Abstract
Background
Several recent studies have provided evidence that polymorphisms in the telomerase reverse transcriptase (TERT) gene sequence are associated with cancer development, but a comprehensive synopsis is not available. We conducted a systematic review and meta-analysis of the available molecular epidemiology data regarding the association between TERT locus polymorphisms and predisposition to cancer.
Methods
A systematic review of the English literature was conducted by searching PubMed, Embase, Cancerlit, Google Scholar, and ISI Web of Knowledge databases for studies on associations between TERT locus polymorphisms and cancer risk. Random-effects meta-analysis was performed to pool per-allele odds ratios for TERT locus polymorphisms and risk of cancer, and between-study heterogeneity and potential bias sources (eg, publication and chasing bias) were assessed. Because the TERT locus includes the cleft lip and palate transmembrane 1-like (CLPTM1L) gene, which is in linkage disequilibrium with TERT, CLPTM1L polymorphisms were also analyzed. Cumulative evidence for polymorphisms with statistically significant associations was graded as “strong,” “moderate,” and “weak” according to the Venice criteria. The joint population attributable risk was calculated for polymorphisms with strong evidence of association.
Results
Eighty-five studies enrolling 490 901 subjects and reporting on 494 allelic contrasts were retrieved. Data were available on 67 TERT locus polymorphisms and 24 tumor types, for a total of 221 unique combinations of polymorphisms and cancer types. Upon meta-analysis, a statistically significant association with the risk of any cancer type was found for 22 polymorphisms. Strong, moderate, and weak cumulative evidence for association with at least one tumor type was demonstrated for 11, 9, and 14 polymorphisms, respectively. For lung cancer, which was the most studied tumor type, the estimated joint population attributable risk for three polymorphisms (TERT rs2736100, intergenic rs4635969, and CLPTM1L rs402710) was 41%. Strong evidence for lack of association was identified for five polymorphisms in three tumor types.
Conclusions
To our knowledge, this is the largest collection of data for associations between TERT locus polymorphisms and cancer risk. Our findings support the hypothesis that genetic variability in this genomic region can modulate cancer susceptibility in humans.
CONTEXT AND CAVEATS
Prior knowledge
Previous studies have provided abundant evidence that polymorphisms in the telomerase reverse transcriptase (TERT) gene are associated with cancer development, but a comprehensive synopsis is currently not available.
Study design
Publications in English were searched for associations between TERT locus polymorphisms and risk of cancer. A systematic review and meta-analysis of literature was conducted to assess the cumulative evidence for associations. Because TERT locus also includes the cleft lip and palate transmembrane 1-like (CLPTM1L) gene, CLPTM1L polymorphisms were also analyzed.
Contribution
Eighty-five studies contributed data for 67 TERT locus polymorphisms from 24 tumor types for a total of 221 unique combinations of polymorphisms and cancer types. Meta-analysis showed that 22 polymorphisms were associated with risk of at least one cancer type. Cumulative evidence for associations with at least one tumor type was strong for 11 polymorphisms.
Implication
This synopsis confirms that genetic variation in the TERT locus can modulate cancer risk.
Limitations
Some publications may have been overlooked. For some polymorphisms and tumors, data were insufficient for pooling.
From the Editors
Human telomeres consist of repetitive TTAGGG DNA sequences that associate with a series of telomere binding proteins (shelterin complex) believed to provide genomic stability by protecting the linear chromosome ends from being recognized as DNA breaks to be repaired (1,2). The inability of the DNA replication machinery to copy the extreme ends of chromosomes, often referred to as the “end replication problem,” is consistent with the observation that cells can lose telomeric repeats without initially affecting cell function (2). Thus, most human somatic cells show progressive telomere shortening with ongoing cell division until a subset of telomeres reach a critically shortened length and induce a DNA damage signal triggering a tumor protein p53 (TP53)–dependent G1/S cell cycle arrest referred to as replicative senescence. Thus, telomeres not only serve as chromosome “caps” to protect chromosome ends from being recognized as DNA damage but also serve as a gauge for the mitotic (replication) age of a cell (1,2).
The gene encoding the enzyme telomerase reverse transcriptase (TERT), which synthesizes the TTAGGG DNA sequences onto the ends of chromosomes in cooperation with other proteins of the core telomerase complex (eg, telomerase RNA component [TERC] and dyskerin [DKC1]), is located on chromosome 5 (locus 5p15.33). With its activity, telomerase helps maintain the integrity of the genome in embryonic stem cells and in proliferating progenitor cells derived from quiescent normal stem cells (3,4). Telomerase is silent in the vast majority of human tissues and is only expressed in a small number of normal cell types such as dividing male germline spermatocytes and a subset of proliferating somatic adult stem cells (4).
In the early 1990s, investigators proposed a connection between telomeres, telomerase, aging, and cancer (5,6). The hypothesis was that most normal human cells lack telomerase activity and their telomeres shorten with each cell division, until they enter replicative senescence. Cells that lose critical cell cycle checkpoint functions escape this initial growth arrest (replicative senescence) and continue to divide; cells that bypass senescence eventually enter a second growth arrest state (crisis) when many shortened chromosome ends fuse, leading to chromosome bridge–breakage–fusion cycles, which almost universally result in apoptosis (5). In human cells, these two mechanisms to restrict cell growth (replicative senescence and crisis) are potent anticancer protection mechanisms. Most human cells remain in this crisis period with cell growth being balanced by cell death until a rare cell acquires a mechanism, such as telomerase expression, that can maintain or lengthen telomeres. Cells that have escaped crisis generally have two defining hallmarks, telomere stability and reactivation of telomerase; this rare cell type that can maintain telomeres is then able to grow continuously (ie, becomes immortal), and this is generally believed to be a critical step in cancer progression (5).
In light of the already abundant evidence linking telomerase activity to the development of many tumor types, many researchers are devising a variety of methods to target telomerase as a novel therapeutic approach potentially useful in a range of cancers (7,8); moreover, other investigators are testing the hypothesis that variability of the TERT gene sequence might be a general mechanism affecting individual cancer predisposition (9). Regarding the latter field of investigation, tens of thousands of patients affected with different cancer histotypes have been so far enrolled in molecular epidemiology studies, and some TERT polymorphisms have been reported to be associated with cancer risk, although findings are not always concordant (9). Because there is no synopsis available on this subject, we systematically reviewed the data published to date on the relationship between TERT locus polymorphisms and cancer risk and quantitatively summarized the available evidence by performing a formal meta-analysis.
Materials and Methods
Search Strategy, Eligibility Criteria, and Data Extraction
We followed the methods proposed by the Human Genome Epidemiology Network (HuGENet) (10) as well as the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (11) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (12). A two-step search strategy was adopted. First, a systematic review of original articles, reviews, and meta-analyses analyzing the association between TERT locus polymorphisms and cancer risk was performed by searching PubMed, Embase, Cancerlit, Google Scholar, and ISI Web of Knowledge databases (Supplementary Figure 1, available online). The search included the following keywords: “cancer,” “tumor,” “carcinoma,” “melanoma,” “sarcoma,” “lymphoma,” “leukemia,” “polymorphism,” “SNP,” “variant,” “risk,” “association,” “TERT,” “telomerase,” “locus,” “5p15.33,” and “gene.” The 5′-end of TERT resides in a 62-kb region of high linkage disequilibrium (LD) that encompasses the upstream gene cleft lip and palate transmembrane 1-like (CLPTM1L) (9) (Supplementary Figure 2, available online). Therefore, polymorphisms not belonging to the TERT gene but localizing within this LD area might be tagging relevant TERT polymorphisms; accordingly, CLPTM1L was used as an additional search term in the second step. Finally, in the light of the growing diffusion of high-throughput technological platforms for the investigation of gene polymorphisms, the expression “genome-wide association study” and its acronym “GWAS” were also used as key words. We then performed the following to retrieve other potentially relevant data: 1) the name or identity of each polymorphism was used as a keyword to further refine the search; 2) cited references from selected articles were reviewed; 3) publicly available databases dedicated to associations between genotype and phenotype (eg, Database of Genotypes and Phenotypes [dbGaP], http://www.ncbi.nlm.nih.gov/gap) were searched; 4) authors were contacted whenever unreported data were potentially useful for the systematic review or to rule out overlapping data reported in different publications.
Studies dealing with the association between any TERT locus polymorphism and predisposition to any type of cancer in humans were considered eligible, provided that the raw or summary data necessary to calculate the risks were available. Exclusion criteria were non-English language and data published in abstract form. For each polymorphism, exclusion criteria were less than 5% minor allele frequency (MAF) in control subjects (rare polymorphisms) and violation of Hardy–Weinberg equilibrium.
The following data were extracted from eligible studies: authors’ names; region or country where the study was conducted; year of publication; number of case subjects with cancer and healthy control subjects; ethnicity; allelic frequency in both case subjects and control subjects (if no raw data were available, summary data were collected; ie, odds ratios [ORs] and 95% confidence intervals [CIs]); MAF and Hardy–Weinberg equilibrium in control subjects; study design; genotyping; and statistical methods. For analysis purposes, the database, which will be updated on a yearly basis and will be publicly available on the Melanoma Molecular Map Project website [(13); http://www.mmmp.org], was frozen in April 2011. Data were extracted independently by the two investigators (D. Verdi and S. Mocellin) to ensure homogeneity of data collection and to rule out the effect of subjectivity in data gathering and entry. Disagreements were resolved by iteration, discussion, and consensus. To unravel potential systematic biases, a third investigator (D. Nitti) performed a concordance study by independently reviewing all eligible studies; complete concordance (100%) was reached for all variables assessed.
Statistical Analysis
Meta-analysis.
Because all the investigated gene variants were biallelic polymorphisms, per-allele odds ratios and corresponding 95% confidence intervals were used to assess the strength of association between each genetic variant and cancer risk, where protective and risk alleles were associated with ORs less than or greater than 1, respectively. Per-allele ORs were calculated for each study and each polymorphism, assuming a codominant genetic model. This assumption was suggested by the following reasons: 1) for some studies (including many GWAS), neither raw nor summary genotype data were available (only per-allele ORs were reported, which only allows to explore the codominant model); 2) the codominant model is widely used as a conservative choice between the recessive and dominant models; 3) the codominant model does not require adjustment for multiple hypotheses (which is necessary when different models are tested); 4) methods that let the data dictate the genetic model (ie, model-free approach) require raw data on genotype distributions (which were not available for many identified studies).
For each allelic contrast (ie, data regarding a specific polymorphism and a given tumor type), summary per-allele ORs (meta-risks) were calculated by performing random-effects meta-analysis as per Der Simonian and Laird (ie, using the inverse variance method to weight the studies), a Z test being used to formally prove the statistical significance. The choice of the random-effects model was suggested by three main reasons: 1) the variety of histological cancer types, which was far from being fully represented for each genetic variant; 2) because the Q test for between-study heterogeneity is characterized by low statistical power, which is especially relevant when few studies are available; 3) in general, the random-effects model is a more conservative choice when heterogeneity is present, whereas it reduces to the fixed effect model when heterogeneity is absent.
For each polymorphism (whose allele of interest is indicated by the corresponding nucleotide letter in squared brackets), a meta-analysis was performed if at least two data sources were available. Stratification by ethnicity and histological subtype was done if data permitted. When a publication reported the data in an ancestry-specific way, each ancestral subset was considered as a distinct study. Similarly, with regard to GWAS, if data were available, discovery (hypothesis testing) and replication (validation) phases were considered as separate studies.
