Abstract
Human immunodeficiency virus (HIV) infection is a major cause of acceleration of hepatitis C virus-related liver disease, cirrhosis, and death. However, studies of liver disease pathogenesis in HIV/HCV coinfection have thus far been limited. Emerging data support multiple derangements attending HIV coinfection, including increases in profibrogenic cytokine expression and secretion, generation of enhanced oxidative stress, and increases in hepaotcyte apoptosis. These derangements may be further augmented in the presence of increased microbial translocation in the setting of HIV disease. New insight into the mechanisms of HIV/HCV pathogenesis causing accelerated liver fibrosis could lead to new therapeutic strategies designed to retard ths process.
Keywords: Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV), Hepatic Fibrogenesis
Hepatitis C virus (HCV) infects approximately 180 million people, and human immunodeficiency virus (HIV) infects about 40 million people worldwide. Among these, 5 million persons are co-infected with HIV/HCV; 1 million persons co-infected with HIV/HCV reside in the United States [1–3]. HCV is a leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Persons with HIV/HCV have an increased mortality rate compared to those with either infection alone [4]. Liver biopsy studies have shown that HIV hastens HCV-related liver disease [1]. Data are conflicting as to whether HCV accelerates HIV progression; it seems that HCV does not increase the rate of AIDS-defining events nor AIDS-related deaths, but CD4 counts may be lower in HIV/HCV persons than HIV-monoinfected persons [4–6].
It has been shown that HIV/HCV coinfection leads to accelerated hepatic fibrosis progression, higher rates of liver failure, and death compared to patients with HCV monoinfection [7]; this acceleration can be hindered with successful control of HIV with highly active antiretroviral therapy (HAART); as those with undetectable HIV RNA tend to progress to cirrhosis more slowly than those with detectable viremia [7]. Since the introduction of HAART in 1996, HIV has been converted effectively from a fatal disease into a chronic condition. End-stage liver disease, predominantly attributable to HCV, is a leading cause of mortality among HIV-infected persons, regardless of HAART status [6]. Studies of the natural history of HCV-related liver disease in HIV coinfection have demonstrated that fibrosis progresses more rapidly to cirrhosis, and that even in the setting of decompensated cirrhosis, co-infected persons have higher mortality rates [8, 9]. A major consequence has been increased referral rates to and mortality rates on the liver transplant wait list among persons who are HIV/HCV coinfected, an added drain on healthcare resources.
Another alarming source of mortality among HCV cirrhotic patients is the rising incidence of hepatocellular carcinoma (HCC); persons with HIV/HCV coinfection develop HCC at younger ages and are more symptomatic at presentation than those with HCV monoinfection, suggesting a synergy between the two viruses that increases the likelihood of oncogenesis [10]. Compounding matters for the co-infected patient is the observation that the historical standard treatment for HCV, peginterferon and ribavirin, had decidedly inferior success rates in HIV-coinfected hosts [1]. Clinical trials currently underway demonstrate improved sustained virologic response rates in co-infected patients with the addition either of the recently approved protease inhibitors, telaprevir or boceprevir. In short, HCV-related liver disease poses a major health burden in the HIV-infected person living in the twenty-first century.
Various pathways and interactions have been implicated in the mechanisms of accelerated hepatic fibrosis progression in HIV/HCV co-infected patients, including direct viral effects, immune dysregulation, alteration of the cytokine milieu towards a profibrotic state, HIV-related depletion of gut CD4 cells and microbial translocation, oxidative stress, and hepatocyte apoptosis [1]. In this article, we review what is known about the interactions between HIV, HCV, and the liver. In particular, we will focus on our understanding of the accelerated pathogenesis of HCV liver fibrosis in the setting of HIV coinfection.
