
INTRODUCTION
Ischemic stroke may result from transient or permanent reductions of regional cerebral blood flow (1). In humans, stroke is the second most common cause of death worldwide (2) and often occurs in the territory supplied by the middle cerebral artery (3). Inflammatory processes have been implicated in the pathophysiology of cerebral ischemia, involving the recruitment and influx of leukocytes from the circulation to the ischemic brain tissue.
In the early stages of ischemia, polymorphonuclear neutrophils (PMNs) are the main type of infiltrating cells. Several studies report the later presence of monocytes/macrophages, activation of resident brain cells and the expression of proinflammatory cytokines, chemokines and adhesion molecules in the injured brain (4). Leukocyte infiltration, especially of PMNs, seems to play a deleterious role during an ischemic event in the central nervous system (5). PMNs are a potential source of radical oxygen species, proteinases and cytokines that may contribute to the brain injury (6). Furthermore, strategies affecting the recruitment of PMNs are of great interest for controlling various inflammatory diseases. Several authors have postulated that the expression of certain chemokines, mainly CXC ligand 8 (CXCL8) in humans and CXCL1 and CXCL2 in rodents, by central nervous system cells is required for PMN accumulation and activation (7). Furthermore, human patients exhibit increased circulating levels of CXCL8 following stroke (8).
In the last decade, several studies have suggested that anti-inflammatory strategies could be of paramount importance to prevent brain damage following cerebral ischemia (9,10). Indeed, anti-chemokine approaches successfully conferred neuroprotection in rodent models of brain ischemia (11-13).
Reparixin is a chemical derivative of phenyl propionic acids that acts as noncompetitive allosteric antagonist of the chemokine receptors CXCR1 and CXCR2, which bind to the chemokines CXCL1 (previously known as KC) and CXCL2 (previously known as MIP-2). Therefore, the aim of this study was to investigate the effect of reparixin on inflammatory and behavioral outcomes in a model of middle cerebral artery occlusion and reperfusion (MCAo) in mice.
MATERIAL AND METHODS
Animals: Male C57BL/6J mice (8-10 weeks old/20-25 g) obtained from the Animal Care Facilities of the Institute of Biological Science (ICB-UFMG) were maintained in a temperature controlled room (24±1°C) under standard laboratory conditions with free access to food and water and a 12-h light/12-h dark cycle (lights on at 7 a.m.). All experiment procedures were conducted in accordance with the Animal Ethics Committee (CETEA-UFMG).
Drugs: Reparixin (30 mg/kg – Dompé Pharma, L'Aquila, Italy), a CXCR1/2 allosteric antagonist, was dissolved in saline and administered subcutaneously.
Transient focal cerebral ischemia: Mice were anesthetized by intraperitoneal injection of ketamine hydrochloride (150 mg/kg) and xylazine (10 mg/kg). The surgical procedure was performed on a warming mantle with all animals spontaneously breathing. Transient focal cerebral ischemia was induced by MCAo, using the method previously described for rats by Longa et al., 1989 (10). Briefly, a blunted silicon tip (Silon2 APS Fluido, Petrópolis, Brazil) and a 5-0 nylon monofilament catalyst (Silon2 APSC, Petrópolis, Brazil) were advanced to the level of the carotid bifurcation via the internal carotid artery until light resistance was felt. The monofilament was removed after 90 minutes of occlusion. In the sham group, the arteries were visualized but not disturbed.
SHIRPA screening test: The primary SmithKline/Harwell/Imperial College/Royal Hospital/Phenotype Assessment (SHIRPA) screen consists of a wide range of tests, organized into the following five functional categories: neuropsychiatric state (i.e., spontaneous activity, transfer arousal, touch escape, positional passivity, biting, fear, irritability, aggression, vocalizations), motor behavior (i.e., body position, tremor, locomotor activity, pelvic elevation, gait, tail elevation, trunk curl, limb grasping, wire maneuver, negative geotaxis), reflex and sensory function (i.e., startle response, visual placing, pinna reflex, corneal reflex, toe pinch, righting reflex), autonomic function (i.e., respiratory rate, defecation, urination, palpebral closure, piloerection, skin color, heart rate, lacrimation, salivation, body temperature) and muscle tone and strength (i.e., grip strength, body tone, limb tone, abdominal tone). The SHIRPA provides a basic behavioral and functional profile through the observational assessment of individual performance (14). All measures were performed in a Plexiglas square arena (40 cm × 40 cm × 30 cm).