Meta-analysis also included evaluation of between-study heterogeneity, sensitivity analysis, and examination for bias. Heterogeneity (true variance of effect size across studies) was formally investigated using a Q test (to assess whether observed variance exceeds expected variance) and I 2 statistic (it indicates the percentage of the variability in effect estimates because of true heterogeneity rather than sampling error) (14). The Q test for heterogeneity was also used to formally compare effects (ie, meta-risk) between groups of interest (eg, different ethnicities).
The extent to which the combined risk estimate might be affected by individual studies was assessed by consecutively omitting every study from the meta-analysis (leave-one-out sensitivity analysis). This approach would also capture the effect of the oldest or first positive study (first study effect).
Funnel plot was used to detect the so-called “small study effect.” Publication and selection biases in meta-analysis are more likely to affect small studies, which also tend to be of lower methodological quality (15). Funnel plot asymmetry was formally investigated with the Egger linear regression approach (Egger test) and the Begg rank correlation test (Begg test). The impact of small study effect bias on the summary effects was formally assessed by means of the trim and fill method described by Duval and Tweedie (16). The excess of statistically significant findings (potentially indicating the so-called “chasing bias”) was evaluated by the test proposed by Ioannidis and Trikalinos (Ioannidis and Trikalinos test) (17).
The population attributable risk (PAR) was calculated using the following formula:
where Pr is the proportion of control subjects exposed to the allele of interest and the relative risk (RR) was estimated using the summary estimates (ORs) calculated by the meta-analysis. The joint PAR for combinations of polymorphisms was calculated as follows:
where PARi corresponds to the individual PAR of the ith polymorphism and n is the number of polymorphisms considered (18). Because PAR is a relative measure of effect and thus it does not account for the absolute risk of disease, we also calculated the attributable community risk, according to the following formula:
where I c is the crude risk in the general population (probability of developing the disease during the entire lifetime) and I 0 is the risk of disease in nonexposed (ie, in people carrying the protective allele) (19).
P values less than .05 were considered statistically significant for all tests, except for Q test for heterogeneity, Egger test, Begg test, and Ioannidis and Trikalinos test, for which a less stringent 10% alpha level of statistical significance was applied. The latter three tests were performed if at least four studies were available. All tests were two-sided. The Bonferroni method was used for P value adjustment in case of multiple testing. Statistical analyses were performed with STATA 11.0/SE software (College Station, TX).
Assessment of Cumulative Evidence.
We used the Venice criteria (10,20) to evaluate the epidemiological credibility of each statistically significant association identified by meta-analysis. Briefly, credibility was defined as “strong,” “moderate,” or “weak” based on grades A, B, or C in three categories: 1) amount of evidence, 2) replication of the association, and 3) protection from bias. Amount of evidence (which roughly depends upon the study sample size) was graded by the sum of test allele among case and control subjects in the meta-analysis: grades A, B, or C were assigned for values greater than 1000, 100–1000, and less than 100, respectively. Replication was graded by the heterogeneity statistic: grades A, B, and C were assigned for I 2 less than 25%, 25–50%, and greater than 50%, respectively. Protection from bias was graded as grade A if there was no observable bias (bias was unlikely to explain the presence of the association), grade B if bias could be present, or grade C if bias was evident. Assessment of protection from bias also considered the magnitude of association; grade “C” was assigned to an association with a summary odds ratio less than 1.15, or greater than 0.85 in case of protective effect.
Overall, the cumulative epidemiological evidence for statistically significant associations upon meta-analysis were considered to be “strong” if all three grades were A, “weak” if any grade was C, and “moderate” in all other cases. In case of no statistically significant association upon meta-analysis, the minimum detectable risk was calculated considering a hypothetical study with sample size equal to the combined sample sizes of the studies reporting on a given polymorphism (error alpha and power were set at 5% and 90%, respectively). If no heterogeneity was found (I 2 <25% and Q test P >.10) and the detectable alternative included non-negligible associations (ie, OR ≥1.15 or ≤0.87), we considered the cumulative evidence sufficient to rule out any meaningful relationship between that polymorphism and cancer risk (strong evidence); in the other cases, the evidence for lack of association was considered weak (ie, more data are necessary before the association can be ruled out).
Results
Literature Search
We found 6497 potentially relevant articles, and retrieved 66 articles (18,21–84) reporting on 85 case–control studies that assessed the association between TERT locus polymorphisms and cancer risk, and met the eligibility criteria (Supplementary Figure 1, available online). They were published in a relatively limited time span (2003–2011), witnessing the recent interest in this molecular epidemiology field. We identified 494 allelic contrasts after including ethnicity and tumor subtype–specific data, whose details are reported in Supplementary Table 1, available online.
Overall, data were available on 67 polymorphisms and 24 tumor types, with 490 901 subjects being genotyped (195 305 case subjects and 295 596 control subjects): the 221 unique combinations of TERT locus polymorphisms with cancer types are depicted in a heat map (Supplementary Figure 3, available online), which also includes information on number of studies, type of association (statistically significant vs non–statistically significant), and the level of evidence (strong, moderate, and weak) for association. Considering the total number of case and control subjects enrolled in the eligible studies, TERT locus polymorphisms were most frequently investigated in lung cancer (56 682 subjects; 29.0%), the only tumor type for which data on histological subtypes could be included in the meta-analysis; the other five most frequently studied tumor types were prostate (47 294 subjects; 24.2%), colon (16 279 subjects; 8.3%), breast (15 090 subjects; 7.7%), and bladder (10 202 subjects; 5.2%) carcinomas, and central nervous system (CNS) tumors (8795 subjects; 4.5%).
The median number of subjects enrolled per study was 2771 (range: 125–79 600). White subjects of European ancestry was by far most frequently involved (425 010 subjects; 86.5%), Asian ethnicity representing virtually all the remaining subjects. In the majority of the studies (58 of 85), selected TERT locus polymorphisms were investigated, whereas the remaining 27 were GWAS. All variants were single-nucleotide polymorphism (SNP), except for two minisatellite polymorphisms (TERT MNS16a and VNTR2-2nd) and one deletion (TERT Glu441del).
Among the 485 allelic contrasts for which an odds ratio could be calculated, 233 (48%) showed a statistically significant association with risk of cancer (Supplementary Table 1, available online). Available data enabled us to perform 118 meta-analyses (including 41 subgroup analyses based on specific ancestries and histological subtypes) for 35 polymorphisms tested in one or more different tumor types and for which at least two studies were available. The main findings of these meta-analyses reporting 75 (64%) statistically significant associations and 43 (36%) non–statistically significant associations between TERT locus polymorphisms and cancer are shown in Tables 1 and 2, respectively.
Table 1.
TERT locus polymorphisms for which the meta-analysis demonstrated a statistically significant association with cancer risk*
| Polymorphism data | Study features | Meta-analysis findings | ||||||||||
| Polymorphism ID | Gene | Allele | Race or ethnicity† | No. of studies | Case subjects, No. | Control subjects, No. | Cancer type‡ | OR (95% CI) | P for Z test§ | I 2, % | P for Q test§ | Level of evidence║ |
| rs13167280 | TERT | T | White | 2 | 1581 | 1889 | Bladder | 1.23 (1.07 to 1.42) | .004 | 0 | .45 | BAA/moderate |
| rs1801075 | Intergenic | C | Miscellany | 4 | 3552 | 4340 | Lung (miscellany) | 0.88 (0.80 to 0.96) | .006 | 0 | .49 | AAC/weak |
| rs2242652 | TERT | T | White | 4 | 33600 | 32419 | Prostate | 0.82 (0.76 to 0.89) | 9.0E-07¶ | 61 | .05 | ACA/weak |
| rs2735845 | Intergenic | G | Miscellany | 3 | 7213 | 7523 | Lung (miscellany) | 1.11 (1.05 to 1.17) | 2.3E-04¶ | 0 | .39 | AAC/weak |
| rs2736098 | TERT | A | Miscellany | 3 | 4617 | 10134 | Bladder | 1.19 (1.12 to 1.25) | 8.6E-10¶ | 0 | .87 | AAA/strong |
| rs2736098 | TERT | A | Miscellany | 4 | 6949 | 12492 | Lung (miscellany) | 1.21 (1.15 to 1.27) | 2.2E-13¶ | 0 | .99 | AAA/strong |