HEPATIC FIBROSIS AND INJURY
A comprehensive review of hepatic fibrogenesis is beyond the scope of this article (See Hernandez-Gea 2011 and Friedman 2008 for further review), but certain background information is essential to understanding the interactions between HIV and HCV that lead to fibrosis. Hepatic fibrosis is a dynamic response to the liver injury that results in deposition of extracellular matrix (ECM) into the space of Disse, the area between the hepatocytes and the hepatic sinusoids in which hepatic stellate cells (HSCs) reside [11]. Although recent studies have demonstrated that multitude of hepatic cells are responsibly for hepatic fibrogenesis, the driver of this process remains the HSC. In the quiescent phase, HSCs act as the main reservoir for vitamin A in the liver. HSCs are activated by cytokines produced in response to cell injury; hepatocytes and Kupffer cells (KCs—hepatic macrophages) serve as the main intrahepatic cellular sources. Upon activation, HSCs release a cytokine milieu that promotes inflammation, fibrosis, contraction, and mitosis [11]. Transforming growth factor β1 (TGF-β1) is the most well-characterized of these fibrotic cytokines; its activation of HCV through Smad signaling leads to increased production of collagen I and α-smooth muscle actin, 2 main components of ECM [12]. TGF-β1 expression is governed through a variety of signal proteins/complexes including NF-κB, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38MAPK), which tend to be upregulated in the setting of cellular injury [13].
Initiation of the hepatic fibrosis cascade is fueled by a variety of substrates. Reactive oxygen species (ROS) are key contributors to liver injury. They are oxygen-containing free radicals that cause injury through DNA mutations and oxidation of lipids and proteins and produced in diverse liver diseases including viral hepatitis, alcohol-induced and fatty liver disease. Dysregulation of the electron transport chain in hepatic mitochondria is the most well-known source of ROS in liver injury [14]. In HCV-infected hepatocytes, core protein attaches to the mitochondrial membrane and alters calcium homeostasis, increasing the production of ROS via the electron transport chain (ETC) [14]. HCV NS5A has also been demonstrated to enhance ROS by increasing cellular calcium levels, suggesting an indirect interaction with the mitochondrial ETC [15]. It has recently been shown that ROS can be generated in HCV-infected hepatocytes independent of mitochondria in a NADPH oxidase-dependent (Nox) manner [14]. Nox1 and Nox4 are regulated by TGFβ1, suggesting a possible positive feedback system for injury and fibrosis between ROS, TGF-β1, HIV, and HCV.
EFFECT OF HIV ON HEPATIC CELLS AND THE ROLE OF HCV MODELS ON THE STUDY OF HIV/HCV PATHOGENESIS
HCV infects hepatocytes but does not replicate in T cells or hepatic stellate cells. Once HCV has entered the hepatocyte, the cellular immune system is the dominant means of limiting viral spread. The interactions between HCV and the cellular immune system are described in further details in this issue by Lauer et al. HIV infects human CD4 T lymphocytes, macrophages, and dendritic cells; in contrast to HCV, HIV does not replicate in human hepatocytes. Although the cognate HIV-1 receptor CD4 is not expressed on hepatocytes or HSCs, the chemokine HIV-1 co-receptors, CCR5 and CXCR4, are expressed on hepatocytes, HSC, and other resident liver cells. Both HIV and its envelope protein gp120 have been demonstrated to induce cell signaling within hepatocytes, HSCs and other immune cells through its interactions with the CCR5 and CXCR4 chemokine receptors [2, 16–20].
While numerous studies have examined the effects of HIV and its associated proteins upon hepatic cells, basic models for HIV/HCV pathogenesis were hindered for years by the lack of a robust HCV cell culture model. HCV has been notoriously difficult to grow in vitro. Prior to the discovery of the JFH1 (genotype 2) virus in 2005—still the only reliable self-replicating cell culture system—all in vitro studies were performed in subgenomic and full genomic replicon models that required selection adaptations—usually resistance to a readily available antibiotic. The replicon system still remains the best in vitro model for the study of HCV genotype 1. The findings described below have used both the JFH1 and replicon models to monitor HCV replication, signal transduction, and cytokine production.
