Abstract
Background. Most patients with respiratory syncytial virus (RSV) bronchiolitis requiring admission to the pediatric intensive care unit (PICU) have no risk factors for severe disease. We sought to investigate the relationship between serum cytokine concentrations, innate immune responsiveness, and RSV disease severity.
Methods. Previously healthy infants (median age, 2.6 months) with RSV bronchiolitis (PICU, n = 20; floor, n = 46) and healthy matched controls (n = 14) were enrolled, and blood samples were obtained within 24 hours of admission to measure plasma tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 8 (IL-8), and interleukin 10 (IL-10) concentrations and, whole blood lipopolysaccharide-stimulated cytokine production capacity.
Results. Plasma IL-6, IL-8, and IL-10 concentrations were comparable between PICU and floor patients, but higher than in healthy controls (P < .05). In contrast, TNF-α, IL-6, and IL-8 production capacity was significantly decreased in PICU compared with both floor patients and healthy controls. In adjusted analyses, only impaired TNF-α and IL-8 production capacity were associated with longer length of stay (P = .035) and greater disease severity scores (P = .001).
Conclusions. Infants with severe RSV bronchiolitis had increased plasma cytokine concentrations and yet impaired innate immunity cytokine production capacity, which predicted worse disease outcomes. Immune monitoring of otherwise healthy infants with RSV lower respiratory tract infection could help identify patients at risk for severe disease at the time of hospitalization.
Keywords: RSV, bronchiolitis, immunoparalysis, innate immunity, disease severity
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection (LRTI) in young children worldwide and an increasingly recognized cause of death in developing countries [1, 2]. Despite that selected groups of infants have increased risk for severe disease and mortality, the majority of children hospitalized with RSV LRTI are previously healthy with no known risk factors for severe disease [3, 4]. Of those hospitalized infants, 10%–20% will develop a disease severe enough to require admission to the pediatric intensive care unit (PICU) [5, 6].
It has been proposed that the combination of both viral factors and the host immune response probably contribute to the severity of RSV disease [7–11]. The importance of the innate immune response in the pathogenesis of severe RSV disease is being increasingly recognized. To date, however, studies addressing the role of innate immunity cytokines on RSV disease severity have been focused on determining concentrations of blood and respiratory cytokines, with inconsistent results [10–13]. Studies in critically ill patients with bacterial sepsis suggest that innate immune hyporesponsiveness, defined by decreased whole blood tumor necrosis factor α (TNF-α) production after ex vivo lipopolysaccharide (LPS) stimulation, is associated with worse clinical outcomes [14, 15]. Whether the functional capacity of the innate immune response is impaired in children with severe RSV disease, and how it correlates with plasma concentrations of innate immunity cytokines has not been characterized. The overall aims of this study were to determine whether patients with severe RSV bronchiolitis admitted to the PICU had decreased whole blood functional innate immune responses and to evaluate the relationships between innate immune dysfunction and disease outcomes.
MATERIALS AND METHODS
Study Subjects and Study Design
This was a prospective observational study of previously healthy children <24 months old hospitalized at Nationwide Children's Hospital with a first episode of RSV bronchiolitis during the 2010–2011 respiratory season. We excluded children with history of prematurity, wheezing, and/or hospitalizations for bronchiolitis, immunodeficiency, chronic conditions (eg, congenital heart disease; chronic lung disease; chronic renal insufficiency) and children who received immunomodulatory drugs, including systemic steroids, within 15 days before admission.
Monday through Friday, patients were identified using the daily virology laboratory report. Patients fulfilling inclusion criteria were enrolled and sampled within 24 hours of admission in the inpatient floor or the PICU. We obtained demographic and clinical information, performed physical examination, and collected a nasal wash sample for confirmation of RSV infection and RSV quantitation by real-time reverse-transcription polymerase chain reaction (RT-PCR) [10, 16, 17]. A blood sample was obtained simultaneously to determine white blood cell (WBC) and differential counts and to measure cytokine concentrations before and after LPS stimulation. A radiologist blinded to the study read all chest radiographs from children who underwent radiologic evaluation at admission.
Disease severity was assessed by using standard criteria, such as length of hospitalization and the need for and duration of supplemental oxygen, and by applying a disease severity score that classified patients as having mild (0–5), moderate (6–10), or severe (11–15) RSV bronchiolitis [13, 18]. Healthy control infants of comparable age, sex, and race, with no history of respiratory illness in the preceding 2 weeks, were enrolled while in the operating room for elective minor surgical procedures, or at the primary care physician office during well-child visits. Blood and nasal wash samples were also obtained in controls subjects to measure cytokine production and to assure their negative viral status. The study was approved by the Nationwide Children's Hospital institutional review board (IRB 10–00028) and classified as a risk level 1 clinical study (no greater than minimal risk, pursuant under 45 CFR 46.404 and 21 CFR 50.51); written informed consent was obtained from parents or legal guardians, in compliance with Nationwide Children's Hospital guidelines for the responsible conduct of research.