| rs2736100 | TERT | C | Miscellany | 2 | 970 | 1072 | Bladder | 1.19 (1.05 to 1.34) | .007 | 0 | .64 | BAA/moderate |
| rs2736100 | TERT | C | Miscellany | 6 | 6871 | 12449 | CNS | 1.34 (1.23 to 1.46) | 4.4E-11¶ | 60 | .029 | ACA/weak |
| rs2736100 | TERT | C | White | 5 | 5918 | 11413 | CNS | 1.35 (1.22 to 1.50) | 1.2E-08¶ | 65 | .021 | ACA/weak |
| rs2736100 | TERT | C | Miscellany | 23 | 50917 | 72598 | Lung (miscellany) | 1.19 (1.14 to 1.23) | <1.0E-16¶ | 77 | 2.9E-11 | ACA/weak |
| rs2736100 | TERT | C | White | 11 | 30650 | 41248 | Lung (miscellany) | 1.11 (1.08 to 1.15) | 1.1E-12¶ | 35 | .12 | AAC/weak |
| rs2736100 | TERT | C | Asian | 12 | 20267 | 31350 | Lung (miscellany) | 1.26 (1.20 to 1.32) | <1.0E-16¶ | 68 | 3.5E-04 | ACB/weak |
| rs2736100 | TERT | C | Miscellany | 14 | 22045 | 68197 | Lung (adenocarcinoma) | 1.30 (1.25 to 1.36) | <1.0E-16¶ | 62 | .001 | ACB/weak |
| rs2736100 | TERT | C | Miscellany | 13 | 11645 | 57483 | Lung (squamous) | 1.06 (1.01 to 1.12) | .017 | 54 | .011 | ACC/weak |
| rs2736100 | TERT | C | Miscellany | 29 | 35509 | 61787 | Lung (NSCLC) | 1.19 (1.14 to 1.25) | 3.3E-14¶ | 80 | 1.1E-16 | ACA/weak |
| rs2736100 | TERT | C | White | 6 | 9540 | 37840 | Lung (adenocarcinoma) | 1.22 (1.17 to 1.27) | <1.0E-16¶ | 0 | .42 | AAA/strong |
| rs2736100 | TERT | C | Asian | 8 | 12505 | 30357 | Lung (adenocarcinoma) | 1.35 (1.29 to 1.42) | <1.0E-16¶ | 47 | .06 | ABA/moderate |
| rs2736100 | TERT | C | White | 6 | 6626 | 37840 | Lung (squamous) | 1.05 (1.00 to 1.10) | .043 | 30 | .21 | ABA/moderate |
| rs2736100 | TERT | C | White | 13 | 16572 | 37840 | Lung (NSCLC) | 1.15 (1.09 to 1.22) | 1.2E-06¶ | 73 | 9.2E-06 | ACA/weak |
| rs2736100 | TERT | C | Asian | 16 | 18937 | 23947 | Lung (NSCLC) | 1.24 (1.16 to 1.31) | 2.4E-12¶ | 74 | 5.2E-07 | ACA/weak |
| rs2736100 | TERT | C | White | 2 | 3851 | 3934 | Pancreas | 1.12 (1.04 to 1.20) | .001 | 0 | .77 | AAC/weak |
| rs2736100 | TERT | C | White | 3 | 1959 | 8679 | Testis | 0.75 (0.70 to 0.81) | 4.4E-16¶ | 0 | .62 | AAA/strong |
| rs2853668 | Intergenic | T | White | 2 | 11067 | 12517 | Colon | 0.90 (0.82 to 0.99) | .034 | 72 | .05 | ACC/weak |
| rs2853668 | Intergenic | T | Miscellany | 2 | 5531 | 6284 | Lung (miscellany) | 1.09 (1.02 to 1.15) | .009 | 0 | .66 | AAC/weak |
| rs2853668 | Intergenic | T | White | 2 | 3851 | 3934 | Pancreas | 0.92 (0.85 to 0.99) | .046 | 0 | .68 | AAC/weak |
| rs2853676 | TERT | A | Miscellany | 4 | 6010 | 8313 | CNS | 1.26 (1.21 to 1.32) | <1.0E-16¶ | 0 | .58 | AAA/strong |
| rs2853676 | TERT | A | Miscellany | 3 | 11205 | 11957 | Lung (miscellany) | 1.06 (1.01 to 1.10) | .008 | 0 | .79 | AAC/weak |
| rs31489 | CLPTM1L | A | Miscellany | 6 | 13929 | 16047 | Lung (miscellany) | 0.83 (0.78 to 0.88) | 4.2E-09¶ | 55 | .05 | ACA/weak |
| rs31489 | CLPTM1L | A | White | 4 | 10594 | 11070 | Lung (miscellany) | 0.84 (0.78 to 0.90) | 1.5E-06¶ | 58 | .07 | ACA/weak |
| rs31489 | CLPTM1L | A | Asian | 2 | 3335 | 4977 | Lung (miscellany) | 0.80 (0.70 to 0.91) | .001 | 44 | .18 | ABA/moderate |
| rs31489 | CLPTM1L | A | White | 2 | 3851 | 3934 | Pancreas | 1.18 (1.06 to 1.32) | .003 | 70 | .06 | ACA/weak |
| rs31489 | CLPTM1L | A | White | 2 | 1191 | 5805 | Testis | 1.28 (1.16 to 1.41) | 1.4E-06¶ | 18 | .27 | AAA/strong |
| rs380286 | CLPTM1L | A | Miscellany | 2 | 5529 | 6289 | Lung (miscellany) | 0.85 (0.80 to 0.91) | 5.8E-07¶ | 0 | .84 | AAA/strong |
| rs401681 | CLPTM1L | T | Miscellany | 4 | 8645 | 41188 | Bladder | 0.89 (0.86 to 0.92) | 9.2E-11¶ | 0 | .81 | AAC/weak |
| rs401681 | CLPTM1L | T | White | 3 | 4152 | 32824 | Pancreas | 1.14 (1.01 to 1.29) | .037 | 77 | .013 | ACC/weak |
| rs401681 | CLPTM1L | T | White | 3 | 11235 | 74276 | Prostate | 0.92 (0.89 to 0.95) | 3.8E-06¶ | 0 | .63 | AAC/weak |
| rs401681 | CLPTM1L | T | White | 3 | 3941 | 32808 | BCC (skin) | 0.82 (0.76 to 0.89) | 4.2E-06¶ | 49 | .14 | ABA/moderate |
| rs401681 | CLPTM1L | T | White | 5 | 8012 | 38925 | Melanoma (skin) | 1.12 (1.03 to 1.22) | .006 | 72 | .007 | ACC/weak |
| rs401681 | CLPTM1L | T | White | 3 | 1478 | 30525 | SCC (skin) | 0.92 (0.84 to 0.99) | .046 | 0 | .57 | AAC/weak |
| rs401681 | CLPTM1L | T | Miscellany | 12 | 25674 | 58027 | Lung (miscellany) | 0.87 (0.84 to 0.90) | <1.0E-16¶ | 33 | .13 | ABA/moderate |
| rs401681 | CLPTM1L | T | White | 8 | 18641 | 48866 | Lung (miscellany) | 0.87 (0.84 to 0.90) | 3.5E-14¶ | 34 | .15 | ABA/moderate |
| rs401681 | CLPTM1L | T | Asian | 4 | 7033 | 9161 | Lung (miscellany) | 0.86 (0.81 to 0.93) | 2.9E-05¶ | 46 | .13 | ABA/moderate |
| rs401681 | CLPTM1L | T | Miscellany | 6 | 5900 | 43343 | Lung (adenocarcinoma) | 0.87 (0.81 to 0.93) | 4.3E-05¶ | 52 | .06 | ACA/weak |
| rs401681 | CLPTM1L | T | Miscellany | 5 | 5319 | 40522 | Lung (squamous) | 0.85 (0.76 to 0.96) | .010 | 72 | .006 | ACA/weak |
| rs401681 | CLPTM1L | T | Miscellany | 12 | 11852 | 53575 | Lung (NSCLC) | 0.86 (0.82 to 0.91) | 1.9E-07¶ | 56 | .01 | ACA/weak |
| rs401681 | CLPTM1L | T | White | 3 | 2231 | 37259 | Lung (adenocarcinoma) | 0.90 (0.82 to 0.98) | .019 | 45 | .16 | ABC/weak |
| rs401681 | CLPTM1L | T | Asian | 3 | 3669 | 6084 | Lung (adenocarcinoma) | 0.84 (0.76 to 0.93) | 7.5E-04 | 57 | .10 | ACA/weak |
| rs401681 | CLPTM1L | T | Asian | 2 | 736 | 4184 | Lung (squamous) | 0.85 (0.75 to 0.96) | .010 | 0 | .95 | BAA/moderate |
| rs401681 | CLPTM1L | T | White | 7 | 7447 | 43307 | Lung (NSCLC) | 0.87 (0.80 to 0.95) | .001 | 67 | .01 | ACA/weak |
| rs401681 | CLPTM1L | T | Asian | 5 | 4405 | 10268 | Lung (NSCLC) | 0.84 (0.79 to 0.90) | 1.5E-07¶ | 14 | .32 | AAA/strong |
| rs402710 | CLPTM1L | T | Miscellany | 2 | 974 | 1055 | Bladder | 0.85 (0.75 to 0.98) | .022 | 0 | .36 | BAA/moderate |
| rs402710 | CLPTM1L | T | Miscellany | 12 | 31364 | 35462 | Lung (miscellany) | 0.88 (0.83 to 0.92) | 8.1E-07¶ | 65 | .002 | ACC/weak |
| rs402710 | CLPTM1L | T | White | 8 | 24471 | 26161 | Lung (miscellany) | 0.89 (0.84 to 0.94) | 5.2E-05¶ | 69 | .004 | ACC/weak |
| rs402710 | CLPTM1L | T | Asian | 4 | 6893 | 9301 | Lung (miscellany) | 0.87 (0.82 to 0.91) | 8.2E-08¶ | 9 | .35 | AAA/strong |
| rs402710 | CLPTM1L | T | Miscellany | 3 | 4929 | 15011 | Lung (adenocarcinoma) | 0.84 (0.80 to 0.89) | 5.9E-10¶ | 0 | .42 | AAA/strong |
| rs402710 | CLPTM1L | T | Miscellany | 3 | 2461 | 15011 | Lung (squamous) | 0.83 (0.77 to 0.89) | 4.1E-07¶ | 0 | .94 | AAA/strong |
| rs402710 | CLPTM1L | T | Miscellany | 8 | 8945 | 31783 | Lung (NSCLC) | 0.84 (0.81 to 0.88) | 2.2E-16¶ | 0 | .78 | AAA/strong |
| rs4246742 | TERT | T | Miscellany | 2 | 5528 | 6274 | Lung (miscellany) | 1.08 (1.01 to 1.15) | .018 | 0 | .83 | AAC/weak |
| rs451360 | CLPTM1L | A | Miscellany | 3 | 6983 | 8058 | Lung (miscellany) | 0.80 (0.70 to 0.92) | .001 | 70 | .035 | ACB/weak |
| rs451360 | CLPTM1L | A | White | 2 | 4652 | 4981 | Lung (miscellany) | 0.85 (0.76 to 0.95) | .004 | 48 | .16 | ABA/moderate |
| rs452384 | CLPTM1L | C | Miscellany | 2 | 5302 | 6823 | Lung (miscellany) | 0.82 (0.74 to 0.90) | 7.4E-05¶ | 52 | .15 | ACA/weak |
| rs452932 | CLPTM1L | C | Miscellany | 2 | 5302 | 6823 | Lung (miscellany) | 0.82 (0.74 to 0.90) | 7.4E-05¶ | 52 | .15 | ACA/weak |
| rs4635969 | Intergenic | A | Miscellany | 4 | 12893 | 13126 | Lung (miscellany) | 0.86 (0.82 to 0.90) | 8.1E-10¶ | 8 | .35 | AAA/strong |
| rs4635969 | Intergenic | A | Miscellany | 2 | 3674 | 8230 | Lung (adenocarcinoma) | 0.81 (0.75 to 0.88) | 1.2E-06¶ | 0 | .87 | AAA/strong |
| rs4635969 | Intergenic | A | Miscellany | 2 | 1594 | 8230 | Lung (squamous) | 0.87 (0.78 to 0.97) | .010 | 0 | .45 | AAA/strong |
| rs4635969 | Intergenic | A | Miscellany | 4 | 5268 | 8230 | Lung (NSCLC) | 0.83 (0.78 to 0.89) | 6.0E-08¶ | 0 | .69 | AAA/strong |
| rs4635969 | Intergenic | A | White | 2 | 3851 | 3934 | Pancreas | 1.22 (1.14 to 1.32) | 1.1E-07¶ | 0 | .86 | AAA/strong |
| rs4635969 | Intergenic | A | White | 3 | 1729 | 9079 | Testis | 1.61 (1.47 to 1.75) | <1.0E-16¶ | 0 | .77 | AAA/strong |
| rs465498 | CLPTM1L | G | Miscellany | 4 | 11579 | 13190 | Lung (miscellany) | 0.79 (0.74 to 0.84) | 2.6E-12¶ | 49 | .12 | ABA/moderate |
| rs465498 | CLPTM1L | G | Asian | 3 | 8608 | 9444 | Lung (miscellany) | 0.76 (0.72 to 0.81) | <1.0E-16¶ | 0 | .76 | AAA/strong |
| rs467095 | CLPTM1L | C | Miscellany | 3 | 7739 | 9271 | Lung (miscellany) | 0.83 (0.78 to 0.87) | 3.9E-11¶ | 0 | .37 | AAA/strong |
| rs4975615 | Intergenic | G | Miscellany | 3 | 6983 | 8058 | Lung (miscellany) | 0.81 (0.76 to 0.87) | 2.7E-10¶ | 26 | .26 | ABA/moderate |
| rs4975616 | Intergenic | G | Miscellany | 7 | 12743 | 14130 | Lung (miscellany) | 0.83 (0.79 to 0.88) | 1.1E-11¶ | 41 | .12 | ABA/moderate |
| rs4975616 | Intergenic | G | White | 2 | 3851 | 3934 | Pancreas | 0.85 (0.78 to 0.94) | .001 | 57 | .13 | ACA/weak |
| rs4975616 | Intergenic | G | White | 2 | 1328 | 5861 | Testis | 1.20 (1.03 to 1.40) | .016 | 55 | .13 | ACA/weak |
| MNS16a | TERT | S | White | 2 | 1764 | 1664 | CNS | 1.19 (1.07 to 1.33) | .001 | 8 | .30 | AAA/strong |
Telomerase reverse transcriptase (TERT) locus includes TERT and cleft lip and palate transmembrane 1-like (CLPTM1L) genes. BCC = basal cell carcinoma; CI = confidence interval; CNS =central nervous system; ID = identification; OR = odds ratio; NSCLC = non–small cell lung cancer; SCC =squamous cell carcinoma; SCLC = small cell lung cancer.