TGF-β1 EXPRESSION IN HIV/HCV MODELS, ITS ROLE IN HCV REPLICATION, AND ITS STIMULATION BY ROS THROUGH NF-κB DEPENDENT PATHWAYS
As mentioned above, TGF-β1 is a central mediator of liver fibrogenesis. It has been shown that both HCV monoinfection and HIV/HCV coinfection are associated with a significant increase in TGF-β1 expression in the liver and serum of patients [2, 16]. Overexpression of HCV core and nonstructural (NS) proteins, including NS3/4A and NS5A, and HCV replication in the JFH and replicon cell models have been demonstrated to increase TGF-β1 expression in hepatocytes [16, 21]. Both heat-inactivated HIV (HI-HIV) and HIV envelope protein gp120 increase TGF-β1 levels in hepatocytes [16]. These agents also further augment HCV-induced TGF-β1 expression in replicon cells; similarly, HI-HIV reproduces these effects in the JFH1 model [16]. These in vitro studies suggest that while HCV monoinfection leads to increased TGF-β1 expression, HIV/HCV coinfection will increase TGF-β1 levels even further.
TGF-β1 has also been shown to positively regulate HCV RNA replication in cell culture models [16, 21]; the effects of TGF-β1 upon viral replication have been documented in other viruses as well, such as respiratory syncytial virus and JC virus. As previously described, HI-HIV and HIV envelope protein independently increase TGF-β1 levels in uninfected hepatocytes and further increase HCV-induced TGF-β1 expression. In both models, exposure to HIV gp120 and HI-HIV consistently resulted in 2–3-fold increases in HCV RNA replication [16]. This proviral effect of HIV and gp120 on HCV replication is neutralized by antibodies to CCR5 or CXCR4, indicating that CXCR4 or CCR5 coreceptor engagement by HIV or its envelope protein is necessary for its indirect stimulation of HCV replication [16]. Moreover, this stimulatory effect of HIV upon HCV replication is abrogated by neutralizing antibody to TGF-β1, indicating that HIV increases HCV replication in a TGF-β1-dependent manner [16]. TGF-β1 exerts stimulatory action on HCV replication, implicating a positive feedback loop operative in HIV/HCV coinfection; this portends a highly plausible means by which TGF-β1 promotes both HCV replication and hepatic fibrosis [16].
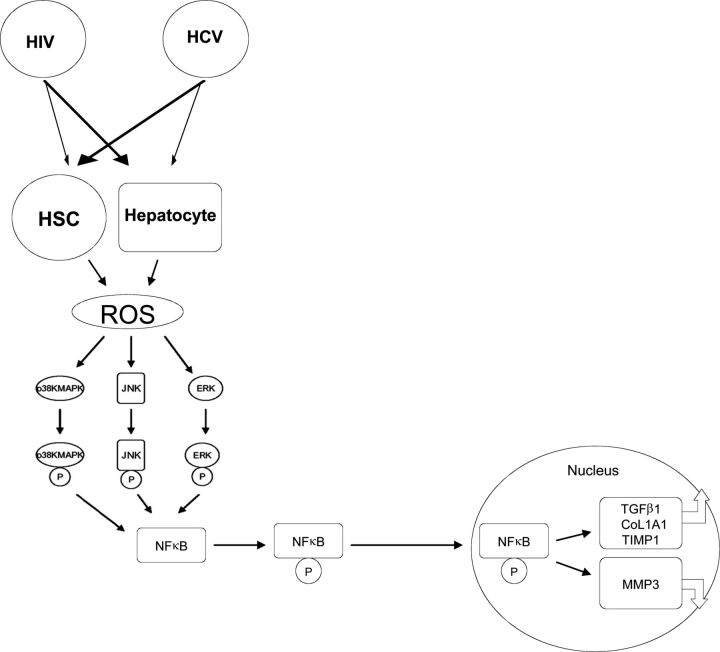
HCV induces mitochondrial oxidative stress and ROS principally through the actions of core and NS5A proteins [15]. Induction of ROS and TGF-β1 expression has been demonstrated in the JFH1 model [13]. HCV infection stimulates the phosphorylation of p38 MAPK, JNK, ERK, and NF-κB—all components of the TGF-β1 signaling cascade. By using the ROS inhibitor DPI and siRNAs designed to knock down these pathways, it was demonstrated that HCV upregulates TGF-β1 through the induction of ROS via 3 separate NF-κB dependent pathways—p38 MAPK, JNK, and ERK1/2 (Figure 1). These findings provide further evidence for the hypothesis that HCV promotes hepatic fibrosis progression through the generation of ROS, which utilizes 3 distinct pathways leading to upregulation of NF-κB, which induces TGF-β1, initiating the fibrogenic cascade [13].