Plasma Cytokine Determination and Cytokine Production Capacity
Blood was collected into heparinized tubes (Vacutainer; BD). Within 1 hour of collection, 50 μL of heparinized whole blood was mixed with 500 μL of standardized stimulation solution containing phenol-extracted LPS from Salmonella abortus equi (500 pg/mL) and incubated at 37°C for 4 hours. After centrifugation for 5 minutes at 3000 rpm, the unstimulated plasma and the LPS-stimulated supernatant were stored at −80°C for subsequent measurement of TNF-α, interleukin 6 (IL-6), interleukin (IL-8), and interleukin 10 (IL-10) concentrations by chemiluminescence with the Immulite automated chemiluminometer (Siemens Medical Solutions Diagnostics) [19]. The lower limit of detection was 5 pg/mL for TNF-α, IL-10, and IL-8 and 2 pg/mL for IL-6. All samples were assayed in duplicate.
Virus Quantitation
Nasal wash samples were obtained from patients and controls according to a standardized protocol [10, 20], and RSV loads measured with RT-PCR. Known concentrations of RSV A2 and B were used to derive a standard curve. Standards and negative controls were tested together with each PCR assay. One-step real-time RT-PCR (Qiagen Quantitect) targeting the conserved region of the RSV N gene was performed using an ABI-7000 sequence detector (Applied Biosystems) with 5 µL of complementary DNA in a total volume of 50 µL of master mix, according to the manufacturer's instructions [17, 21]. Briefly, RSV A forward (5′-AGA TCA ACT TCT GTC ATC CAG CAA) and reverse (5′-TTC TGC ACA TCA TAA TTA GGA GTA TCA AT) primers amplified an 85–base pair region containing the 25-mer FAM-labeled probe (5′-CAC CAT CCA ACG GAG CAC AGG AGA T). RSV B forward (5′-AAG ATG CAA ATC ATA AAT TCA CAG GA), and reverse (5′-TGA TAT CCA GCA TCT TTA AGT ATC TTT ATA GTG) primers were also amplified using the 25-mer FAM-labeled probe (5′-AGG TAT GTT ATA TGC TAT GTC CAG GTT AGG AAG GGA A).
Statistical Analysis
Descriptive analyses, means (± standard deviations), medians (interquartile ranges [25th–75th percentile]) and frequency distributions were used to summarize the patient demographic and baseline characteristics. Group (PICU patients, floor patients, and controls) were compared using either χ2 or Fisher exact tests for categorical variables or Mann-Whitney tests for continuous variables, and correlations were determined using Spearman's rank correlation coefficients, because most of the data did not follow a normal distribution. Multivariable logistic regression models were built using (a) length of stay (dichotomized as ≤2 or > 2 days), (b) clinical disease severity score (CDSS; dichotomized as ≤10 and >10), and (c) TNF-α and IL-8 production capacity (both dichotomized as <1000 and ≥1000, reflecting their median concentrations) as primary outcomes. TNF-α and IL-8 were highly correlated, and thus separate models were built for each individual cytokine. The following covariates were introduced in the models if they were associated with the outcomes in unadjusted analyses or if they were clinically important: age, sex, duration of symptoms, RSV loads, presence of fever, and cytokine production. For all predictors and associations between variables, differences were considered significant at P < .05. All analyses were performed with SAS 9.2 (SAS Institute) or Stata/SE 10.0 (StataCorp) software.
RESULTS
Study Subjects
From December 2010 to September 2011, 66 patients with RSV bronchiolitis and 14 healthy controls were enrolled. Blood samples were obtained in healthy controls and all patients within 24 ± 12 hours of admission (PICU, n = 20 [30%]; floor, n = 46 [70%]) for cytokine measurements. Nasal wash samples were obtained simultaneously for quantification of RSV loads by RT-PCR. There were no significant differences in age, sex, and race between study patients and healthy controls or between children with RSV bronchiolitis admitted to the PICU or floor (Tables 1 and 2). Overall, children with RSV bronchiolitis had significantly higher percentage of bands and lower lymphocyte and eosinophil percentages than controls (Tables 1 and 2).
Table 1.
Demographic and Clinical Characteristics of Study Patients and Controls
| Variable | RSV-Infected Patients (n = 66) | Controls (n = 14) | P |
|---|---|---|---|
| Age, mo | 2.6 (1.6–4.45) | 6.8 (2.7–9.2) | .062a |
| Sex, No. (%) male | 40 (60) | 9 (45) | .303b |
| Race/ethnicity, No. (%) | |||
| White | 45 (68) | 6 (43) | .547c |
| Black | 12 (18) | 7 (50) | |
| Hispanic | 6 (9) | 1 (7) | |
| Other | 3 (5) | 0 | |
| WBCs/μL | 11 900 (9170–17 200) | 8.6 (7.3–10) | .067d |
| Segmented neutrophils, % | 26 (16–32) | 17.5 (11.75–22.75) | .065d |
| Bands, % | 4 (1–10.5) | 0 (0–0) | <.001d |
| Lymphocytes, % | 53 (44.25–62) | 73 (61.25–77.5) | .002d |
| Eosinophils, % | 0 (0–1) | 2 (1–3.25) | <.0001d |
| Monocytes, % | 10.5 (8–15) | 8.5 (4.75–12) | .088d |
Unless otherwise specified, data represent median values (interquartile ranges [25th–75th percentile]).
Abbreviations: Mo, months; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus; WBCs, white blood cells.
a Kruskal-Wallis comparison between RSV-infected patients (PICU, floor) and controls.
b Fisher's exact test.
c χ2 test.
d Mann–Whitney rank sum test.
Table 2.