Experimental procedures: The subcutaneous administration of reparixin (30 mg/kg) was performed 60 minutes before cerebral ischemia induction (15). The animals were divided into the following three experimental groups: Sham (i.e., the group in which the arteries were visualized, but there was no occlusion of the middle cerebral artery), Vehicle (i.e., the group pre-treated with the vehicle, phosphate buffer solution, 60 minutes before MCAo) and Reparixin (i.e., the group pre-treated with the drug 60 minutes before MCAo). To evaluate neurological signs secondary to MCAo, the animals were assessed with the SHIRPA battery 24 h after reperfusion (16). Immediately after behavioral testing, all animals were sacrificed, and the whole brain was removed for biochemical and histological analyses. Neutrophil infiltration in the brain was estimated by myeloperoxidase activity test (MPO), and the results were expressed in relative units. The concentration of IL-1β was measured in brain tissue using a commercially available enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (Duo-Set Kits; R&D Systems, Minneapolis, MN). For histological analysis, a brain fragment was preserved in 10% buffered formalin. Sections (4 μm thick) were subject to routine hematoxylin and eosin staining. Ischemia/reperfusion-induced lesions and the infiltration of inflammatory cells were analyzed qualitatively.
Statistical analysis: Data are represented as the means ± SEM. One-way ANOVA followed by the Newman-Keuls post-hoc test was used for multiple comparisons. The statistical significance was set at p<0.05.
RESULTS
When subjected to MCAo, vehicle-treated mice exhibited higher MPO activity, an index of PMN infiltration in the brain, in comparison with the sham animals. Reparixin significantly prevented the increase of MPO induced by MCAo (Figure 1.
Figure 1.
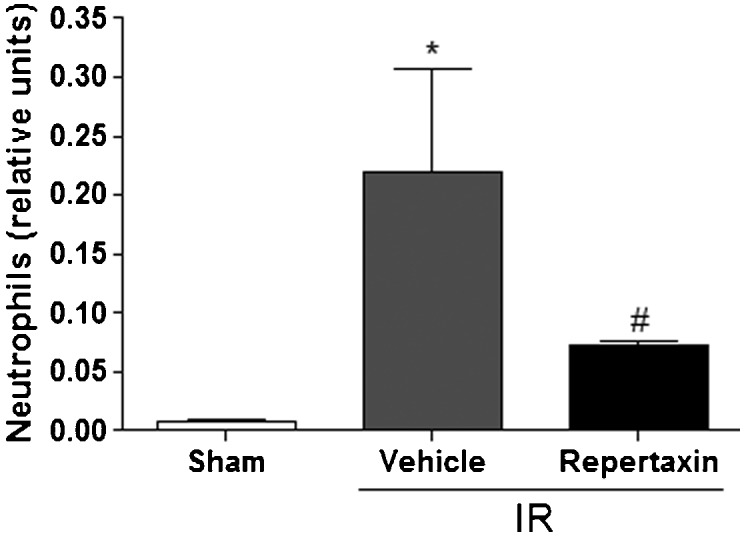
Reparixin reduces neutrophil activity in the brain after middle cerebral artery occlusion/reperfusion (MCAo) in mice. The figure shows myeloperoxidase (MPO) activity in mice not subjected to MCAo (Sham) or subjected to MCAo with pre-administration of vehicle (Vehicle) or reparixin (30 mg/kg, s.c.) 60 minutes before MCAo procedures. The results are expressed as the means ± SEM (n = 6). * p<0.05 compared to Sham group. # p<0.05 compared to vehicle. (ANOVA followed by Newman-Keuls posthoc test).
Vehicle-treated animals subjected to the MCAo procedures also displayed higher brain levels of IL1-β compared to sham mice. Similar to the MPO results, pretreatment with reparixin significantly reduced the levels of this inflammatory cytokine (Figure 2.
Figure 2.