Figure 1.
Proposed pathway of human immunodeficiency virus/hepatitis C virus (HIV/HCV) regulation of hepatic fibrogenesis. HIV and HCV activate reactive oxygen species (ROS) productions in hepatic stellate cells (HSCs) and hepatocytes. ROS stimulates the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK), c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). The phosphorylated p38 MAPK, JNK, and p42/44 ERK activate the phosphorylation of NF-κB. The induced NF-κB is then translocated to the nucleus, increasing the expression of profibrogenic genes (eg, transforming growth factor β1[TGF-β1], procollagen α1, and tissue inhibitor of MMPs [TIMP-1]) and inhibiting the production of antifibrogenic genes (eg, MMP-3).
HIV AND HCV COLLABORATE TO PROMOTE HEPATIC FIBROSIS
Liver fibrosis is characterized by an excessive accumulation of ECM components, a reduction of ECM-removing matrix metalloproteinases (MMPs), and an upregulation of tissue inhibitors of MMPs (TIMPs), mainly TIMP-1. HIV and HCV each can induce liver fibrosis through regulation of the production and deposition of ECM components. HI-HIV and HCV JFH1 were used to examine the impact of HIV and HCV on fibrogenesis-related gene activity in HSC LX2 and Huh7.5.1 hepatoma cells [17]. CXCR4 and CCR5 HIV strains increased Col1A1 messenger RNA (mRNA) in both HSC and hepatocytes; Col1A1encodes procollagen α1 (I), a component of type I collagen that is regulated, in part, by TGF-β, produced in abundance by fibroblasts, and a major component of ECM [17]. Furthermore, HI-HIV also increased TIMP-1 mRNA and protein expression in both HSC and Huh7.5.1 cells; the addition of JFH1 further increased Col1A1 and TIMP-1 expression in each cell type, again suggesting that HIV and HCV act synergistically to foster hepatic fibrogenesis as evidenced by the increase in ECM components [17].
Similar to TGF-β1 and the above-mentioned ECM components, HI-HIV significantly induced ROS production in HSCs and hepatocytes; the addition of JFH1 HCV to HI-HIV further enhanced ROS production in these cells [17]. The potent ROS inhibitor DPI abrogated HIV- and HCV-induced ROS enhancement and TIMP-1 expression in HSCs, hepatocytes, and JFH1-infected hepatocytes. In each of the previous models, TIMP-1 expression was each silenced by NF-κB siRNA [17]. Taken together, these data provide further evidence that HIV and HCV independently regulate hepatic fibrosis progression through the generation of ROS; this regulation occurs in an NF-κB-dependent fashion in both hepatocytes and HSCs. These data suggest that novel strategies to limit each virus’ induction of ROS and NF-κB are warranted to slow fibrosis. We therefore propose a unique model in which HIV and HCV catalyze a fibrogenic state: HIV and HCV, through a variety of proposed mechanisms, act upon hepatocytes and HSCs to augment the production of ROS; this, in turn, activates the phosphorylation of p38 MAPK, JNK, and ERK. The phosphorylated p38 MAPK, JNK, and p42/44 ERK subsequently induce the phosphorylation of NFκB. Activated NF-κB is then translocated to the nucleus in order to regulate other genes including increased production of profibrogenic genes TGF-β1, procollagen a1, and TIMP-1; and decreased expression of anti-profibrogenic genes such as MMP-3 (Figure 1).
HCV AND HIV INCREASE HEPATOCYTE APOPTOSIS THROUGH INCREASED DEATH RECEPTOR EXPRESSION
Hepatocyte apoptosis is ubiquitous in liver diseases—both acute and chronic—with growing evidence that it is a significant component in hepatic fibrogenesis [22]. Apoptosis occurs via two intracellular signaling pathways—through the cytosol or the mitochondria; both pathways are catalyzed by the death inducing signaling complex (DISC), which is formed after the binding of specific extracellular ligands (eg, tumor necrosis factor -related apoptosis-inducing ligand [TRAIL]) to associated cell-membrane death receptors (DR). In situ studies using liver biopsy specimens from persons with chronic HCV demonstrate a positive correlation between grade of inflammation and the expression of pro-apoptotic proteases, caspases 3 and 7, members of the extrinisic (cytosolic) apoptotic pathway [22].