Demographic, Clinical, and Virologic Variables in Children Admitted to the Inpatient Floor or Pediatric Intensive Care Unit
| Variable | Floor Patients (n = 46) | PICU Patients (n = 20) | P |
|---|---|---|---|
| Demographics | |||
| Age, mo | 2.5 (1.3–4.4) | 2.6 (1.7–5.9) | .471a |
| Sex, No. (%) male | 27 (58.7) | 13 (65.0) | .630b |
| Race/ethnicity, No. (%) | |||
| White | 32 (69.6) | 13 (65.0) | .492c |
| Black | 8 (17.4) | 4 (20.0) | |
| Hispanic | 5 (10.9) | 1 (5.0) | |
| Other | 1 (2.2) | 2 (10.0) | |
| Laboratory and radiographic characteristics | |||
| WBCs/μL | 11 800 (9200–14 800) | 10 950 (5250–13 350) | .322a |
| Segmented neutrophils, % | 27 (17.0–31.0) | 22.5 (10.0–40.0) | .988a |
| Bands, % | 3 (0–6.0) | 6.5 (2.50–19.50) | .042a |
| Lymphocytes, % | 54.5 (47.0–64.5) | 50.5 (38.5–60.5) | .131a |
| Monocytes, % | 11.0 (8–15.5) | 10.5 (6.3–14.5) | .662a |
| Eosinophils, % | 0 (0––0.75) | 0 (0–1) | .776a |
| Chest radiographic findings, No. (%) | |||
| Normal | 1 (3) | 1 (5) | .397b |
| BWT/atelectasis | 31 (91) | 15 (75) | |
| Lobar consolidation | 2 (6) | 4 (20) | |
| Clinical characteristics | |||
| Total duration of symptoms, d | 5 (4–6) | 5 (4–7) | .529a |
| Presence of fever, No. (%) | 29 (63.0) | 19 (95.0) | .007c |
| Use of antibiotics, No. (%) | 16 (35) | 14 (70) | .015c |
| Antibiotics >48 h, No. (%) | 2 (12) | 9 (64) | .006c |
| Disease severity | |||
| Length of stay, d | 2 (2.0–3.0) | 6 (5.0–7.0) | <.001a |
| Need for O2, No. (%) | 34 (73.9) | 20 (100.0) | .013c |
| Duration of O2, d | 0.8 (0–1.5) | 4 (2.8–5.0) | <.001a |
| CDSS, No. (%) | |||
| Mild (0–5) | 32 (69.6) | 0 | <.001c |
| Moderate (6–9) | 14 (30.4) | 2 (10.0) | |
| Severe (11–15) | 0 | 18 (90.0) | |
| RSV load, log10 copies/mL | 4.05 × 105 (6.8 × 104 to 3.2 × 106) | 1.18 × 105 (1.36 × 104 to 1.98 × 106) | .311a |
Unless otherwise specified, data represent median values (interquartile ranges [25th–75th percentile]).
Abbreviations: BWT, bronchial wall thickening; CDSS, clinical disease severity score; d, days; Mo, months; O2, oxygen; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus; WBCs, white blood cells.
a Mann–Whitney U or Wilcoxon rank sum test.
b χ2 test.
c Fisher's exact test.
Clinical Characteristics of Children Hospitalized With RSV Bronchiolitis
We compared laboratory, radiologic, microbiologic, and disease severity parameters between children hospitalized in the PICU and on the floor (Table 2). Total WBC counts and neutrophil, lymphocyte, monocyte, and eosinophil percentages did not differ significantly between PICU and floor patients. However, the percentage of bands was significantly higher in PICU compared with floor patients (P = .042). There were no differences in absolute neutrophil, lymphocyte or monocyte counts between the 2 groups (data not shown).
All patients in the PICU and 74% (n = 34) of patients on the floor underwent chest radiography. The most common radiologic finding was bronchial wall thickening and/or atelectasis. There were no significant differences in chest radiographic findings between PICU and floor patients. Except for 2 positive tracheal aspirate cultures from 2 patients who required mechanical ventilation, one with Streptococcus pneumoniae and the other with Moraxella catarrhalis and Pseudomonas aeruginosa, all blood (n = 29 [44%]), urine (n = 15 [23%]), and cerebrospinal fluid (n = 7 [11%]) cultures were negative.
The median duration of symptoms at the time of sampling was similar for both groups. However, patients admitted to the PICU were more likely to have fever during the hospitalization and were treated with antibiotics more frequently and for longer periods than patients admitted to the floor. In addition, PICU patients had more prolonged hospital stays, required supplemental oxygen more frequently and for longer periods, and had lower oxygen saturation levels at admission than floor patients (median, 85% [interquartile range, 79%–90%] vs 89% [86%–92%]; P = .005).
Among PICU patients, 15 (75%) required noninvasive ventilation for a median of 2.3 days (range, 1.2–3.25 days), and 4 (20%) required invasive mechanical ventilation for a median of 4.5 days (range, 3.6–5.9 days). The noninvasive ventilation methods used included continuous positive airway pressure in 4 patients (20%) and bilevel positive airway pressure (BiPAP/SiPAP) in 11 (55%). Disease severity was assessed using a CDSS at the time of sampling [13]. Most PICU patients had disease classified as severe, whereas most floor patients had nonsevere disease. RSV loads measured by RT-PCR in nasal wash samples showed no significant differences between floor and PICU patients. There was no difference in RSV types either; RSV A was the most prevalent type in both units (floor, 72% [33 of 46 patients]; PICU, 60% [12 of 20 patients]; Table 2).
