Abstract
The Centers for Disease Control and Prevention recommends hepatitis A virus (HAV) vaccination for all children at age 1 year and for high-risk adults. The vaccine is highly effective; however, protection duration is unknown. We report HAV antibody concentrations 17 years after childhood immunization, demonstrating that protective antibody levels remain and have stabilized over the past 7 years.
Keywords: hepatitis A, inactivated hepatitis A vaccine, enterovirus infections, immunogenicity, infectious hepatitis
Historically, incidence rates of hepatitis A virus (HAV) infection were up to 40 times greater in Alaska Native people as compared to similarly aged non–Alaska Natives in Alaska [1, 2]. Large epidemic outbreaks occurred in rural Alaska approximately every 7–10 years. In 1996, universal childhood HAV vaccination was introduced statewide in Alaska, and the incidence of acute hepatitis A declined dramatically [3]. In the early 1990s, we recruited a prospective cohort of Alaska Native children (45% male) for long-term follow-up who had participated in a HAV prelicensure vaccine trial. All were HAV antibody (anti-HAV) seronegative, did not have preexisting medical conditions (eg, none were immunocompromised or long-term recipients of steroids), and were 3–6 years of age at the time they were vaccinated. Previous reports from this group have described the interim anti-HAV levels at 10 and 15 years [4, 5]. Here, we compare anti-HAV geometric mean concentrations (GMCs) by vaccination schedule in aggregate and also specifically at 10, 12, 14, and 17 years to propose revised interpretations.
METHODS
Study Population
This study was approved by the institutional review boards of the Alaska Area Native Health Service, the Indian Health Service, and the Centers for Disease Control and Prevention, as well as by 2 Alaska Native health organizations, the Southcentral Foundation and the Alaska Native Tribal Health Consortium. Consent to participate was received from 1 parent or guardian at the start of the study, and consent was again obtained at the 10-year follow-up time point. When participants reached 18 years of age, consent was obtained from the participants themselves. Initially, 144 Alaska Native children were recruited for long-term follow-up and invited to be tested yearly for the first 5 years then approximately every 2 years thereafter.
HAV Vaccination
Patients were randomized to receive the inactivated HAV vaccine HAVRIX® (360 EU, GlaxoSmithKline Biologicals, Rixenart, Belgium) on 1 of 3 schedules: schedule A (0, 1, and 2 months; 51 participants), schedule B (0, 1, and 6 months; 46 participants), and schedule C (0, 1, and 12 months; 47 participants). At each follow-up time point, participants were queried regarding any illness that included jaundice or icterus and whether they had received a diagnosis of acute hepatitis. In addition, medical records for each participant were reviewed for any illness consistent with acute hepatitis A.
Analysis
Sera were tested for anti-HAV by using a modified enzyme-linked immunosorbent assay (DiaSorin). The method for this assay has not changed over the course of the study, and the results are quantitatively expressed in milli-international units per milliliter, with anti-HAV concentrations of ≥20 mIU/mL considered protective. All anti-HAV levels were log transformed and analyzed using simple and repeated-measures analysis of variance. Data are reported as GMCs, by vaccination schedule, over time.
RESULTS
At the 17-year follow-up time point, there were 58 participants from the original cohort of 144. The 58 participants did not differ from those who were no longer participating in the study with respect to sex, study group, initial peak response, or age at first dose of vaccine. Thirty participants from the original cohort were ineligible at 17 years because they had received a booster dose of the inactivated HAV vaccine after completion of primary vaccination series. Fifty-four of the 58 participants (93%) had anti-HAV levels of ≥20 mIU/mL. Specifically, 20 of 23 schedule A participants (87%) had protective anti-HAV titers at 17 years, which is similar to results at 12 years (13 of 16 [81%]) and 14 years (18 of 21 [86%]), whereas at 10 years all persons (21 of 21 [100%]) had protective levels. Comparatively, 17 of 17 schedule B participants (100%) and 17 of 18 schedule C participants (94%) had protective antibody levels after 17 years. Participants vaccinated with either schedule B or C demonstrated full (100%) protective antibody levels at 10 years (B, 21 of 21 participants; C, 20 of 20 participants), 12 years (B, 17 of 17 participants; C, 16 of 16 participants) and 14 years (B, 22 of 22 participants; C, 13 of 13 participants). A summary of our cumulative data over the course of this study is presented in Table 1. At each of the follow-up time points, we recruited all available participants; thus, the numbers of participants varied at each of these time points.
Table 1.