The apoptotic effect of HIV upon HCV-infected hepatocytes was evaluated in vitro in the JFH1 model in the presence of HI-HIV. Apoptosis activity was assessed through various assays evaluating caspase 3/7 activity and expression of TRAIL, DR4, DR5, and cleaved poly ADP-ribose polymerase (PARP). PARP is involved in DNA repair; it is inactivated by caspase 3 and serves as an indirect marker of caspase activity [22]. Caspase 3/7 activity was increased in the HIV/HCV coinfection model (JFH1 cells grown in the presence of HI-HIV) compared to both the HCV model (JFH1 cells) and the HIV model (uninfected hepatocytes grown in the presence of HI-HIV); all 3 models demonstrated increased caspase activity compared to uninfected hepatocytes [22]. Similarly, DR4 and DR5 mRNA and protein expression were increased in the in vitro coinfection model (JFH1 cells grown in the presence of HI-HIV) compared to the 3 other models (HCV, HIV, and null infection) [22]. Conversely, while the TRAIL receptors DR4/DR5 were upregulated in the all of the infectious models, TRAIL expression itself was decreased in the HCV model while increased in the HIV model [22]. It is likely that there is a feedback mechanism in place in HCV infection that leads to a downregulation of TRAIL in the setting of increased DR4/DR5 expression; these brakes are removed in the presence of HIV. Taken together, these findings suggest that hepatocyte apoptosis may be increased in the livers of persons with HCV/HIV coinfection compared to those with each infection alone. The increases in apoptosis outlined above may contribute to the accelerated fibrosis observed in HIV/HCV coinfection.
HEPATIC FIBROSIS IS INFLUENCED BY IMMUNE ACTIVATION AND MICROBIAL TRANSLOCATION
In addition to the intrahepatic mechanisms described above, there is increasing evidence that HIV and HCV may promote hepatic fibrosis through augmentation of microbial translocation. Recent models of HIV-1 immunopathogenesis have focused on the massive depletion of lymphoid tissue associated with the gastrointestinal tract, the largest lymphoid organ, leading to disrupted epithelium and increased microbial translocation. In conjunction with T-cell depletion, HIV infection leads to increased T-cell activation; this immune activation occurs throughout all phases of HIV infection and is regulated by bacteria and their by-products. Immune activation is often indirectly ascertained through plasma levels of lipopolysaccharide (LPS), a well-characterized immunostimulant and component of bacterial cell wall [23]. In HIV-infected persons, as plasma LPS increases, so does activation of CD8 T cells [23]. In corollary, persons initiated on HAART exhibit falling plasma LPS levels with CD4 T-cell restoration and HIV viral load decline [23].
It has been documented that LPS can trigger the cascade for hepatic fibrogenesis. LPS binds to Toll-like receptor 4 (TLR4) on the surface of quiescent HSCs leading to simultaneous TGF-β1 stimulation through an NF-κB-dependent pathway and KC activation, which, in turn, can produce further fibrogenic (eg, TGF-β1) and inflammatory cytokines [24]. There is indirect evidence that HIV alone may be associated with some degree of liver damage, possibly mediated by LPS; a study evaluating APRI (aspartate aminotransferase-to-platelet ratio index), a surrogate marker for hepatic fibrosis, demonstrated similar APRI between HIV and HCV monoinfected persons; those with HIV/HCV coinfection had higher APRI then both [25]. Indeed, HCV monoinfected persons have elevated plasma LPS compared to uninfected controls [26]. In persons with HIV/HCV coinfection, advanced liver disease correlated with higher plasma LPS [27]. To complicate the picture, it appears that KCs are depleted with uncontrolled HIV [28]. Though a key stimulator of HSC and consequently fibrosis, KCs are also responsible for engulfment of LPS. In the setting of KC depletion, LPS can activate HSC uninhibited [28]. Research on the gut-liver conduit is a growing field; therapeutics targeting this relationship could have a great impact on those with HIV/HCV coinfection.