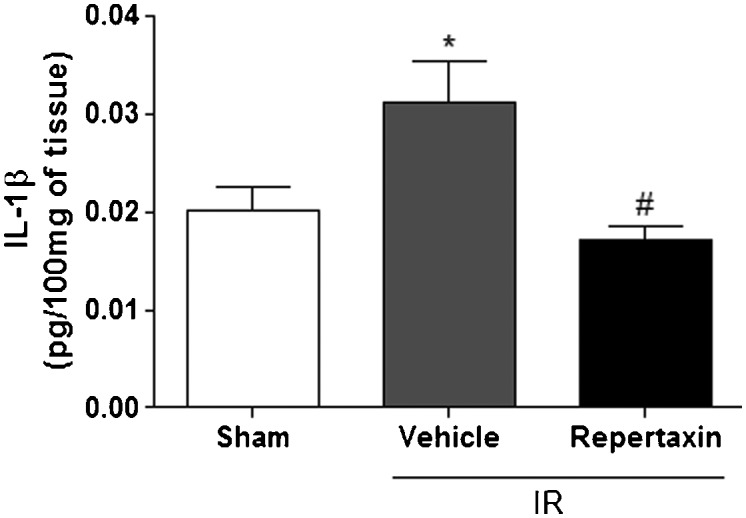
Reparixin reduces the levels of IL-1β in the brain after middle cerebral artery occlusion/reperfusion (MCAo) in mice. Bars represent levels of IL-1β (pg/100 mg) measured by ELISA in the brain tissues of mice subjected or not (SHAM) to MCAo and pretreated with vehicle or reparixin (30 mg/kg, s.c.). The results are expressed as the means ± SEM (n = 6). *p<0.05 compared to Sham group. # p<0.01 compared to Vehicle. (ANOVA followed by Newman-Keuls posthoc test).
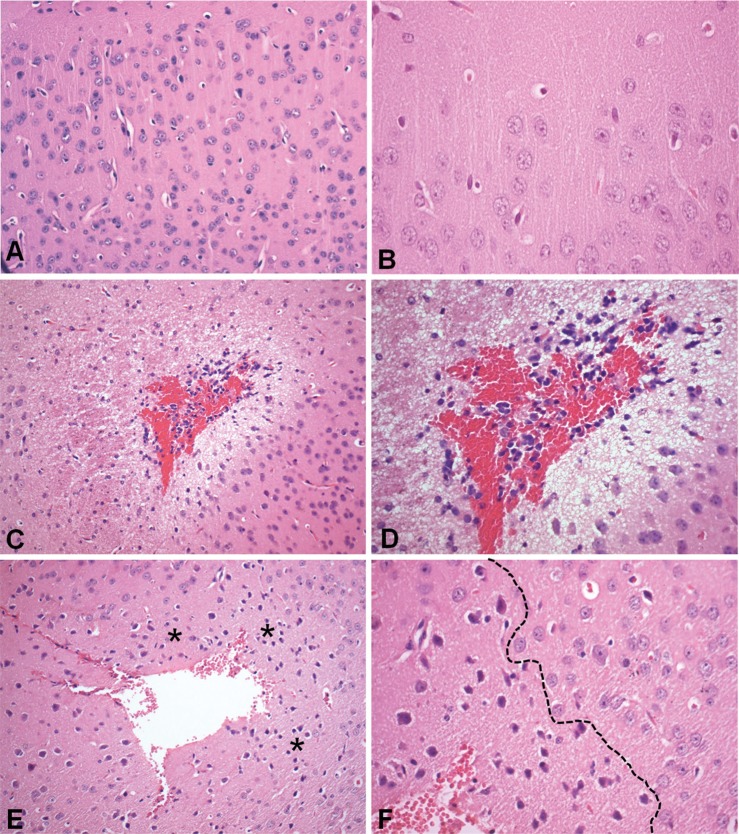
The ischemia/reperfusion procedure induced an extensive hemorrhagic necrosis in the brain (Figures 3 A-D). This lesion was circumscribed by inflammatory cells, mainly PMNs, surrounded by degenerative tissue (Figures 3 C and D). Corroborating the MPO and IL1-β results, reparixin treatment attenuated the ischemia/reperfusion-induced lesions and the infiltration of inflammatory cells (Figures 3 E and F).
Figure 3.
Histopathological analysis of cerebral sections of sham (A-B), ischemia/reperfusion (C-D) and reparixin-treated mice subjected to middle cerebral artery occlusion/reperfusion (E-F). Hematoxylin and eosin-stained sections. Cerebral cortex of sham mice showing normal tissue (A) and healthy neural cells (B). Cerebral section of an ischemia/reperfusion mouse exhibits large hemorrhagic necrosis (C). Note the inflammatory cells and vacuolated tissue around the infarct area (D). Reparixin-treated animals present focal infarct surrounded by ischemic neural cells (asterisks) (E). The dotted line delineates dark and shrunken neural cells (left) from normal neural cells (right) (F). Original magnifications: A, C and E: x200. B, D and F: x400.
Consistent with these pathological results, the MCAo procedure induced motor impairment, which was attenuated by pretreatment with reparixin (Figure 4. Other SHIRPA categories did not change after MCAo.