Increased Plasma Cytokine Concentrations in Patients With RSV Bronchiolitis
Unstimulated plasma IL-6, IL-8, and IL-10 concentrations were modestly but significantly increased in RSV-infected patients compared with controls, with no differences between PICU and floor patients (Table 3). Unstimulated plasma TNF-α was detected at low concentrations or was below the limit of detection of the assay in RSV-infected patients and controls and did not correlate with clinical parameters.
Table 3.
Unstimulated Plasma Cytokine Concentrations
| Plasma Cytokine Concentration, Median (IQR), pg/mL |
|||||
|---|---|---|---|---|---|
| Cytokine | PICU Patients (n = 20) | Floor Patients (n = 46) | Controls (n = 14) | Pa | Pb |
| TNF-α | 27.65 (21–36.23) | 32 (27.38–36.78) | 31.55 (26.8–39.35) | .401 | NS |
| Interleukin 6 | 21.65 (12–31.78) | 17.75 (12.68–31.40) | 12 (6–12) | <.001 | NS |
| Interleukin 8 | 40.55 (30–62.9) | 40 (30–56.23) | 30 (18.23–30) | <.001 | NS |
| Interleukin 10 | 40.1 (30–56.75) | 30.95 (30–40) | 30 (15–30) | .024 | NS |
Abbreviations: IQR, interquartile range; NS, not statistically significant; PICU, pediatric intensive care unit; TNF-α, tumor necrosis factor α.
a Kruskal–Wallis analysis of variance, with Dunn's multiple posttest comparisons among groups.
b Posttest significance for comparison between PICU and floor patients.
Univariate analyses showed that higher IL-6 concentrations were correlated with longer duration of hospitalization (r = 0.6; P = .005) and PICU length of stay (r = 0.55; P = .001), only in PICU patients. Similarly, increased plasma IL-8 concentrations were correlated with longer duration of hospitalizations (r = 0.57; P = .009) and higher CDSS (r = 0.45; P = .036) only in PICU patients. No associations were found in multivariable analyses (data not shown).
TNF-a and IL-8 Production Capacity Correlates With Disease Severity
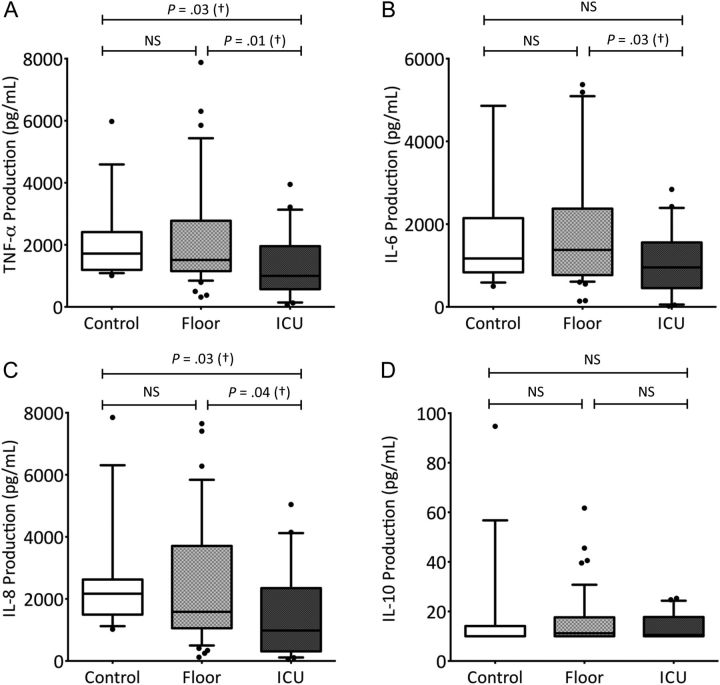
Ex vivo LPS-induced IL-10 production was low and not different between PICU patients, floor patients, and controls [22]. However, production of TNF-α, IL-6, and IL-8 was significantly decreased only in PICU patients compared with floor patients and healthy controls. No significant differences were found in cytokine production capacity between floor patients and healthy controls (Figure 1A–D)
Figure 1.
Lipopolysaccharide-stimulated cytokine concentrations in whole blood. Horizontal lines represent median values; top and bottom whiskers, 10th and 90th percentile values, respectively, for each group. P values were determined with the Mann–Whitney rank sum test. Abbreviations: IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; NS, not statistically significant; PICU, pediatric intensive care unit; TNF-α, tumor necrosis factor α.
In univariate analyses, IL-8 production capacity inversely correlated with duration of supplemental oxygen in PICU patients (r = −0.46; P = .038) but not in those admitted to the floor. Independent of the admission unit, TNF-α production was inversely, and weakly, correlated with duration of hospitalization (r = −0.3; P = .046). Moreover, TNF-α production was significantly lower in patients with prolonged hospital stay and was associated with the duration of hospitalization in all patients, including patients admitted to the PICU (Figure 2). There was no association between IL-6 production and clinical outcomes.
Figure 2.