Age and Concentration of Antibody to Hepatitis A Virus (Anti-HAV) During Follow-up, by Vaccination Schedule, Among Alaska Native People Who Completed the Primary HAV Vaccination Series
| Vaccination Schedule (mo) |
||||||||
|---|---|---|---|---|---|---|---|---|
| A (0, 1, 2) |
B (0, 1, 6) |
C (0, 1, 12) |
||||||
| Follow-up, y, Meana | Participant Age, y, Mean (Range) | Anti-HAV GMC, mIU/mL (95% CI) | Participants, No. | Anti-HAV GMC, mIU/mL (95% CI) | Participants, No. | Anti-HAV GMC, mIU/mL (95% CI) | Participants, No. | Pb |
| 0.1 | 5.2 (3.3–7.5) | 993 (661–1495) | 49 | 5503 (3744–8087) | 42 | 7778 (6009–10 057) | 45 | <.001 |
| 0.5 | 5.4 (3.7–7.8) | 518 (366–733) | 46 | 1597 (1012–2520) | 35 | 2793 (85–91 950) | 2 | <.001 |
| 1 | 6.1 (4.2–8.2) | 462 (310–689) | 44 | 2465 (1003–6057) | 9 | 1806 (1258–2592) | 38 | <.001 |
| 1.9 | 7.0 (5.1–9.0) | 728 (502–1056) | 42 | 1053 (670–1656) | 33 | 1286 (855–1931) | 8 | .117 |
| 2.9 | 8.1 (6.1–10.3) | 598 (395–903) | 38 | 766 (498–1179) | 33 | 897 (627–1282) | 41 | .324 |
| 3.9 | 9.0 (7.1–11.1) | 430 (282–658) | 36 | 651 (428–990) | 35 | 896 (610–1317) | 35 | .04 |
| 4.8 | 9.2 (8.0–11.2) | 390 (222–687) | 28 | 655 (371–1157) | 23 | 259 (57–1187) | 6 | .248 |
| 8.2 | 13.2 (11.3–15.8) | 189 (113–317) | 25 | 322 (188–550) | 22 | 466 (258–844) | 19 | .057 |
| 10.2 | 15.3 (13.0–17.3) | 145 (88–239) | 21 | 240 (124–467) | 21 | 368 (193–699) | 20 | .087 |
| 12.4 | 17.6 (15.1–20.3) | 107 (51–225) | 16 | 233 (141–385) | 17 | 323 (139–750) | 16 | .066 |
| 14.2 | 19.1 (17.1–21.9) | 132 (66–266) | 21 | 227 (145–357) | 22 | 213 (89–505) | 13 | .37 |
| 17.3 | 22.4 (20.3–24.6) | 129 (61–270) | 23 | 235 (125–445) | 17 | 354 (143–880) | 18 | .143 |
At each follow-up time, we recruited all available participants who had not received additional booster doses of inactivated HAV vaccine.
Abbreviations: CI, confidence interval; GMC, geometric mean concentration.
a Defined as the time since receipt of the last HAV vaccine dose.
b For comparison of schedules A, B, and C.
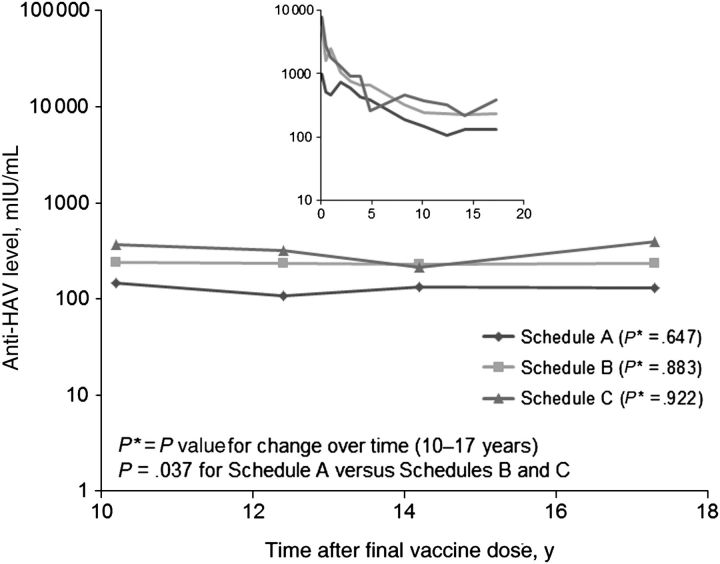
Schedule A participants had an anti-HAV GMC of 129 mIU/mL at 17 years, compared with 235 mIU/mL and 354 mIU/mL for schedule B and C participants, respectively, but this difference did not reach significance (P = .143). However, comparison of schedule A participants with schedule B and C participants in aggregate over the past 7-year follow-up period revealed that the anti-HAV GMC among schedule A participants was significantly lower than that among schedule B and C participants (P = .037; Figure 1). If we adjust for peak anti-HAV levels, the antibody levels did not differ for participants in the 3 schedules over 17 years of follow-up (P = .788). Previous analysis of this group showed a linear decrease in anti-HAV levels over time, through 10 years after completion of the vaccine schedules. However, between 10–17 years, the anti-HAV GMC for schedule A (P = .647), B (P = .883), and C (P = .922) participants demonstrated no significant decreases and instead plateaued (P = .836; Figure 1). No participants reported having an illness with acute jaundice, and review of clinical medical and laboratory records did not identify any participant who had acute hepatitis during the entire follow-up period.