Miscellany indicates a mix of different races.
Lung (miscellany) indicates a mix of different histological subtypes.
All P values calculated by Z test or Q test were two-sided.
A, B, and C represent the Venice criteria grades for amount of evidence, replication of association and protection from bias, which ultimately define the level of cumulative evidence (strong, moderate, weak).
Statistically significant P values after Bonferroni adjustment for multiple testing.
Table 2.
TERT locus polymorphisms for which the meta-analysis demonstrated no nominally statistically significant association with cancer risk*
| Polymorphism data | Study characteristic | Meta-analysis findings | ||||||||||
| Polymorphism ID | Gene | Allele | Race or ethnicity† | No. of studies | Case subjects, No. | Control subjects, No. | Cancer type‡ | OR (95% CI) | P for Z test§ | I 2, % | P for Q test§ | Level of evidence║ |
| rs10073340 | CLPTM1L | T | White | 2 | 3851 | 3934 | Pancreas | 0.95 (0.73 to 1.23) | .68 | 89 | .002 | Weak |
| rs13167280 | TERT | T | White | 2 | 2989 | 3360 | Breast | 1.02 (0.91 to 1.16) | .69 | 0 | .82 | Weak |
| rs2075786 | TERT | T | White | 2 | 2953 | 3333 | Breast | 1.01 (0.94 to 1.09) | .77 | 0 | .45 | Weak |
| rs2075786 | TERT | T | Miscellany | 4 | 3134 | 3130 | Lung (miscellany) | 1.31 (0.98 to 1.75) | .06 | 74 | .01 | Weak |
| rs2075786 | TERT | T | Asian/Black | 3 | 2770 | 2750 | Lung (miscellany) | 1.45 (0.88 to 2.37) | .14 | 82 | .004 | Weak |
| rs2242652 | TERT | T | Miscellany | 2 | 4619 | 5360 | Lung (miscellany) | 1.06 (0.94 to 1.19) | .36 | 48 | .17 | Weak |
| rs2735940 | Intergenic | T | White | 2 | 1453 | 2584 | Bladder | 0.96 (0.87 to 1.05) | .37 | 0 | .77 | Weak |
| rs2735940 | Intergenic | T | White | 2 | 3180 | 3405 | Breast | 0.99 (0.93 to 1.07) | .92 | 0 | .99 | Weak |
| rs2735940 | Intergenic | T | Miscellany | 6 | 3682 | 4099 | Lung (miscellany) | 1.08 (0.95 to 1.23) | .25 | 59 | .032 | Weak |
| rs2735940 | Intergenic | T | White | 3 | 2746 | 3166 | Lung (miscellany) | 1.04 (0.89 to 1.22) | .62 | 65 | .06 | Weak |
| rs2735940 | Intergenic | T | Asian/Black | 3 | 936 | 933 | Lung (miscellany) | 1.20 (0.84 to 1.72) | .32 | 64 | .06 | Weak |
| rs2736098 | TERT | A | Miscellany | 2 | 1102 | 1182 | CNS | 1.07 (0.77 to 1.48) | .69 | 56 | .13 | Weak |
| rs2736098 | TERT | A | White | 2 | 8708 | 48804 | Prostate | 1.10 (1.00 to 1.20) | .05 | 83 | .015 | Weak |
| rs2736100 | TERT | C | Miscellany | 9 | 6002 | 50492 | Lung (SCLC) | 1.05 (0.99 to 1.10) | .06 | 13 | .33 | Strong |
| rs2736100 | TERT | C | Asian | 7 | 5019 | 19643 | Lung (squamous) | 1.08 (0.98 to 1.18) | .11 | 53 | .05 | Weak |
| rs2736100 | TERT | C | White | 2 | 3341 | 4533 | Melanoma (skin) | 1.03 (0.95 to 1.11) | .46 | 0 | .62 | Weak |
| rs2736109 | Intergenic | A | White | 2 | 2967 | 3331 | Breast | 0.96 (0.75 to 1.23) | .76 | 90 | .001 | Weak |
| rs2736122 | TERT | T | Miscellany | 3 | 11215 | 11908 | Lung (miscellany) | 0.97 (0.92 to 1.01) | .19 | 0 | .80 | Strong |
| rs2736122 | TERT | T | Miscellany | 2 | 3851 | 3934 | Pancreas | 1.00 (0.93 to 1.08) | .94 | 0 | .37 | Weak |
| rs2853669 | Intergenic | C | White | 3 | 3787 | 3824 | Breast | 1.02 (0.91 to 1.14) | .76 | 52 | .12 | Weak |
| rs2853676 | TERT | A | Miscellany | 2 | 3851 | 3934 | Pancreas | 0.93 (0.86 to 1.00) | .06 | 0 | .40 | Weak |
| rs2853676 | TERT | A | White | 2 | 3349 | 4542 | Melanoma (skin) | 1.16 (0.81 to 1.66) | .42 | 88 | .003 | Weak |
| rs2853677 | TERT | C | White | 2 | 2963 | 3340 | Breast | 1.03 (0.96 to 1.10) | .43 | 0 | .53 | Weak |
| rs2853677 | TERT | C | Miscellany | 4 | 2252 | 1809 | Lung (miscellany) | 1.31 (0.97 to 1.75) | .073 | 63 | .046 | Weak |
| rs2853677 | TERT | C | White | 2 | 2045 | 1615 | Lung (miscellany) | 1.34 (0.88 to 2.06) | .17 | 76 | .04 | Weak |
| rs2853677 | TERT | C | Asian/Black | 2 | 207 | 194 | Lung (miscellany) | 1.38 (0.62 to 3.05) | .43 | 74 | .05 | Weak |
| rs2853690 | TERT | T | White | 2 | 2983 | 3340 | Breast | 0.98 (0.89 to 1.08) | .74 | 0 | .60 | Weak |
| rs2853690 | TERT | T | Miscellany | 2 | 459 | 483 | Lung (NSCLC) | 0.89 (0.63 to 1.25) | .51 | 0 | .75 | Weak |
| rs31484 | CLPTM1L | T | Miscellany | 2 | 5302 | 6823 | Lung (miscellany) | 0.95 (0.61 to 1.48) | .83 | 97 | 2.1E-10 | Weak |
| rs401681 | CLPTM1L | T | White | 3 | 10254 | 36832 | Breast | 1.01 (0.97 to 1.05) | .65 | 0 | .47 | Strong |
| rs401681 | CLPTM1L | T | White | 4 | 5124 | 33326 | Colon | 1.05 (0.98 to 1.12) | .15 | 36 | .20 | Strong |
| rs401681 | CLPTM1L | T | White | 2 | 1095 | 30463 | Endometrium | 0.94 (0.73 to 1.21) | .61 | 86 | .007 | Weak |
| rs401681 | CLPTM1L | T | White | 2 | 1328 | 5861 | Testis | 1.18 (0.99 to 1.40) | .06 | 67 | .08 | Weak |
| rs401681 | CLPTM1L | T | Miscellany | 3 | 1573 | 37295 | Lung (SCLC) | 0.98 (0.90 to 1.07) | .66 | 9 | .33 | Weak |
| rs401681 | CLPTM1L | T | White | 3 | 4583 | 36338 | Lung (squamous) | 0.85 (0.70 to 1.04) | .11 | 86 | .001 | Weak |
| rs402710 | CLPTM1L | T | White | 2 | 3851 | 3934 | Pancreas | 1.14 (0.98 to 1.33) | .10 | 83 | .014 | Weak |
| rs466502 | CLPTM1L | G | Miscellany | 2 | 5302 | 6823 | Lung (miscellany) | 0.96 (0.65 to 1.42) | .84 | 97 | 8.2E-09 | Weak |
| rs4975605 | TERT | A | Miscellany | 3 | 11177 | 11835 | Lung (miscellany) | 0.96 (0.92 to 1.00) | .06 | 0 | .94 | Strong |
| rs4975605 | TERT | A | White | 2 | 3851 | 3934 | Pancreas | 1.03 (0.96 to 1.10) | .39 | 0 | .64 | Weak |
| rs7712562 | Intergenic | C | White | 2 | 2965 | 3341 | Breast | 1.00 (0.82 to 1.21) | .97 | 70 | .06 | Weak |
| rs7727912 | CLPTM1L | T | White | 2 | 4652 | 4981 | Lung (miscellany) | 1.01 (0.74 to 1.39) | .94 | 85 | .01 | Weak |
| MNS16a | TERT | S | Miscellany | 2 | 2058 | 2194 | Breast | 1.20 (0.84 to 1.71) | .32 | 85 | .01 | Weak |
| MNS16a | TERT | S | Miscellany | 2 | 990 | 1015 | Lung (NSCLC) | 0.99 (0.62 to 1.60) | .98 | 61 | .11 | Weak |
Telomerase reverse transcriptase (TERT) locus includes TERT and cleft lip and palate transmembrane 1-like (CLPTM1L) genes. ID = identification; CI = confidence interval; CNS = central nervous system; NSCLC = non–small cell lung cancer; OR =odds ratio; SCLC = small cell lung cancer.
Miscellany indicates a mix of different races.
Lung (miscellany) indicates a mix of different histological subtypes.
All P values calculated by Z test or Q test were two-sided.
Levels of cumulative evidence (strong vs weak) were defined according to statistical power and between-study heterogeneity.
Overall, 22 polymorphisms were associated with the risk of developing one or more types of cancer but only 11 showed strong cumulative evidence according to the international guidelines known as Venice criteria (moderate and weak cumulative evidence was found for nine and 14 polymorphisms, respectively). In particular, among the 75 allelic contrasts for which the meta-analysis showed statistically significant associations with cancer risk, the cumulative evidence was strong, moderate, and weak for 20 (27%), 15 (20%), and 40 (53%) allelic contrasts, respectively. The results for polymorphisms with strong evidence of an association with at least one tumor type are described below. Findings for each polymorphism are detailed in the Supplementary Results (available online). Finally, for five (12%) of the 43 meta-analyses that showed no statistically significant association, the cumulative evidence for lack of association with cancer risk was strong.
Associations between TERT locus Polymorphisms and Cancer Risk
rs2736098.
Although rs2736098 is a synonymous polymorphism (G>A; Ala305Ala), this TERT SNP has been shown to be associated with telomere length but not with TERT expression (41). On the other hand, rs2736098 is in LD with rs2853669 (pairwise correlation coefficient r 2 = 0.79) (24), an SNP granted functional relevance (see Supplementary Results, available online). Therefore, rs2736098 may simply be a marker tagging the relevant polymorphism.