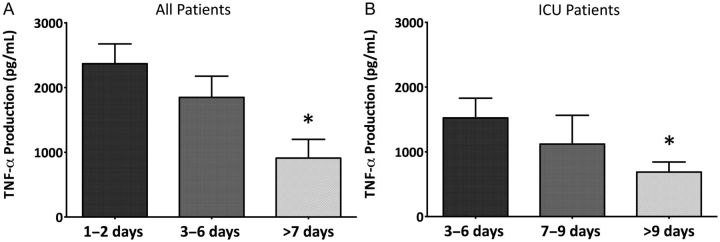
Ex vivo tumor necrosis factor α production grouped by total hospital length of stay in all patients (A) and those hospitalized in the pediatric intensive care unit (B). *Statistically significant by Mann–Whitney rank sum test compared with production in patients with a length of stay 1–2 (A) or 3–6 (B) days. P < .05. Abbreviations: PICU, pediatric intensive care unit; TNF-α, tumor necrosis factor α.
Decreased Production Capacity of TNF-α and IL-8 as Independent Predictors of Disease Severity
Univariate and multivariable logistic regression analyses identified lower TNF-α and IL-8 production capacity to be independent predictors of disease severity as defined by longer length of stay (>2 days) and higher CDSS (>10), after adjustment for several covariables (Tables 4 and 5). Children with impaired TNF-α production capacity (<1000 pg/mL) had almost 6-fold higher odds of being in the hospital >2 days (odds ratio [OR], 5.82 [95% confidence interval, 1.42–23.85]; P = .014), and worse disease severity as evidenced by a CDSS >10 (OR, 20.27 [3.63–113.28]; P = .001) after adjustment for age, sex, days of symptoms at the time of sample collection, presence of fever, and RSV loads (Table 4). Similarly, impaired IL-8 production capacity predicted longer length of stay (OR, 3.72 [1.10–12.62]; P = .035) and a CDSS >10 (OR, 18.44 [3.51–96.91]; P = .001), after adjustment for the same covariates (Table 5).
Table 4.
Univariate and Multivariate Analysis With Tumor Necrosis Factor α Responses Included in Models
| Length of Stay |
CDSS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis (Unadjusted) |
Multivariate Analysis (Adjusted) |
Univariate Analysis (Unadjusted) |
Multivariate Analysis (Adjusted) |
|||||
| Predictors | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Age (in mo) | 1.02 (.92–1.15) | .650 | 1.01 (.89–1.16) | .815 | 1.08 (.96–1.22) | .175 | 1.12 (.96–1.31) | .154 |
| Female sex | .86 (.32–2.31) | .760 | .51 (.14–1.7) | .266 | .97 (.32–2.95) | .959 | .22 (.04–1.25) | .088 |
| Presence of fever | 1.71 (.57–5.15) | .342 | 1.62 (.46–5.72) | .455 | NAa | NA | NA | NA |
| Duration of symptoms (<5 d) | 1.11 (.42––2.95) | .833 | .70 (.2–2.4) | .577 | 1.12 (.38–3.34) | .839 | .46 (.09–2.47) | .366 |
| TNF-α (<1000 pg/mL) | 4.50 (1.27–15.95) | .020 | 5.82 (1.42–23.85) | .014 | 8.75 (2.47–30.95) | .001 | 20.27 (3.63–113.28) | .001 |
| RSV loads (log10) | .82 (.52–1.31) | .424 | .87 (.5–1.49) | .603 | .89 (.53–1.49) | .650 | .91 (.46–1.8) | .789 |
Length of stay >2 days and a CDSS >10 were selected as outcomes. Age and viral loads were analyzed as continuous variables, and presence of fever as a categorical variable. Bold type indicates the parameters that were significantly different in unadjusted and adjusted analysis.
Abbreviations: CDSS, clinical disease severity score; CI, confidence interval; NA, not applicable; OR, odds ratio; TNF-α, tumor necrosis factor α.
a Fever was not included in the model for CDSS, because all subjects without fever had a CDSS <10.
Table 5.
Univariate and Multivariate Analysis With Interleukin 8 Responses Included in Models
| Length of Stay |
CDSS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis (Unadjusted) |
Multivariate Analysis (Adjusted) |
Univariate Analysis (Unadjusted) |
Multivariate Analysis (Adjusted) |
|||||
| Predictor | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Age (in mo) | 1.02 (.92–1.15) | .650 | 1.00 (.87–1.14) | .991 | 1.08 (.96–1.22) | .175 | 1.06 (.81–1.23) | .455 |
| Female sex | .86 (.32–2.31) | .760 | .59 (.18–1.95) | .391 | .97 (.32–2.95) | .959 | .26 (.05–1.37) | .112 |
| Presence of fever | 1.71 (.57–5.15) | .342 | 1.36 (.40–4.66) | .262 | NAa | NA | NA | NA |
| Duration of symptoms (<5 d) | 1.11 (.42–2.95) | .833 | .95 (.29–3.12) | .931 | 1.12 (.38–3.34) | .839 | .92 (.19–4.43) | .918 |
| IL-8 (<1000 pg/mL) | 3.00 (1.01–8.89) | .047 | 3.72 (1.10–12.62) | .035 | 8.67 (2.56–29.32) | .001 | 18.44 (3.51–96.91) | .001 |
| RSV loads (log10) | .82 (.52–1.31) | .424 | .78 (.45–1.33) | .355 | .89 (.53–1.49) | .650 | .67 (.33–1.37) | .277 |
Length of stay >2 days and a CDSS >10 were selected as outcomes. Age and viral loads were analyzed as continuous variables, and presence of fever as a categorical variable. Bold type indicates the parameters that were significantly different in unadjusted and adjusted analysis.