Figure 1.
Geometric mean concentrations of antibody to hepatitis A virus (anti-HAV) in Alaska Native volunteers over time, by vaccine schedule. These graphs depict all available data for each time point. The large graph shows data from years 10–17 after receipt of the final dose, whereas the inset graph shows anti-HAV levels over the entire study period.
DISCUSSION
The findings of our study demonstrate that protective anti-HAV levels persist 17 years after primary vaccination, indicating that booster doses of HAV vaccine are not currently needed. The state of Alaska instituted a large-scale vaccination program in 1996, with high levels of compliance, as evidenced by completion of the HAV vaccination series by 93% of adolescents [6]. Together, the high vaccination rates among children, persistence of anti-HAV levels in vaccinated persons, low statewide incidence of acute hepatitis A (approximately 0.6 cases per 100 000 persons annually since 2004), and no epidemics of acute hepatitis A in Alaska since 1994 support the assumption that HAV is no longer circulating in the general Alaskan population [7–9]. Moreover, these findings support the conclusion that persistence of protective levels of anti-HAV is likely due to the immunogenic properties of the vaccine and that it is unlikely that levels of anti-HAV could have been artificially enhanced by exposure of participants to infected persons (ie, natural boosting).
On the basis of a repeated measures log-linear model of rates of decline, we have previously predicted that anti-HAV GMCs for vaccination schedules would persist above seroprotective levels for 22–32 years, depending on the vaccination schedule, for at least half of the persons immunized [4, 5]. In light of stable anti-HAV GMC levels over the past 7 years of follow-up, we must now consider that the plateauing anti-HAV GMCs suggest that the rates of antibody decline diminish over time and that, potentially, these vaccination schedules are adequate to provide protection to children for durations significantly beyond those predicted earlier, into later adulthood. Moreover, inactivated HAV vaccine is thought to elicit cellular immunity, and immunologic memory may provide protection beyond the period of high levels of antibody circulation [10]. To test this, it is imperative that interval reassessments are conducted to determine how long protective antibody levels persist and when booster vaccination doses, if any, will be needed for later protection.
There are recent reports of long-term follow up studies in adults at 15 years with a 2-dose vaccination schedule [10], in children at 10 years with a 2-dose schedule in different geographical regions [11, 12], and in children in different geographical regions at 5 years with a 3-dose schedule [13, 14]. Whereas these studies support the long-term immunogenicity of HAV vaccine, our cohort is uniquely poised to address the long-term protection of the vaccine, because the cohort consists of individuals who received the vaccine during early childhood and continue to be followed into adulthood. Because children are frequently associated with HAV transmission and we would like to evaluate whether early immunization lasts through adulthood, this is a critical cohort to follow and impacts the immunization strategy of children in the United States and abroad [7].
A limitation of this study is the decreasing number of participants who remain involved as the time from vaccination increases. Nevertheless, this is the longest childhood-administered HAV vaccine immunogenicity study in the world, and the participants will be a valuable cohort to monitor in the future. We plan to continue to follow this cohort of now young adults for at least the next 5 years and, if possible, longer. However, 86 of the original 144 participants (60%) have been lost to follow-up (n = 56) or have inadvertently received subsequent doses of HAV vaccine after the original immunization series (n = 30). As this study continues and the number of participants continues to decrease, it might not be possible to detect statistically significant differences between participants with different immunization schedules. Although future recruitment is a concern as these Alaskans continue into adulthood and possibly become more mobile, our data indicate that while initial anti-GMCs in the schedule A participants never reached the peak anti-HAV GMCs observed in schedule B and C participants, the 3 schedules have similar rates of decline in anti-HAV levels. Thus, in the future, if we do not have sufficient numbers of participants to compare these groups separately, combined analysis of the 3 groups could be reported.