Meta-analysis by tumor type revealed a statistically significant association between the minor allele rs2736098[A] and both lung (OR = 1.21, 95% CI = 1.15 to 1.27) and bladder cancer (OR=1.19, 95% CI = 1.12 to 1.25) (Table 1). According to the Venice criteria, the cumulative evidence for the association between rs2736098[A] and the risk of these two tumor types was strong. Because the MAF of rs2736098 was 26%, the estimated PAR for lung and bladder cancer was 6% and 5%, respectively. Meta-analysis of available data showed no association between this SNP and either prostate cancer or CNS tumors (Table 2).
rs2736100.
SNP rs2736100 is located in intron 2 of TERT and, on the basis of the Evolutionary and Sequence Pattern Extraction through Reduced Representation (ESPERR) score (85), is located within a putative regulatory region (40). This polymorphism has also been linked to idiopathic pulmonary fibrosis, a disease associated with increased risk of developing lung cancer (86). It is also the most studied polymorphism of the TERT gene, as it was described in 46 studies enrolling 74 785 case subjects with 11 tumor types and 115 726 control subjects.
Thirty-two studies reported a statistically significant association between the minor allele rs2736100[C] and cancer susceptibility. Notably, for testicular cancer, this allele was associated with a decreased disease risk, whereas for all other tumor types, it was associated with an increased disease risk. Upon meta-analysis, the association between this TERT polymorphism and cancer risk was statistically significant for lung, bladder, pancreatic, testis, and CNS tumors (Table 1), but not melanoma (data for melanoma is presented in Table 2). Lung cancer was by far the most studied tumor type, with 50 917 case subjects and 72 598 control subjects enrolled in 23 studies. The meta-risk was highly statistically significant (OR = 1.19, 95% CI = 1.14 to 1.23), but with a large amount of heterogeneity (I 2 = 77%, P < .001), which made the cumulative evidence for association weak. A stronger association was noted in Asians (OR = 1.26, 95% CI = 1.20 to 1.32) compared with whites (OR = 1.11, 95% CI = 1.08 to 1.15; P heterogeneity for difference between ORs < .001); however, the cumulative evidence remained weak for both ancestries because of the small overall association among whites and the large heterogeneity (I 2 = 68%, P < .001) coupled with potential chasing bias (Ioannidis and Trikalinos test P = .035) among Asian studies.
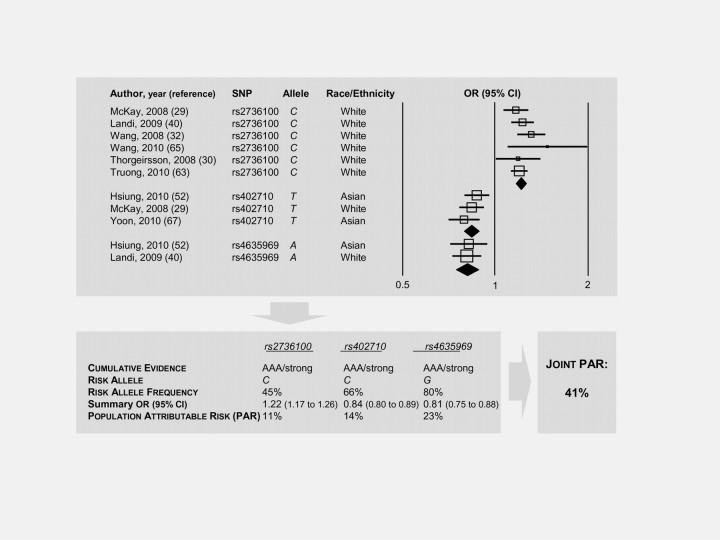
Because data were permissive, we investigated this SNP also in relation with lung cancer subtypes. The risk of adenocarcinoma was highest among all subtypes (OR = 1.30, 95% CI = 1.25 to 1.36), but again, the cumulative evidence was weak as a result of lack of replication and large heterogeneity (I 2 = 62%, P = .001). However, when ethnicity was taken into account, the risk among whites was highly statistically significant (OR = 1.22, 95% CI = 1.17 to 1.27) and the cumulative evidence was strong (estimated PAR = 11%, MAF = 45%). The association of rs2736100 with adenocarcinoma was reported to be stronger among never smokers (65), a population subset where this is the most common form of lung cancer. Moreover, the estimated joint PAR for three polymorphisms including TERT rs2736100, intergenic rs4635969, and CLPTM1L rs402710 was 41% (Figure 1). Among Asians, the meta-risk for this tumor subtype was higher (OR = 1.35, 95% CI = 1.29 to 1.42, P heterogeneity < .001), but heterogeneity remained statistically significant (I 2 = 47%, P = .067) and the cumulative evidence for association was moderate.
Figure 1.
Flow chart for the estimation of the joint risk of lung adenocarcinoma in the general population attributable to three TERT locus polymorphisms. Top panel. A forest plot depicting the meta-analysis of the studies that contributed to define the association between the minor alleles of three single-nucleotide polymorphisms (SNPs) and the risk of developing lung adenocarcinoma. Open squares represent odds ratios (ORs) of single studies (the width of each square is proportional to the weight of the corresponding study; the horizontal line represents the 95% confidence interval [CI] of the study OR); solid black diamonds represent summary OR for each SNP (the width of each diamond is proportional to the 95% CI of the corresponding summary OR). Bottom left panel. Only SNP showing strong cumulative evidence for association with lung adenocarcinoma were selected. Cumulative evidence was assessed as per the Venice criteria (see text for more details). OR refer to risk alleles (alleles associated with increased cancer risk). Bottom right panel. The joint PAR (population attributable risk) represents the proportion of lung adenocarcinoma cases estimated to be attributable to the three SNPs showing strong cumulative evidence of association; it depends on both the magnitude of the association (OR) and the risk allele frequency in the general population.
For squamous cell carcinoma, the meta-risk point estimate was small for rs2736100[C] (OR = 1.06, 95% CI = 1.01 to 1.12) and the cumulative evidence for association was weak. For small cell lung cancer, the cumulative evidence for lack of association (OR = 1.05, 95% CI = 0.99 to 1.09) was strong (Table 2). Other subgroup analyses performed for lung cancer are reported in Table 1.
rs2736100[C] was statistically significantly associated with reduced risk of testicular germ cell carcinoma (TGCC) (OR= 0.75, 95% CI = 0.70 to 0.81), the cumulative evidence for association was strong (estimated PAR for the risk allele rs2736100[A] = 22%, risk allele frequency = 55%).
For other tumor types, no strong evidence could be demonstrated. For pancreatic cancer, the available data were in favor of a small risk increase (OR = 1.12, 95% CI = 1.04 to 1.20) and the cumulative evidence was weak. The association of rs2736100[C] with the risk of bladder carcinoma was also statistically significant (OR = 1.19, 95% CI = 1.05 to 1.34), but because of the relatively low number of subjects enrolled (100 < n< 1000, n= subjects carrying the minor allele), the cumulative evidence was moderate. Finally, the association with risk of CNS tumors was even stronger (OR = 1.34, 95% CI = 1.22 to 1.46), but the cumulative evidence for association was weak as a result of large heterogeneity (I 2 = 60%, P = .02).
rs2853676.
The association of this TERT intronic polymorphism with risk of cancer was evaluated in 18 studies, including 31 481 case subjects (11 tumor types) and 44 122 control subjects. Four studies on CNS tumors and one on cutaneous melanoma reported a statistically significantly increased risk for carriers of the minor allele (A), whereas one study described a reduced risk for TGCC. Meta-analysis by tumor type revealed a strong association between rs2853676[A] and increased risk of CNS tumors (OR = 1.26, 95% CI = 1.21 to 1.32), and the cumulative evidence was strong (estimated PAR = 7%, MAF = 24%).
In contrast, the cumulative evidence for lung cancer risk was weak, because of a small meta-risk (OR = 1.06, 95% CI = 1.01 to 1.10). Finally, no association could be demonstrated for pancreatic cancer (OR = 0.93, 95% CI = 0.86 to 1.00) and melanoma (OR = 1.16, 95% CI = 0.81 to 1.66).
rs31489.
This intronic SNP of the CLPTM1L gene was chosen because it is located in a DNA region in LD with the TERT promoter and the 5′-end of TERT gene (Supplementary Figure 1, available online) (9). Nine studies reported statistically significant association between cancer risk and the minor allele (A), but with opposite risk directions for lung cancer and skin basal cell carcinoma (BCC) (decreased) as compared with pancreatic cancer and TGCC (increased).
Meta-analysis by tumor type confirmed that rs31489[A] is associated with an increased risk of pancreatic cancer (OR = 1.18, 95% CI = 1.06 to 1.32) and TGCC (OR = 1.28, 95% CI = 1.16 to 1.41) but is statistically significantly associated with reduced risk of lung cancer (OR = 0.83, 95% CI = 0.78 to 0.88) (Table 1). The cumulative evidence for association was strong only for testicular cancer (estimated PAR = 11%, MAF = 36%). For the other tumor types, no meta-analysis could be performed.
rs380286.
This SNP is located in an intronic region of CLPTM1L. Two studies reported that subjects carrying the minor allele (A) were associated with reduced risk of lung cancer (OR = 0.85, 95% CI = 0.80 to 0.91), and the cumulative evidence was strong (estimated PAR for risk allele rs380286[G] = 13%, major allele frequency = 63%) (note that in this case the “risk” allele coincides with the “major” allele).
rs401681.
This SNP is located in an intronic region of CLPTM1L. Like rs2736098, rs401681 was reported to be associated with telomere length (but not TERT expression) (41), which supports its relevance for telomere biology and potentially cancer development. At present, rs401681 is the most widely studied TERT locus polymorphism because it was assessed for cancer risk in 56 series enrolling 89 903 case subjects (23 tumor types) and 155 202 control subjects (Supplementary Table 1, available online). In 29 studies, a statistically significant association was observed between the minor allele (T) and cancer risk; however, 21 studies described a decreased cancer risk, whereas eight described an increased risk.
Meta-analysis by cancer type revealed that rs401681[T] carriers have a modestly increased risk of pancreatic carcinoma (OR = 1.14, 95% CI = 1.01 to 1.29) and skin melanoma (OR = 1.12, 95% CI = 1.03 to 1.22). Conversely, a modest risk reduction was observed for bladder (OR = 0.89, 95% CI = 0.86 to 0.92), lung (OR = 0.87, 95% CI = 0.84 to 0.89), and prostate (OR = 0.92, 95% CI = 0.89 to 0.95) cancer and skin squamous cell carcinoma (OR = 0.92, 95% CI = 0.84 to 0.99). Finally, a more pronounced risk reduction was detected for BCC (OR = 0.82, 95% CI = 0.76 to 0.89). In no case was the cumulative evidence strong because of between-study heterogeneity and/or small overall association (Table 1).