Abbreviations: CDSS, clinical disease severity score; CI, confidence interval; IL-8, interleukin 8; NA, not applicable; OR, odds ratio; RSV, respiratory syncytial virus.
a Fever was not included in the model for CDSS, because all subjects without fever had a CDSS <10.
Finally, to identify which factors were independently associated with TNF-α and IL-8 production capacity we built logistic regression models with LPS-induced production of TNF-α and IL-8 as outcomes. Female sex and PICU admission were identified as independent predictors for decreased production capacity of TNF-α and IL-8 in multivariable analysis after adjustment for all other covariates including age, days of symptoms, presence of fever, and RSV viral loads (Table 6).
Table 6.
Univariate and Multivariate Analysis for Decreased Tumor Necrosis Factor α and Interleukin 8 Production Capacity
| TNF-α Production <1000 pg/mL |
IL-8 Production <1000 pg/mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis (Unadjusted) |
Multivariate Analysis (Adjusted) |
Univariate Analysis (Unadjusted) |
Multivariate Analysis (Adjusted) |
|||||
| Predictors | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Age (in mo) | 1.03 (.91–1.17) | .588 | 1.05 (.89–1.25) | .573 | 1.14 (1.01–1.30) | .036 | 1.09 (.93–1.28) | .269 |
| Female sex | 2.50 (.79–7.87) | .118 | 11.23 (1.89–66.68) | .008 | 2.94 (1.01–8.61) | .047 | 5.91 (1.30–26.88) | .021 |
| PICU admission | 6.67 (1.96–22.3) | .002 | 24.67 (3.56–170.89) | .001 | 6.17 (1.95–19.55) | .002 | 14.88 (2.81–78.77) | .001 |
| Presence of fever | 1.17 (.32–4.23) | .815 | .22 (.03–1.52) | .124 | 3.00 (.76–11.81) | .116 | .68 (.12–3.88) | .660 |
| Duration of symptoms (<5 d) | 2.10 (.67–6.57) | .203 | 5.70 (.93–34.85) | .060 | .57 (.19–1.68) | .310 | 1.43 (.30–6.86) | .655 |
| RSV loads (log10) | .72 (.42–1.24) | .237 | .99 (.47–2.13) | .989 | 1.26 (.76–2.06) | .368 | 1.48 (.73–3.00) | .280 |
Age and viral loads were analyzed as continuous variables, and sex, PICU admission, and presence of fever as categorical variables. Bold type indicates the parameters that were significantly different in unadjusted and adjusted analysis.
Abbreviations: CI, confidence interval; IL-8, interleukin 8; OR, odds ratio; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus; TNF-α, tumor necrosis factor α.
DISCUSSION
The present study demonstrates that critically ill children with RSV admitted to the PICU had mild increases in the concentrations of circulating proinflammatory cytokines and yet lower ex vivo production capacity of those innate cytokines, compared with both patients with less severe disease admitted to the floor and healthy controls. This reduced cytokine production capacity was documented within 24 hours of admission, suggesting that it is primarily related to the severity of RSV disease rather than subsequent changes associated with PICU management. Although serum cytokine concentrations were not associated with disease outcomes in adjusted analyses, impaired production capacity of both TNF-α and IL-8 were found to be independent predictors of worse disease severity, after adjustment for age, sex, days of symptoms, presence of fever, and RSV loads.
The relative contributions of the direct viral cytopathic effect and the innate and adaptive immune responses to disease severity in RSV infection are still a matter of debate. Early studies showed the presence of a robust inflammatory response in the airways of children with RSV LRTI, with neutrophil infiltration and production of cytokines such as TNF-α, IL-6, IL-8, macrophage inflammatory protein 1α, and interferon (IFN) γ [10, 23–25]. Recently, different groups of investigators, including our own, found decreased concentrations of inflammatory cytokines and other markers of cell injury, such as lactate dehydrogenase, in the respiratory tract in the more severe forms of RSV disease [11, 13, 26], suggesting that a defective rather than an excessive airway innate immune response might be associated with disease severity.
Several studies attempted to link RSV disease severity with unstimulated serum cytokines concentrations, with contradictory results, most likely related to differences in study design. Those studies showed a phenotype defined by increased plasma concentrations of T-helper 1 and 2 cytokines, chemokines and soluble activation markers [27–29]. In the present study, we also found a modest but significant increase in plasma IL-6, IL-8, and IL-10 concentrations in RSV-infected patients compared with controls. However, only after LPS stimulation were we able to demonstrate that children with severe disease admitted to the PICU had decreased production of TNF-α and IL-8 compared with floor patients and with controls. These differences in immune responsiveness were not related to differences in the number of cells in peripheral blood, because PICU and floor patients had comparable numbers of WBCs, monocytes, or neutrophils.
Data are limited regarding blood cytokine responses in children with RSV LRTI, which probably reflects the technical challenges of performing this type of studies with young infants. A Finnish study attempted to identify infants at high risk for severe RSV infection at birth by measuring innate and T-helper 2 cytokine production in 1084 cord blood samples. They found that 1.3% of children (14 of 1084) required hospitalization for RSV infection in the first 6 months of life and had significantly higher IL-8 and IL-6 responses to LPS at birth, compared with 10 infants who developed mild RSV disease and were not hospitalized and healthy controls [30].