Figure 4.
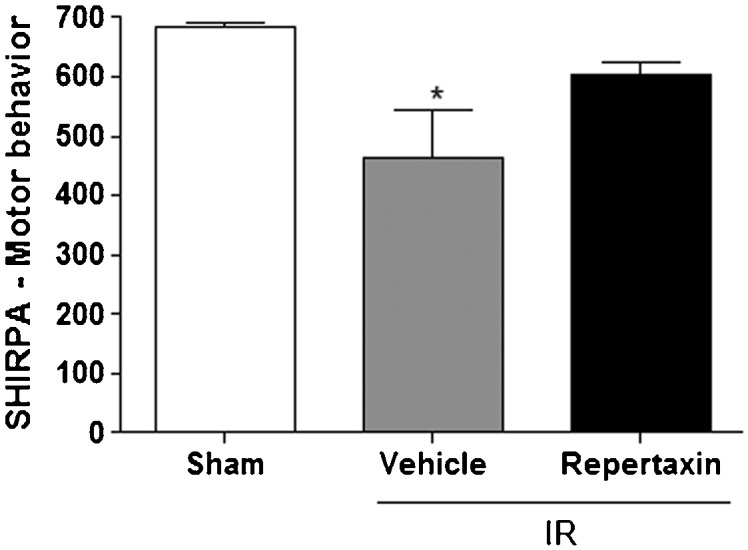
Reparixin prevents motor impairment after middle cerebral artery occlusion/reperfusion (MCAo) in mice. Bars indicate levels of motor deficits as assessed by SHIRPA in mice subjected or not to MCAo (Sham) or subjected to MCAo pretreated with vehicle or reparixin (30 mg/kg, s.c.). The results are expressed as the means ± SEM (n = 6). * p<0.05 compared to Sham group; ANOVA followed by Newman-Keuls post-test. (ANOVA followed by Newman-Keuls posthoc test).
DISCUSSION
In this work, we demonstrated that the inhibition of CXCR1/2 receptors by reparixin protected the brain after MCAo. The blockade of CXCR1/2 receptors attenuated not only the structural changes in the ischemic brain but also the corresponding behavioral consequences of the brain injury.
Tissue damage after ischemia is mediated through several mechanisms, including hypoxia and inflammatory responses (17). The latter is particularly relevant in the context of reperfusion, in which PMNs infiltrate ischemic/reperfused organs, such as the brain, lung and intestine (10, 18, 19). Once in the tissue, neutrophils release enzymes from their granules that may cause serious damage to the inflamed tissue. The peak of PMN infiltration occurs 24 h after the ischemia (20); this was the time point chosen to perform the analyses for this work.
Strategies against the recruitment of PMNs have been associated with protective effects in ischemia/reperfusion models (21,19). Considering the role of CXCR1 and CXCR2 in PMN recruitment, their blockade could theoretically protect the brains of mice subjected to MCAo (10). Reparixin is a compound that inhibits the effects of CXCL1 and CXCL2 by allosteric modulation of the CXCR1 and CXCR2 receptors. In rats, reparixin reduced brain PMN infiltration and the associated tissue damage in a cerebral ischemia/reperfusion model (10). The administration of SB225002, a CXCR2 antagonist, was also associated with reduced neutrophil infiltration in the brains of rats 24 h after cerebral ischemia–reperfusion (22). Our results are in agreement with these preliminary findings in rats (10,13). In parallel with the decrease of PMN infiltration, as assessed by the MPO assay, reparixin treatment decreased tissue damage and motor compromise. Therefore, this study reinforces the role of PMNs in stroke physiopathology and suggests that reparixin is a promising agent for future experimental and clinical studies. A better understanding of the mechanisms involved in stroke pathogenesis can foster the search for candidate drugs to prevent or attenuate the deficits caused by cerebral ischemia. This is of paramount importance because quality of life for stroke sufferers is largely dependent on the severity of their motor and/or cognitive deficits (23).
Members of the IL-1 family of cytokines, such as IL-1β, are considered major effectors of injury in stroke. Inhibiting the signaling of IL-1α and IL-1β with an IL-1 receptor antagonist was protective in experimental models of cerebral ischemia (24). We found that reparixin-pretreated mice had lower brain levels of IL-1β in conjunction with less structural and functional compromise.