For lung cancer, available data also enabled us to perform meta-analysis by ancestry and histological subtype. SNP rs401681[T] was associated with statistically significantly reduced risk of both adenocarcinoma (OR = 0.87, 95% CI = 0.81 to 0.93) and squamous cell carcinoma (OR = 0.85, 95% CI = 0.76 to 0.96), but not with small cell lung cancer (OR = 0.98, 95% CI = 0.90 to 1.07). Nevertheless, the cumulative evidence for the first two lung cancer subtypes was weak because of heterogeneity (Table 1). When ethnicity was taken into account, a reduced risk of non–small cell lung carcinoma was observed only among Asians (OR = 0.84, 95% CI = 0.79 to 0.90), and the cumulative evidence for association was strong (estimated PAR for risk allele rs401681[C] = 13%, major allele frequency = 60%).
Finally, no statistically significant relationship was observed for the other tumor types (breast, colon, endometrial cancer, and TGCC) for which a meta-analysis could be performed; for breast and colon carcinomas, the cumulative evidence for lack of association was strong (Table 2).
rs402710.
This CLPTM1L intronic SNP is in LD with many other CLPTM1L polymorphisms, including the above described rs401681 (see Supplementary Figure 2, available online), but not with TERT polymorphisms such as rs2736100, which led some authors to postulate that that the 5p15.33 locus might host two independent cancer risk SNPs (ie, rs2736100 and rs402710) (29). Ten studies reported a statistically significant association between the minor allele (T) and a decreased risk of lung, bladder, and nasopharyngeal tumors, whereas two other studies described the opposite relationship with pancreatic and testicular cancer (Supplementary Table 1, available online).
Upon meta-analysis, rs402710[T] was associated with reduced risk of both bladder (OR = 0.85, 95% CI = 0.75 to 0.98) and lung cancer (OR = 0.87, 95% CI = 0.83 to 0.92), but in neither case was the cumulative evidence for association strong (Table 1).
Available data allowed a meta-analysis of lung cancer histological subtypes for risk assessment. Interestingly, rs402710[T] showed a homogeneous and statistically significantly reduced risk of both adenocarcinoma (OR = 0.84, 95% CI = 0.80 to 0.89) and squamous cell carcinoma (OR = 0.83, 95% CI = 0.77 to 0.89), the cumulative evidence being strong in both subtypes (estimated PAR for risk allele rs402710[C] = 14%, risk allele frequency = 66%). Finally, no statistically significant association was found with the risk of pancreatic cancer (OR = 1.14, 95% CI = 0.98 to 1.32).
rs4635969.
Considering the minor allele (A) of this TERT/CLPTM1L intergenic SNP, available studies reported opposing but statistically significant findings for lung cancer (decreased risk) and both pancreatic cancer and TGCC (increased risk). Meta-analysis by tumor type confirmed an association between rs4635969[A] and reduced risk of lung cancer (OR = 0.86, 95% CI = 0.82 to 0.90), although the cumulative evidence was weak because of a small overall association. As also reported for rs2736100, the reduced risk was more pronounced in lung adenocarcinoma (OR = 0.81, 95% CI = 0.75 to 0.88), for which the cumulative evidence for association could be classified as strong (estimated PAR for risk allele rs4635969[G] = 23%, major allele frequency = 80%).
Among rs4635969[A] carriers, the risk was statistically significantly increased for pancreatic (OR = 1.22, 95% CI = 1.14 to 1.32) and testicular (OR = 1.61, 95% CI = 1.46 to 1.76) carcinomas. In these two cancers, the overall evidence for association was strong, the estimated PAR being 5% and 14%, respectively. Of note, the meta-risk for TGCC associated with this SNP was the strongest association found in the present meta-analysis.
rs465498.
All four studies evaluating this intronic CLPTM1L SNP found a statistically significantly reduced cancer risk in people carrying the minor allele (G). Pooling the summary data confirmed this association (OR = 0.79, 95% CI = 0.74 to 0.84), although the cumulative evidence for association appeared moderate as a result of heterogeneity (I 2 = 49%, P = .12). Restricting the analysis to the Asian ancestry by pooling data from three series (a GWAS with one discovery and two independent replication phases) resulted in a homogeneous (I 2 = 0%, P = .76) and statistically significant association with reduced risk of cancer (OR = 0.76, 95% CI = 0.72 to 0.81), which was statistically significantly lower (P heterogeneity = .021) than that reported in the study restricted to white subjects (OR = 0.85, 95% CI = 0.79 to 0.92), and the cumulative evidence was strong (estimated PAR for risk allele rs465498[A] among Asians = 36%, major allele frequency among Asians = 83%).
rs467095.
The three studies reporting on the allele distribution of this intronic CLPTM1L polymorphism described a reduced cancer risk in people carrying the minor allele (C). Pooling the summary data confirmed the association of rs467095[C] with reduced cancer risk (OR = 0.83, 95% CI = 0.78 to 0.85), and the cumulative evidence for association was strong (estimated PAR for the risk allele rs467095[T] = 17%, major allele frequency ≈ 72%).
MNS16a.
This variable tandem repeat polymorphism (short [S] vs long [L] polymorphism) is located downstream of the TERT gene and was reported to affect promoter activity in lung cancer cell lines (21), although the functional importance of the antisense transcript activity is unclear. Two studies on CNS tumors and one on breast cancer reported that the minor allele (S) was associated with an increased risk of disease development.
The meta-analysis showed that MNS16a[S] is associated with a homogeneously reported (I 2 = 8%, P = .30) statistically significantly increased risk of CNS tumors (OR = 1.20, 95% CI = 1.07 to 1.33; I 2 = 8%, P = .30), but not breast (OR = 1.20, 95% CI = 0.84 to 1.71) or lung (OR = 0.99, 95% CI = 0.62 to 1.60) carcinomas. The cumulative evidence for association between MNS16a[S] and CNS tumors was strong (estimated PAR = 7%, MAF = 34%).
Discussion
This work, to our knowledge, is the first synopsis of the literature on the role of polymorphisms at the TERT locus (5p15.33) in cancer predisposition. Upon systematic review and meta-analysis of the data from 85 molecular epidemiology studies, enrolling almost half a million people tested for one or more of 67 polymorphisms, which generated 494 allelic contrasts, we found that 22 polymorphisms were associated with the risk of developing one or more types of cancer but only 11 showed strong cumulative evidence for association, according to the Venice criteria.
The risks were relatively low, with odds ratios for risk alleles ranging between 1.05 and 1.61 and those for protective alleles between 0.92 and 0.75. Accordingly, PAR, which takes into account both magnitude of the risk and risk allele frequency in the general population, varied from 4% to 36%. Although these figures might suggest at first glance that the TERT locus does not play a major role in cancer susceptibility, a couple of considerations should be made. First, it is widely recognized that single common polymorphisms are generally associated with low risks (ie, OR < 1.5) (20), which calls for considering the effect of combinations of multiple polymorphisms. A few studies (29,37,54,63) addressed this issue by assessing the effect of TERT locus haplotypes on cancer risk: However, the haplotypes considered by different authors are heterogeneous, and thus no meta-analysis could be performed. Other investigators have verified that some polymorphisms (eg, rs2736100 and rs2736098) carry a risk independently of others [eg, rs402710 (29), rs4635969 (40), and rs401681 (41)] using multivariable logistic regression analysis, but unfortunately, the models (ie, the combination of included covariates) are not equal and thus their results cannot be merged. Nevertheless, to provide readers with an idea of the potential predictive value of multiple polymorphisms, we considered three unrelated (based on pairwise correlation coefficient r 2 <0.1 and multivariable analysis) polymorphisms (ie TERT rs2736100, intergenic rs4635969, and CLPTM1L rs402710) with a strong cumulative evidence for association with lung adenocarcinoma, and we found that the estimated joint PAR defined by these polymorphisms is 41% (see Figure 1), which corresponds to a 0.5% attributable community risk (considering a 1.2% lifetime risk of lung adenocarcinoma). Although we could calculate only a per-allele PAR (ie, only the codominant model was tested), this result highlights the pivotal role that TERT locus polymorphisms play in the determination of the most frequent histological subtype of lung cancer and exemplifies the importance of further investigation on the 5p15.33 region with regard to cancer predisposition in general.
Another key point is that the TERT gene alone (without considering the rest of the TERT locus) has more than 500 known SNPs, whereas thus far the relationship with cancer risk has been investigated only for 67 SNPs, which is a minority of these polymorphisms. It should also be remembered that only 24 tumor types were investigated and that on average, each polymorphism was tested for about three tumor types (range = 1–23) (see the heat map in Supplementary Figure 3, available online). Furthermore, as we reported above, for many polymorphisms, some evidence of association with cancer risk already exists, although more data are necessary to conclusively define their role (see Table 1 for polymorphisms with moderate or weak cumulative evidence and Supplementary Table 1, available online, for polymorphisms with statistically significant association in a single study). In contrast, only for a minority of the polymorphisms investigated to date (5 [7%] of 67) the available results are compatible with no relevance for the susceptibility of three tumor types (see Table 2 for strong cumulative evidence), which does not rule out that these SNPs might be associated with the risk of other tumor types. Therefore, this synopsis, which strongly supports the relevance of the TERT locus to define the genetic architecture of cancer predisposition, also underscores that much work remains to be done before we can entirely appreciate the importance of this DNA region in cancer development.
The 5p15.33 locus is characterized by a 62-kb LD block including the 5′-end of TERT, its promoter, and the entire gene CLPTM1L (Supplementary Figure 2, available online); consequently, there are two genes that can be involved in the tumor promoting effects epidemiologically linked to the above described polymorphisms. Certainly, TERT is the more appealing candidate because of its well-known role in telomere and tumor biology. However, we must underscore the discrepancy between the epidemiological findings associating TERT polymorphisms to cancer risk and the relatively scarce (29) and sometime conflicting evidence (59,87) associating the same polymorphisms to telomere length, a key aspect linking these chromosomal structures to cancer biology. In light of these controversies, more work is warranted to elucidate the molecular mechanisms possibly responsible for these epidemiological observations. For example, rs2736100 and other SNP in the locus are close to mutations known to alter telomerase activity (88). The complete sequencing of this locus in cancer patients will help investigators to fully elucidate the relationship between the TERT locus polymorphisms and cancer risk.
For the other gene in this region, CLPTM1L might be relevant not only because it is in LD with TERT but also in the light of its own biological activity; its product (cleft lip and palate transmembrane protein 1-like protein) is known to induce cisplatin resistance in ovarian cancer cells (the CLPTM1L product is also called cisplatin resistance related protein [CRR9]) (89). Moreover, its SNP rs402710 has been recently associated with higher levels of bulky aromatic and hydrophobic DNA adducts (47), a typical product of lung cancer carcinogens such as polycyclic aromatic hydrocarbons and tobacco-specific nitrosamines.
Considering that, for both genes, virtually all polymorphisms have no effect on protein sequence (all polymorphisms for which a meta-analysis could be performed were either intronic or exonic-synonymous or intergenic) (Supplementary Table 1, available online), the association of these polymorphisms with cancer susceptibility might derive from either their LD with still undetected functional polymorphisms (ie, exonic non-synonymous) or from their effect on protein expression (although the latter hypothesis does not appear to be supported by the evidence collected thus far, as mentioned above). Overall, the findings collected in this synopsis, which are virtually always based on tagging SNP, might tip the balance in favor of gene-centric strategies, that is, studies based on the use of polymorphisms known to affect protein sequence. However, some investigators have recently reported no meaningful results adopting this approach and have hypothesized that natural selection has rendered non-synonymous alleles so rare (ie, MAF < 5%) that sample sizes greater than those used for common alleles should be used to detect statistically significant associations (90). Remembering that in this synopsis, where virtually all studies regarded polymorphisms with MAF greater than 5%, the mean sample size is approximately 6000 subjects, the practical difficulty of carrying out gene-centric research is self-evident.