Bont et al showed reduced production of blood IFN-γ and IL-4 after phytohemagglutinin stimulation in ventilated infants with RSV LRTI, compared with nonventilated hospitalized infants with RSV LRTI [31]. Ventilated infants were younger than the comparator group (1 vs 4 months), which could have influenced the results. The same investigators also showed an inverse correlation between blood monocyte IL-12 production and the duration of mechanical ventilation in 30 infants with RSV-associated respiratory failure [32]. In this second study a control group was not included. In addition in both studies 22%–43% of patients were premature, with or without chronic lung disease.
Similarly, Bendelja et al showed decreased production of Toll-like receptor 8–mediated TNF-α in peripheral blood monocytes in 10 hospitalized infants with acute RSV bronchiolitis compared with healthy controls, suggesting an inadequate monocyte response in those children [12]. In agreement with those other studies and using a larger cohort of previously healthy infants with RSV LRTI, of similar ages, and a healthy control group, we also found impaired immune responses in blood of children with severe RSV disease, increasing the generalizability of these findings. By measuring serum cytokine concentrations and innate immune function in parallel, we were able to document the coexistence of increased plasma cytokine concentrations with impaired functional innate immune cytokine responses.
Innate immune dysfunction has been associated with poor outcomes in children and adults with bacterial sepsis [33, 34], multiple organ dysfunction syndrome [14, 19], or trauma and after cardiopulmonary bypass [22, 35]. This innate immune dysfunction has been defined by reduced expression of class II major histocompatibility complex (HLA-DR) in monocytes and/or reduced ex vivo LPS-induced TNF-α production capacity in whole blood. Ex vivo LPS-induced TNF-α production has been used as a readout of the overall ability of monocytes to respond to a new challenge, rather than reflecting a specific deficit in TNF-α production itself. Further studies will be required to determine whether children who develop severe RSV disease are born with an already impaired immune response, and RSV just uncovers an abnormal immune system. Nevertheless, the findings of the present study, which included a larger cohort of patients with no predisposing medical conditions, clearly suggest the presence of inadequate, rather than excessive, functional innate immune responses in children with acute RSV disease, directly associated with disease severity.
Immune monitoring of RSV-infected patients at the time of hospitalization could have important clinical implications. Certain therapies, such as corticosteroids, are still misused in an effort to blunt the proinflammatory response to RSV. Our data suggest that children with the most severe forms of RSV disease may in fact be already immunosuppressed, raising the possibility of worsening this immunosuppression with the addition of steroids. Moreover, if the temporal relationship between immune suppression and clinical worsening is confirmed in subsequent studies, prospective immune monitoring may be helpful to identify children with bronchiolitis at high risk for severe disease. Finally, it also suggests the potential for the use of immune-stimulant agents in this population, such as IFN-γ or granulocyte-macrophage colony-stimulating factor. These drugs have been used with some success to reverse innate immune suppression in critically ill adults and children [14, 15, 36].
Our study has a number of limitations. First, we measured innate cytokine production capacity at a single time point only, and thus it is unclear when the innate immune dysfunction associated with RSV infection developed and whether it persisted or worsened with time. However, impaired TNF-α and IL-8 production capacity measured within 24 hours of hospitalization was independently associated with severe disease and predicted longer duration of hospitalization. The study was conducted during a single respiratory season, and thus we were not able to determine whether different viral strains could have played a role in disease outcomes. Despite this limitation, we did not find any associations between viral types or viral loads and any of the parameters measured. In addition, although all blood cultures did not yield positive results, we could not completely exclude the possibility of pulmonary bacterial superinfections, because only 2 children required invasive ventilator support that permitted endotracheal sampling. Nevertheless, comparisons of surrogate markers of bacterial infection between PICU and floor patients, such as WBC counts, percentage of neutrophils, and chest radiographic findings, showed no differences between groups.
In summary, we showed that severe RSV bronchiolitis in critically ill children was associated with a profile of hyporesponsive innate immune function as evidenced by lower ex vivo cytokine production capacity. In addition, we showed that this innate immune suppression predicted worse disease outcomes as defined by higher clinical disease severity scores and longer length of stay. Further studies are needed to elucidate the mechanisms responsible for innate immune suppression observed in critically ill RSV-infected children.
Notes
Acknowledgments. We would like to thank the Clinical Research Department at Nationwide Children's Hospital, in particular Gail Arthur, RN, for her efforts with patient enrollment, Lisa Hanson, BS, for her help with cytokine processing, Sara Mertz, BS, for performing RSV loads determinations, William Shiels, MD, for his assistance with radiology readings, and especially our patients and their families for agreeing to participate in the study.