CXCR1/2 receptor blockade by reparixin pretreatment resulted in a decrease of cerebral damage, as indicated by a reduction of PMN recruitment, IL-1β levels and motor impairment following MCAo. Further studies are needed to determine if reparixin treatment after ischemia could reverse the brain injury and the associated long-lasting neurological consequences of stroke in mice. The lack of discrimination of cortical versus subcortical damage and of identification of the penumbra area in histopathological analysis must be regarded as limitations of this study and deserve further investigation.
In conclusion, these results support the concept that the blockade of inflammatory processes mediated by CXCL8 could be a future intervention for the improvement of the structural and behavioral consequences of human cerebrovascular diseases.
ACKNOWLEDGMENTS
This study was funded by CNPq, Fapemig and Rede IBN-Net.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87(5):779–89. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 3.Durukan A, Tutlisumak T. Ischemic stroke in mice and rats. Methods Mol Biol. 2009;573:95–114. doi: 10.1007/978-1-60761-247-6_6. [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 5.Emerich DF, Dean RL, 3rd, Bartus RT. The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct? Exp Neurol. 2002;173(1):168–81. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- 6.Witki-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80(5):617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 7.Campbell IL. Chemokines as plurifunctional mediators in the CNS: implications for the pathogenesis of stroke. Ernst Schering Res Found Workshop. 2004;(45):31–51. doi: 10.1007/978-3-662-05403-1_3. [DOI] [PubMed] [Google Scholar]
- 8.Kostulas N, Kivisäkk P, Huang Y, Matusevicius D, Kostulas V, Link H. Ischemic stroke is associated with a systemic increase of blood mononuclear cells expressing interleukin-8 mRNA. Stroke. 1998;29(2):462–6. doi: 10.1161/01.str.29.2.462. [DOI] [PubMed] [Google Scholar]
- 9.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19(8):819–34. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Villa P, Triulzi S, Cavalieri B, Di Bitondo R, Bertini R, Barbera S, et al. The interleukin-8 (IL-8/CXCL8) receptor inhibitor repertaxin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Mol Med. 2007;13(3-4):125–33. doi: 10.2119/2007-00008.Villa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto T, Ikeda K, Mukaida N, Harada A, Matsumoto Y, Yamashita J, et al. Prevention of cerebral edema and infarct in cerebral reperfusion injury by an antibody to interleukin-8. Lab Invest. 1997;77(2):119–25. [PubMed] [Google Scholar]
- 12.Yamasaki Y, Matsuo Y, Zagorski J, Matsuura N, Onodera H, Itoyama Y, et al. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997;759(1):103–11. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]
- 13.Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, et al. Neuroprotection with the CXCL8 inhibitor reparixin in transient brain ischemia. Cytokine. 2005;30(3):125–31. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Lalonde R, Dumont M, Staufenbiel M, Strazielle C. Neurobehavioral characterization of APP23 transgenic mice with the SHIRPA primary screen. Behav Brain Res. 2005;157(1):91–8. doi: 10.1016/j.bbr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Coelho FM, Vinho V, Amaral FA, Sachs D, Costa VV, Rodrigues DH, et al. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis and Rheumatism. 2008;58(8):2329–37. doi: 10.1002/art.23622. [DOI] [PubMed] [Google Scholar]
- 16.Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;(10):711–3. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- 17.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80(5):617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 18.Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993;365(6447):654–7. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- 19.Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143(1):132–42. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang N, Chopp M, Chawala S. Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res. 1998;788(1-2):25–34. doi: 10.1016/s0006-8993(97)01503-5. [DOI] [PubMed] [Google Scholar]
- 21.Souza D G, Cassali G D, Pool S, Teixeira MM. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br J Pharmacol. 2001;134(5):985–94. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brait VH, Rivera J, Broughton BR, Lee S, Drummond GR, Sobey CG. Chemokine-related gene expression in the brain following ischemic stroke: no role for CXCR2 in outcome. Brain Res. 2011;1372:169–79. doi: 10.1016/j.brainres.2010.11.087. [DOI] [PubMed] [Google Scholar]
- 23.Kwa VI, Limburg M, de Haan RJ. The role of cognitive impairment in the quality of life after ischaemic stroke. J Neurol. 1996;243(8):599–604. doi: 10.1007/BF00900948. [DOI] [PubMed] [Google Scholar]
- 24.Brough D, Tyrrell PJ, Allan SM. Regulation of interleukin-1 in acute brain injury. Trends Pharmacol Sci. 2011;32(10):617–622. doi: 10.1016/j.tips.2011.06.002. [DOI] [PubMed] [Google Scholar]