An intriguing finding of our work is that the association between a given polymorphism and cancer risk can be very specific not only in terms of ethnicity/histology but also in terms of effect direction (Table 1). Indeed, we found that some 5p15.33 locus polymorphisms correlate (with strong evidence) with cancer risk only in whites (eg, rs2736100 in lung adenocarcinoma) or Asians (eg, rs402710 in lung cancer), and that others predispose to a specific histological subtype of cancer (eg, rs2736100 and rs4027100 in lung adenocarcinoma). More surprisingly, the same polymorphism (eg, rs2736100, rs401681) can result in an increased risk for some cancer types (eg, lung cancer, BCC) and a reduced risk for others (eg, TGCC, melanoma). In the case of melanoma and BCC, this finding, apparently contradictory, is in line with the opposite effect that telomere shortening [which is accelerated in rs401681[C] carriers (41)] can have on the two tumor types (91,92). On the other hand, our observation lends support to a double hypothesis: 1) no common molecular pathway leads to the development of all cancer types, and 2) some pathways favoring the development of some tumor types might even oppose the genesis of others. Some clinical epidemiology evidence is in the same direction: for example, women taking tamoxifen (a drug interfering with the estrogen receptor pathway) are at lower risk of breast cancer but at higher risk of endometrial cancer; and people affected with skin melanoma (and thus with a genetic background predisposing to this tumor) are at higher risk of secondary melanoma but also at lower risk of gastrointestinal and lung tumors (93). Though appealing, this hypothesis clearly warrants further investigation to be validated and thus to be exploitable on the clinical ground.
Finally, the limitations of this synopsis must be addressed. For example, although an exhaustive literature search was performed, it is possible that some publications were overlooked; moreover, not all the GWAS authors we contacted agreed to provide their data; finally, only for a minority of studies genotype data (either as raw or summary data) were provided, which enabled us to test only the codominant model (per-allele risk analysis). We hope that this large collection of data on TERT locus polymorphisms and cancer risk will prompt investigators to share their knowledge in this field, also exploiting the dedicated online data repository above mentioned (available at http://www.mmmp.org) (13). Furthermore, we used allele counts and crude estimates of effect, rather than association estimates adjusted by other polymorphisms, genes, or even environmental factors. As discussed above, for interaction between polymorphisms, the models used in single eligible studies differ in most cases and were therefore unsuitable for pooling. Lung cancer was the only tumor type for which investigators often reported data adjustment for smoking, the most common environmental risk factor. In this case, the impact of TERT locus polymorphisms was generally unaffected by smoking (80), which strengthens the findings of our meta-analysis, although it does not rule out other confounding factors [eg, chronic obstructive pulmonary disease (84)]. Again, even for lung cancer, the adjustment for smoking was reported in different ways (eg, as a single OR adjusted by including smoking behavior in a logistic regression model or by providing multiple ORs obtained separately in smoking and nonsmoking subjects), which precluded any meaningful pooling of summary data.
For gene–gene interactions, it should be remembered that tens of genes are currently known to contribute to telomere biology (94) and thus may contribute to modulate cancer susceptibility. However, to date, only a few authors investigated the relationship between polymorphisms of telomere-related genes (other than TERT) and predisposition to few tumor types (24,28,37,45,60,62,75), and because the available results are sparse (different tumor types), no data merging could be performed, which calls for further research in this field.
Multiple testing is another possible concern. We performed a total of 118 meta-analyses (including ethnicity-specific and cancer subtype–specific analyses): Considering a Bonferroni adjustment, the P value threshold for statistical significance would be .0004, which would reduce statistically significant associations from 75 to 47 (Table 1, P values tagged with symbols). Nevertheless, it should be noted that this P value is overly conservative because many tests were performed in independent datasets. Furthermore, lowering the alpha level of significance increases the possibility of type II error (ie, reduces statistical power). More importantly, polymorphisms statistically significantly associated with cancer risk were graded according to the Venice criteria, providing evidence over and above statistical P values.
In conclusion, this synopsis demonstrates that genetic variation in the TERT locus is likely to play a relevant role in cancer development. However, it also underscores that much work still needs to be done to clarify the molecular mechanisms underlying this epidemiological observation and to define the interactions of this evidence with the other pieces of the cancer predisposition puzzle.
Funding
This work was in part supported by a grant from the Italian Association for Research on Cancer (AIRC Veneto Regional fund 2008-2011 to SM and DN).
Footnotes
We are grateful to the following investigators who provided us with unpublished data from their genome-wide association study (GWAS): Maria Teresa Landi (National Cancer Institute, NIH, Bethesda, MD); Valerie Gaborieau and Paul Brennan (International Agency for Research on Cancer, IARC, Lyon, France); Jesus Lascorz (German Cancer Research Center, DKFZ, Heidelberg, Germany); Robert Clifford (National Cancer Institute, NIH, Bethesda, MD); Jin-Xin Bei and Yi-Xin Zeng (State Key Laboratory of Oncology, Guangzhou, China); Mario Falchi (Kings College of London, London, UK); John Maris and Sharon Diskin (Children's Hospital of Philadelphia, Philadelphia, PA); and Clare Turnbull (Institute for Cancer Research, Sutton, UK).
We also thank our data manager Dr Marta Briarava for setting and filling up the dedicated database we used for the systematic review. The authors are solely responsible for the study design, data collection, analysis and interpretation of the data, writing the article, and decision to submit the article for publication.
References
- 1.Blackburn EH. Switching and signaling at the telomere. Cell. . 2001;106(6):661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T. How telomeres solve the end-protection problem. Science. . 2009;326(5955):948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi A, Shore D. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol Cell. . 2008;31(2):153–165. doi: 10.1016/j.molcel.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Osterhage JL, Friedman KL. Chromosome end maintenance by telomerase. J Biol Chem. . 2009;284(24):16061–16065. doi: 10.1074/jbc.R900011200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. . 2005;6(8):611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 6.Calado RT, Young NS. Telomere diseases. N Engl J Med. . 2009;361(24):2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. . 2008;8(3):167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 8.Liu JP, Chen W, Schwarer AP, Li H. Telomerase in cancer immunotherapy. Biochim Biophys Acta. . 2010;1805(1):35–42. doi: 10.1016/j.bbcan.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Baird DM. Variation at the TERT locus and predisposition for cancer. Expert Rev Mol Med. . 2010;12:e16. doi: 10.1017/S146239941000147X. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis JP, Boffetta P, Little J, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. . 2008;37(1):120–132. doi: 10.1093/ije/dym159. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. . 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. . 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Mocellin S, Rossi CR. The Melanoma Molecular Map Project. Melanoma Res. . 2008;18(3):163–165. doi: 10.1097/CMR.0b013e328300c50b. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. . 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. . 2000;53(11):1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 16.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. . 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. . 2007;4(3):245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 18.Stacey SN, Sulem P, Masson G, et al. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet. . 2009;41(8):909–914. doi: 10.1038/ng.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watcholder S. The impact of a prevention effort on the community. Epidemiology. . 2005;16(1):1–3. doi: 10.1097/01.ede.0000147633.09891.16. [DOI] [PubMed] [Google Scholar]
- 20.Sagoo GS, Little J, Higgins JP. Systematic reviews of genetic association studies. Human Genome Epidemiology Network. PLoS Med. . 2009;6(3):e28. doi: 10.1371/journal.pmed.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Soria JC, Chang YS, Lee HY, Wei Q, Mao L. Association of a functional tandem repeats in the downstream of human telomerase gene and lung cancer. Oncogene. . 2003;22(46):7123–7129. doi: 10.1038/sj.onc.1206852. [DOI] [PubMed] [Google Scholar]
- 22.Carpentier C, Lejeune J, Gros F, et al. Association of telomerase gene hTERT polymorphism and malignant gliomas. J Neurooncol. . 2007;84(3):249–253. doi: 10.1007/s11060-007-9378-3. [DOI] [PubMed] [Google Scholar]
- 23.Hunter DJ, Kraft P, Jacobs KB, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. . 2007;39(7):870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savage SA, Chanock SJ, Lissowska J, et al. Genetic variation in five genes important in telomere biology and risk for breast cancer. Br J Cancer. . 2007;97(6):832–836. doi: 10.1038/sj.bjc.6603934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeager M, Orr N, Hayes RB, et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. . 2007;39(5):645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 26.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. . 2008;40(5):616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eeles RA, Kote-Jarai Z, Giles GG, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. . 2008;40(3):316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 28.Hosgood HD, III, Menashe I, Shen M, et al. Pathway-based evaluation of 380 candidate genes and lung cancer susceptibility suggests the importance of the cell cycle pathway. Carcinogenesis. 2008;29(10):1938–1943. doi: 10.1093/carcin/bgn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKay JD, Hung RJ, Gaborieau V, et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. . 2008;40(12):1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. . 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomlinson IP, Webb E, Carvajal-Carmona L, et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet. . 2008;40(5):623–630. doi: 10.1038/ng.111. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Broderick P, Webb E, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. . 2008;40(12):1407–1409. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. . 2009;41(9):986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson U, Osterman P, Sjostrom S, et al. MNS16A minisatellite genotypes in relation to risk of glioma and meningioma and to glioblastoma outcome. Int J Cancer. . 2009;125(4):968–972. doi: 10.1002/ijc.24363. [DOI] [PubMed] [Google Scholar]
- 35.Andrew AS, Gui J, Sanderson AC, et al. Bladder cancer SNP panel predicts susceptibility and survival. Hum Genet. . 2009;125(5–6):527–539. doi: 10.1007/s00439-009-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. . 2009;106(4):1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi JE, Kang HG, Jang JS, et al. Polymorphisms in telomere maintenance genes and risk of lung cancer. Cancer Epidemiol Biomarkers Prev. . 2009;18(10):2773–2781. doi: 10.1158/1055-9965.EPI-09-0323. [DOI] [PubMed] [Google Scholar]
- 38.Falchi M, Bataille V, Hayward NK, et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat Genet. . 2009;41(8):915–919. doi: 10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin G, Xu L, Shu Y, et al. Common genetic variants on 5p15.33 contribute to risk of lung adenocarcinoma in a Chinese population. Carcinogenesis. 2009;30(6):987–990. doi: 10.1093/carcin/bgp090. [DOI] [PubMed] [Google Scholar]