Financial support. This work was supported in part by intramural grants (AM), grants from the National Institue of Health (AIAI057234 and AI089987) to AM and OR, and by the Clinical and Translational Research Program sponsored by the Research Institute at Nationwide Children's Hospial (grant 270810 to CM).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia CG, Bhore R, Soriano-Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126:e1453–60. doi: 10.1542/peds.2010-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child. 2009;94:99–103. doi: 10.1136/adc.2008.139188. [DOI] [PubMed] [Google Scholar]
- 6.Berger TM, Aebi C, Duppenthaler A, Stocker M. Prospective population-based study of RSV-related intermediate care and intensive care unit admissions in Switzerland over a 4-year period (2001–2005) Infection. 2009;37:109–16. doi: 10.1007/s15010-008-8130-z. [DOI] [PubMed] [Google Scholar]
- 7.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–8. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 8.Houben ML, Coenjaerts FE, Rossen JW, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–71. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larranaga CL, Ampuero SL, Luchsinger VF, et al. Impaired immune response in severe human lower tract respiratory infection by respiratory syncytial virus. Pediatr Infect Dis J. 2009;28:867–73. doi: 10.1097/INF.0b013e3181a3ea71. [DOI] [PubMed] [Google Scholar]
- 10.Sheeran P, Jafri H, Carubelli C, et al. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–22. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Bennett BL, Garofalo RP, Cron SG, et al. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195:1532–40. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 12.Bendelja K, Vojvoda V, Aberle N, et al. Decreased Toll-like receptor 8 expression and lower TNF-alpha synthesis in infants with acute RSV infection. Respir Res. 2010;11:143. doi: 10.1186/1465-9921-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia C, Soriano-Fallas A, Lozano J, et al. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhinovirus bronchiolitis. Pediatr Infect Dis J. 2012;31:86–9. doi: 10.1097/INF.0b013e31822dc8c1. [DOI] [PubMed] [Google Scholar]
- 14.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–32. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Docke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–81. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 16.Gill MA, Long K, Kwon T, et al. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. J Infect Dis. 2008;198:1667–76. doi: 10.1086/593018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estripeaut D, Torres JP, Somers CS, et al. Respiratory syncytial virus persistence in the lungs correlates with airway hyperreactivity in the mouse model. J Infect Dis. 2008;198:1435–43. doi: 10.1086/592714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics. 1983;71:13–8. [PubMed] [Google Scholar]
- 19.Hall MW, Gavrilin MA, Knatz NL, Duncan MD, Fernandez SA, Wewers MD. Monocyte mRNA phenotype and adverse outcomes from pediatric multiple organ dysfunction syndrome. Pediatr Res. 2007;62:597–603. doi: 10.1203/PDR.0b013e3181559774. [DOI] [PubMed] [Google Scholar]
- 20.Jafri HS, Chavez-Bueno S, Mejias A, et al. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis. 2004;189:1856–65. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- 21.Torres JP, Gomez AM, Khokhar S, et al. Respiratory syncytial virus (RSV) RNA loads in peripheral blood correlates with disease severity in mice. Respir Res. 2010;11:125. doi: 10.1186/1465-9921-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen ML, Hoschtitzky JA, Peters MJ, et al. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med. 2006;34:2658–65. doi: 10.1097/01.CCM.0000240243.28129.36. [DOI] [PubMed] [Google Scholar]
- 23.Noah TL, Ivins SS, Murphy P, Kazachkova I, Moats-Staats B, Henderson FW. Chemokines and inflammation in the nasal passages of infants with respiratory syncytial virus bronchiolitis. Clin Immunol. 2002;104:86–95. doi: 10.1006/clim.2002.5248. [DOI] [PubMed] [Google Scholar]
- 24.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis. 2001;184:393–9. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 25.Laham FR, Israele V, Casellas JM, et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis. 2004;189:2047–56. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]
- 26.Laham FR, Trott AA, Bennett BL, et al. LDH concentration in nasal-wash fluid as a biochemical predictor of bronchiolitis severity. Pediatrics. 2010;125:e225–33. doi: 10.1542/peds.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripp RA, Moore D, Barskey At, et al. Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J Infect Dis. 2002;185:1388–94. doi: 10.1086/340505. [DOI] [PubMed] [Google Scholar]
- 28.Alonso Fernandez J, Roine I, Vasquez A, Caneo M. Soluble interleukin-2 receptor (sCD25) and interleukin-10 plasma concentrations are associated with severity of primary respiratory syncytial virus (RSV) infection. Eur Cytokine Netw. 2005;16:81–90. [PubMed] [Google Scholar]
- 29.Pinto RA, Arredondo SM, Bono MR, Gaggero AA, Diaz PV. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117:e878–86. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 30.Juntti H, Osterlund P, Kokkonen J, et al. Cytokine responses in cord blood predict the severity of later respiratory syncytial virus infection. J Allergy Clin Immunol. 2009;; 124 doi: 10.1016/j.jaci.2009.04.014. 52–58.e1–2. [DOI] [PubMed] [Google Scholar]
- 31.Bont L, Heijnen CJ, Kavelaars A, et al. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14:144–9. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 32.Bont L, Kavelaars A, Heijnen CJ, van Vught AJ, Kimpen JL. Monocyte interleukin-12 production is inversely related to duration of respiratory failure in respiratory syncytial virus bronchiolitis. J Infect Dis. 2000;181:1772–5. doi: 10.1086/315433. [DOI] [PubMed] [Google Scholar]
- 33.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquardt DJ, Knatz NL, Wetterau LA, Wewers MD, Hall MW. Failure to recover somatotropic axis function is associated with mortality from pediatric sepsis-induced multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2010;11:18–25. doi: 10.1097/PCC.0b013e3181b06046. [DOI] [PubMed] [Google Scholar]
- 35.Cornell TT, Sun L, Hall MW, et al. Clinical implications and molecular mechanisms of immunoparalysis after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2011;143:1160–6e1. doi: 10.1016/j.jtcvs.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–8. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]