SUMMARY
In summary, HIV alters the natural history of HCV-related liver disease through at least 4 important mechanisms: (1) HIV signals through coreceptors on hepatocytes to upregulate HCV replication in a TGF-β1-dependent manner; (2) HIV augments HCV-related increases in TGF-β1 from hepatocytes; (3) HCV and HIV independently induce TGF-β1 through the generation of ROS, which in turn induces p38 MAPK, JNK, and ERK, and ultimately NF-κB; and (4) HCV and HIV each induce hepatocyte apoptosis. Collectively, they provide a basis by which HIV itself, independent of its effects on cellular immunity, accelerates HCV-related liver disease progression.
Notes
Financial Support. This work was supported by the National Institutes of Health (grants AI069939 and DK098079).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kim AY, Chung RT. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology. 2009;137:795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotman Y, Liang TJ. Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J Virol. 2009;83:7366–74. doi: 10.1128/JVI.00191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman KE, Thomas DL, Chung RT. Human immunodeficiency virus and liver disease forum 2010: conference proceedings. Hepatology. 2011;54:2245–53. doi: 10.1002/hep.24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernando V, Perez-Cachafeiro S, Lewden C, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: Differences by HCV co-infection. J Hepatol. 2012;57:743–51. doi: 10.1016/j.jhep.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Chen TY, Ding EL, Seage Iii GR, Kim AY. Meta-analysis: Increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis. 2009;49:1605–15. doi: 10.1086/644771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. AIDS. [DOI] [PubMed] [Google Scholar]
- 7.Macias J, Berenguer J, Japon MA, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056–63. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 8.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 9.Pineda JA, Romero-Gomez M, Diaz-Garcia F, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779–89. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 10.Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527–37. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 12.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, Tsai WL, Shao RX, et al. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor κB-dependent manner. Gastroenterology. 2010;138:2509–18. doi: 10.1053/j.gastro.2010.03.008. 2518 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J. Oxidative stress, endogenous antioxidants, alcohol, and hepatitis C: pathogenic interactions and therapeutic considerations. Free Radic Biol Med. 2012;52:1135–50. doi: 10.1016/j.freeradbiomed.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Dionisio N, Garcia-Mediavilla MV, Sanchez-Campos S, et al. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signaling alterations in hepatocytes. J Hepatol. 2009;50:872–82. doi: 10.1016/j.jhep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Lin W, Weinberg EM, Tai AW, et al. HIV increases HCV replication in a TGF-β1-dependent manner. Gastroenterology. 2008;134:803–11. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Lin W, Wu G, Li S, et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFκB. J Biol Chem. 2011;286:2665–74. doi: 10.1074/jbc.M110.168286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuyama AC, Hong F, Saiman Y, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–22. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno R, Galastri S, Sacchi P, et al. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513–20. doi: 10.1136/gut.2008.163287. [DOI] [PubMed] [Google Scholar]
- 20.Hong F, Tuyama A, Lee TF, et al. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49:2055–67. doi: 10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Presser LD, Haskett A, Waris G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-β1: role of TGF-β1 in HCV replication. Virology. 2011;412:284–96. doi: 10.1016/j.virol.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang JY, Shao RX, Lin W, et al. HIV infection increases HCV-induced hepatocyte apoptosis. J Hepatol. 2011;54:612–20. doi: 10.1016/j.jhep.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 24.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 25.Price JC, Seaberg EC, Badri S, Witt MD, D'Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis. 2012;205:1005–13. doi: 10.1093/infdis/jir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–30. doi: 10.1053/j.gastro.2011.06.063. 1230 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–33. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balagopal A, Ray SC, De Oca RM, et al. Kupffer cells are depleted with HIV immunodeficiency and partially recovered with antiretroviral immune reconstitution. 2009;23:2397–404. doi: 10.1097/QAD.0b013e3283324344. AIDS. [DOI] [PMC free article] [PubMed] [Google Scholar]