- 40.Landi MT, Chatterjee N, Yu K, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85(5):679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafnar T, Sulem P, Stacey SN, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. . 2009;41(2):221–227. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. . 2009;41(8):899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song W, Ruder AM, Hu L, et al. Genetic epidemiology of glioblastoma multiforme: confirmatory and new findings from analyses of human leukocyte antigen alleles and motifs. PLoS One. 2009;4(9):e7157. doi: 10.1371/journal.pone.0007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Dyke AL, Cote ML, Wenzlaff AS, et al. Chromosome 5p region SNPs are associated with risk of NSCLC among women. J Cancer Epidemiol. . 2009;2009:242151. doi: 10.1155/2009/242151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varadi V, Brendle A, Grzybowska E, et al. A functional promoter polymorphism in the TERT gene does not affect inherited susceptibility to breast cancer. Cancer Genet Cytogenet. . 2009;190(2):71–74. doi: 10.1016/j.cancergencyto.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. . 2009;41(8):905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zienolddiny S, Skaug V, Landvik NE, et al. The TERT-CLPTM1L lung cancer susceptibility variant associates with higher DNA adduct formation in the lung. Carcinogenesis. . 2009;30(8):1368–1371. doi: 10.1093/carcin/bgp131. [DOI] [PubMed] [Google Scholar]
- 48.Bei JX, Li Y, Jia WH, et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. . 2010;42(7):599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 49.Clifford RJ, Zhang J, Meerzaman DM, et al. Genetic variations at loci involved in the immune response are risk factors for hepatocellular carcinoma. Hepatology. . 2010;52(6):2034–2043. doi: 10.1002/hep.23943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gudmundsson J, Besenbacher S, Sulem P, et al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci Transl Med. . 2010;2(62):62–92. doi: 10.1126/scitranslmed.3001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guey LT, Garcia-Closas M, Murta-Nascimento C. Genetic susceptibility to distinct bladder cancer subphenotypes. Eur Urol. . 2010;57:283–292. doi: 10.1016/j.eururo.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsiung CA, Lan Q, Hong YC, et al. The 5p15.33 locus is associated with risk of lung adenocarcinoma in never-smoking females in Asia. PLoS Genet. . 2010;6(8):e1001051. doi: 10.1371/journal.pgen.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnatty SE, Beesley J, Chen X, et al. Evaluation of candidate stromal epithelial cross-talk genes identifies association between risk of serous ovarian cancer and TERT, a cancer susceptibility “hot-spot”. PLoS Genet. . 2010;6(7):e1001016. doi: 10.1371/journal.pgen.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohno T, Kunitoh H, Shimada Y, et al. Individuals susceptible to lung adenocarcinoma defined by combined HLA-DQA1 and TERT genotypes. Carcinogenesis. . 2010;31(5):834–841. doi: 10.1093/carcin/bgq003. [DOI] [PubMed] [Google Scholar]
- 55.Lascorz J, Forsti A, Chen B, et al. Genome-wide association study for colorectal cancer identifies risk polymorphisms in German familial cases and implicates MAPK signalling pathways in disease susceptibility. Carcinogenesis. . 2010;31(9):1612–1619. doi: 10.1093/carcin/bgq146. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Li G, Wei S, et al. Genetic variations in TERT-CLPTM1L genes and risk of squamous cell carcinoma of the head and neck. Carcinogenesis. . 2010;31(11):1977–1981. doi: 10.1093/carcin/bgq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miki D, Kubo M, Takahashi A, et al. Variation in TP63 is associated with lung adenocarcinoma susceptibility in Japanese and Korean populations. Nat Genet. . 2010;42(10):893–896. doi: 10.1038/ng.667. [DOI] [PubMed] [Google Scholar]
- 58.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. . 2010;42(3):224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pooley KA, Tyrer J, Shah M, et al. No association between TERT-CLPTM1L single nucleotide polymorphism rs401681 and mean telomere length or cancer risk. Cancer Epidemiol Biomarkers Prev. . 2010;19(7):1862–1865. doi: 10.1158/1055-9965.EPI-10-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prescott J, McGrath M, Lee IM, Buring JE, De Vivo I. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer. . 2010;116(18):4275–4282. doi: 10.1002/cncr.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothman N, Garcia-Closas M, Chatterjee N, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. . 2010;42(11):978–984. doi: 10.1038/ng.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen J, Gammon MD, Wu HC, et al. Multiple genetic variants in telomere pathway genes and breast cancer risk. Cancer Epidemiol Biomarkers Prev. . 2010;19(1):219–228. doi: 10.1158/1055-9965.EPI-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Truong T, Hung RJ, Amos CI, et al. Replication of lung cancer susceptibility loci at chromosomes 15q25, 5p15, and 6p21: a pooled analysis from the International Lung Cancer Consortium. J Natl Cancer Inst. . 2010;102(13):959–971. doi: 10.1093/jnci/djq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turnbull C, Rapley EA, Seal S, et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet. . 2010;42(7):604–607. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Broderick P, Matakidou A, Eisen T, Houlston RS. Role of 5p15.33 (TERT-CLPTM1L), 6p21.33 and 15q25.1 (CHRNA5-CHRNA3) variation and lung cancer risk in never-smokers. Carcinogenesis. . 2010;31(2):234–238. doi: 10.1093/carcin/bgp287. [DOI] [PubMed] [Google Scholar]
- 66.Yang P, Li Y, Jiang R, Cunningham JM, Zhang F, de Andrade M. A rigorous and comprehensive validation: common genetic variations and lung cancer. Cancer Epidemiol Biomarkers Prev. . 2010;19(1):240–244. doi: 10.1158/1055-9965.EPI-09-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon KA, Park JH, Han J, et al. A genome-wide association study reveals susceptibility variants for non-small cell lung cancer in the Korean population. Hum Mol Genet. . 2010;19(24):4948–4954. doi: 10.1093/hmg/ddq421. [DOI] [PubMed] [Google Scholar]
- 68.Yoon SL, Jung SI, Do EJ, et al. Short rare hTERT-VNTR2-2nd alleles are associated with prostate cancer susceptibility and influence gene expression. BMC Cancer. . 2010;10:393. doi: 10.1186/1471-2407-10-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H, Chen Y, Zhao Y, et al. Association of sequence variants on chromosomes 20, 11, and 5 (20q13.33, 11q23.3, and 5p15.33) with glioma susceptibility in a Chinese population. Am J Epidemiol. . 2011;173(8):915–922. doi: 10.1093/aje/kwq457. [DOI] [PubMed] [Google Scholar]
- 70.Egan KM, Thompson RC, Nabors LB, et al. Cancer susceptibility variants and the risk of adult glioma in a US case-control study. J Neurooncol. . 2011;104(2):535–542. doi: 10.1007/s11060-010-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gago-Dominguez M, Jiang X, Conti DV, et al. Genetic variations on chromosomes 5p15 and 15q25 and bladder cancer risk: findings from the Los Angeles-Shanghai bladder case-control study. Carcinogenesis. . 2011;32(2):197–202. doi: 10.1093/carcin/bgq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofer P, Baierl A, Feik E, et al. MNS16A tandem repeats minisatellite of human telomerase gene: a risk factor for colorectal cancer. Carcinogenesis. . 2011;32(6):866–871. doi: 10.1093/carcin/bgr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu Z, Wu C, Shi Y, et al. A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet. . 2011;43(8):792–796. doi: 10.1038/ng.875. [DOI] [PubMed] [Google Scholar]
- 74.Jin G, Yoo SS, Cho S, et al. Dual roles of a variable number of tandem repeat polymorphism in the TERT gene in lung cancer. Cancer Sci. . 2011;102(1):144–149. doi: 10.1111/j.1349-7006.2010.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nan H, Qureshi AA, Prescott J, De Vivo I, Han J. Genetic variants in telomere-maintaining genes and skin cancer risk. Hum Genet. . 2011;129(3):247–253. doi: 10.1007/s00439-010-0921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanetsky PA, Mitra N, Vardhanabhuti S, et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet. . 2011;20(15):3109–3117. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kohno T, Kunitoh H, Mimaki S, et al. Contribution of the TP53, OGG1, CHRNA3, and HLA-DQA1 genes to the risk for lung squamous cell carcinoma. J Thorac Oncol. . 2011;6(4):813–817. doi: 10.1097/JTO.0b013e3181ee80ef. [DOI] [PubMed] [Google Scholar]
- 78.Kote-Jarai Z, Olama AA, Giles GG, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet. . 2011;43(8):785–791. doi: 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kratz CP, Han SS, Rosenberg PS, et al. Variants in or near KITLG, BAK1, DMRT1, and TERT-CLPTM1L predispose to familial testicular germ cell tumour. J Med Genet. . 2011;48(7):473–476. doi: 10.1136/jmedgenet-2011-100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pande M, Spitz MR, Wu X, Gorlov IP, Chen W, Amos CI. Novel genetic variants in the chromosome 5p15.33 region associate with lung cancer risk. Carcinogenesis. . 2011;32(10):1493–1499. doi: 10.1093/carcin/bgr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters U, Hutter CM, Hsu L, et al. Meta-analysis of new genome-wide association studies of colorectal cancer risk. Hum Genet. . 2012;131(2):217–234. doi: 10.1007/s00439-011-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang K, Diskin SJ, Zhang H, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. . 2011;469(7329):216–220. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wauters E, Smeets D, Coolen J, et al. The TERT-CLPTM1L locus for lung cancer predisposes to bronchial obstruction and emphysema. Eur Respir J. . 2011;38(4):924–931. doi: 10.1183/09031936.00187110. [DOI] [PubMed] [Google Scholar]
- 84.Young RP, Hopkins RJ, Whittington CF, Hay BA, Epton MJ, Gamble GD. Individual and cumulative effects of GWAS susceptibility loci in lung cancer: associations after sub-phenotyping for COPD. PLoS One. . 2011;6(2):e16476. doi: 10.1371/journal.pone.0016476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor J, Tyekucheva S, King DC, Hardison RC, Miller W, Chiaromonte F. ESPERR: learning strong and weak signals in genomic sequence alignments to identify functional elements. Genome Res. . 2006;16(12):1596–1604. doi: 10.1101/gr.4537706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. . 2009;84(1):52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mirabello L, Yu K, Kraft P, et al. The association of telomere length and genetic variation in telomere biology genes. Hum Mutat. . 2010;31:1050–1058. doi: 10.1002/humu.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perona R, Machado-Pinilla R, Manguan C, Carrillo J. Telomerase deficiency and cancer susceptibility syndromes. Clin Transl Oncol. . 2009;11(11):711–714. doi: 10.1007/s12094-009-0432-9. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y. A novel gene, CRR9, which was up-regulated in CDDP-resistant ovarian tumor cell line, was associated with apoptosis. Biochem Biophys Res Commun. . 2001;280(4):1148–1154. doi: 10.1006/bbrc.2001.4250. [DOI] [PubMed] [Google Scholar]
- 90.Webb E, Broderick P, Lubbe S, Chandler I, Tomlinson I, Houlston RS. A genome-wide scan of 10 000 gene-centric variants and colorectal cancer risk. Eur J Hum Genet. . 2009;17(11):1507–1514. doi: 10.1038/ejhg.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han J, Qureshi AA, Prescott J, et al. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. . 2009;129(2):415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bataille V, Kato BS, Falchi M, et al. Nevus size and number are associated with telomere length and represent potential markers of a decreased senescence in vivo. Cancer Epidemiol Biomarkers Prev. . 2007;16(7):1499–1502. doi: 10.1158/1055-9965.EPI-07-0152. [DOI] [PubMed] [Google Scholar]
- 93.Mocellin S, Nitti D. Cutaneous melanoma in situ: translational evidence from a large population-based study. Oncologist. . 2011;16(6):896–903. doi: 10.1634/theoncologist.2010-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riethman H. Human telomere structure and biology. Annu Rev Genomics Hum Genet. . 2008;9:1–19. doi: 10.1146/annurev.genom.8.021506.172017. [DOI] [PubMed] [Google Scholar]