Notes
Acknowledgments. We thank the Alaskan study volunteers and their parents for continued support and participation. Without contributions from the entire staff at the Alaska Native Tribal Health Consortium's Liver Disease and Hepatitis Program, as well as the Centers for Disease Control and Prevention's Arctic Investigations Program, this work would not be possible.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention through a cooperative agreement grant to the Alaska Native Tribal Health Consortium (U50/CCU022279 and U01PS001097).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bulkow LR, Wainwright RB, McMahon BJ, Middaugh JP, Jenkerson SA, Margolis HS. Secular trends in hepatitis A virus infection among Alaska Natives. J Infect Dis. 1993;168:1017–20. doi: 10.1093/infdis/168.4.1017. [DOI] [PubMed] [Google Scholar]
- 2.Peach D, McMahon BJ, Bulkow L, Funk E, Harpaz R, Margolis HS. Impact of recurrent epidemics of hepatitis a virus infection on population immunity levels: Bristol Bay, Alaska. J Infect Dis. 2002;186:1081–5. doi: 10.1086/343815. [DOI] [PubMed] [Google Scholar]
- 3.McMahon BJ, Beller M, Williams J, Schloss M, Tanttila H, Bulkow L. A program to control an outbreak of hepatitis A in Alaska by using an inactivated hepatitis A vaccine. Arch Pediatr Adolesc Med. 1996;150:733–9. doi: 10.1001/archpedi.1996.02170320079014. [DOI] [PubMed] [Google Scholar]
- 4.Hammitt LL, Bulkow L, Hennessy TW, et al. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis. 2008;198:1776–82. doi: 10.1086/593335. [DOI] [PubMed] [Google Scholar]
- 5.Byrd KK, Bruden DL, Bruce MG, et al. Long-term immunogenicity of inactivated hepatitis A vaccine: Follow-up at 15 years. J Pediatr Infect Dis. 2010;5:321–7. [Google Scholar]
- 6.Dorell CG, Yankey D, Byrd KK, Murphy TV. Hepatitis a vaccination coverage among adolescents in the United States. Pediatrics. 2012;129:213–21. doi: 10.1542/peds.2011-2197. [DOI] [PubMed] [Google Scholar]
- 7.Byrd KK, Redd JT, Holman RC, Haberling DL, Cheek JE. Changing trends in viral hepatitis-associated hospitalizations in the American Indian/Alaska Native population, 1995–2007. Public Health Rep. 2011;126:816–25. doi: 10.1177/003335491112600607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin J, Castordale L. Alaska Epidemiol Bull. 2011; 7 2010 annual (January–December) infectious disease report. http://www.epi.alaska.gov/bulletins/docs/b2011_07.pdf. Accessed 3 December 2012.
- 9.Singleton RJ, Hess S, Bulkow LR, Castrodale L, Provo G, McMahon BJ. Impact of a statewide childhood vaccine program in controlling hepatitis A virus infections in Alaska. Vaccine. 2010;28:6298–304. doi: 10.1016/j.vaccine.2010.06.113. [DOI] [PubMed] [Google Scholar]
- 10.Van Herck K, Jacquet JM, Van Damme P. Antibody persistence and immune memory in healthy adults following vaccination with a two-dose inactivated hepatitis A vaccine: long-term follow-up at 15 years. J Med Virol. 2011;83:1885–91. doi: 10.1002/jmv.22200. [DOI] [PubMed] [Google Scholar]
- 11.Van Damme P, Kafeja F, Van Der Wielen M, Leyssen M, Jacquet JM. Long-term immunogenicity and immune memory after two doses of the adult formulation of a combined hepatitis A and B vaccine in children 1 to 11 years of age. Pediatr Infect Dis J. 2011;30:703–5. doi: 10.1097/INF.0b013e3182138296. [DOI] [PubMed] [Google Scholar]
- 12.Bian GL, Ma R, Dong HJ, et al. Long-term clinical observation of the immunogenicity of inactivated hepatitis A vaccine in children. Vaccine. 2010;28:4798–801. doi: 10.1016/j.vaccine.2010.04.096. [DOI] [PubMed] [Google Scholar]
- 13.Chan CY, Lee SD, Yu MI, Wang YJ, Chang FY, Lo KJ. Long-term follow-up of hepatitis A vaccination in children. Vaccine. 1999;17:369–72. doi: 10.1016/s0264-410x(98)00200-x. [DOI] [PubMed] [Google Scholar]
- 14.Fan PC, Chang MH, Lee PI, Safary A, Lee CY. Follow-up immunogenicity of an inactivated hepatitis A virus vaccine in healthy children: results after 5 years. Vaccine. 1998;16:232–5. doi: 10.1016/s0264-410x(97)00179-5. [DOI] [PubMed] [Google Scholar]