Abstract
Background
Medicare expenditures for high-cost diagnostic imaging have risen faster than those for total cancer care and have been targeted for potential cost reduction. We sought to determine recent and long-term patterns in high-cost diagnostic imaging use among elderly (aged ≥65 years) patients with stage IV cancer.
Methods
We identified claims within the Surveillance, Epidemiology, and End Results (SEER)-Medicare database with computed tomography, magnetic resonance imaging, positron emission tomography, and nuclear medicine scans between January 1994 and December 2009 for patients diagnosed with stage IV breast, colorectal, lung, or prostate cancer between January 1995 and December 2006 (N = 100 594 patients). The proportion of these patients imaged and rate of imaging per-patient per-month of survival were calculated for each phase of care in patients diagnosed between January 2002 and December 2006 (N = 55 253 patients). Logistic regression was used to estimate trends in imaging use in stage IV patients diagnosed between January 1995 and December 2006, which were compared with trends in imaging use in early-stage (stages I and II) patients with the same tumor types during the same period (N = 192 429 patients).
Results
Among the stage IV patients diagnosed between January 2002 and December 2006, 95.9% underwent a high-cost diagnostic imaging procedure, with a mean number of 9.79 (SD = 9.77) scans per patient and 1.38 (SD = 1.24) scans per-patient per-month of survival. After the diagnostic phase, 75.3% were scanned again; 34.3% of patients were scanned in the last month of life. Between January 1995 and December 2006, the proportion of stage IV cancer patients imaged increased (relative increase = 4.6%, 95% confidence interval [CI] = 3.7% to 5.6%), and the proportion of early-stage cancer patients imaged decreased (relative decrease = −2.5%, 95% CI = −3.2% to −1.9%).
Conclusions
Diagnostic imaging is used frequently in patients with stage IV disease, and its use increased more rapidly over the decade of study than that in patients with early-stage disease.
More than a quarter of Medicare dollars are spent at the end of life (1). As the American population ages, Medicare enrollment is projected to double between 2000 and 2030 (2). Cancer, currently the second leading cause of death, will affect increasing numbers of people (3,4) and consume an ever greater proportion of overall medical expenditures (3,5). The annualized net costs of cancer are highest in the last year of life (4), and care delivered during this time period is intensifying (6).
Diagnostic imaging is the most rapidly growing sector of Medicare-reimbursed services (2), and among Medicare patients with cancer, imaging costs have risen at a rate outpacing total costs of care (7). Advanced imaging has expanded dramatically in both availability and usage (8). The Medicare Payment Advisory Commission (MedPAC) has designated the following types of procedures as “high-cost imaging services” (9): computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and nuclear medicine (NM). In 2008, these four procedure types accounted for 49% of Medicare physician payments for imaging (2).
Little is currently known about the use of high-cost imaging in cancer patients at the end of life. We sought to characterize imaging use in patients who presented with stage IV disease of the four most common causes of cancer death: breast, colorectal, lung, or prostate cancer. Specifically, our objectives were to describe recent practices in imaging these patients and to examine changes over time. A greater understanding of existing patterns of care in this vulnerable population is critical, given the recent attention both high-cost imaging and end-of-life care have received as potential targets for decreasing healthcare expenditures and the need to ensure maintenance or improvement in the quality of care received.
Methods
Data Source
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program encompasses cancer registries in four regions across the United States (Northeast: Connecticut, New Jersey; Midwest: Detroit, Iowa; West: San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles, greater California; South: Atlanta, rural Georgia, Kentucky, Louisiana), representing 28% of the American population. It provides detailed clinical data such as cancer stage, histology, and vital status follow-up (10). Linkage with Medicare claims enables the longitudinal study of cancer care; SEER-Medicare contains covered inpatient, outpatient, and physician-billed healthcare services for more than 3.3 million cancer patients from Medicare eligibility through death (11).
Cohort
We obtained SEER-Medicare data for patients who were diagnosed with stage IV breast, colorectal, lung, or prostate cancer, the four most common causes of cancer death, between January 1995 and December 2006 (N = 100 594 patients). We extracted claims from January 1994 to December 2009 to capture peridiagnostic events, as well as a minimum of 3 years of follow-up time. To ensure that all billable services rendered for cancer care were captured, we excluded patients with Medicare eligibility because of end-stage renal disease or disability, diagnosis based on autopsy or death certificate, diagnosis occurring before age 65 years, greater than 3-month discrepancies in SEER and Medicare-determined death dates, noncontinuous Part A (inpatient care) and Part B (medically necessary services, eg, physician services, outpatient care, durable medical equipment) Medicare coverage, and Health Maintenance Organization (HMO) coverage at any time during the study period (30 days prediagnosis through death or December 2009).
Claim Identification
We identified International Classification of Diseases, Version 9 (ICD-9; used in the inpatient SEER-Medicare file), Current Procedural Terminology (CPT; used in the outpatient and physician files), or Healthcare Common Procedure Coding System (HCPCS; used in the outpatient and physician files) billing codes corresponding to computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and nuclear medicine (NM). Each PET-CT procedure (ie, PET and CT scans performed concurrently) was counted as a single PET claim. Codes for therapeutic procedures and for imaging guidance for invasive procedures were excluded. We verified our codes and their classification into CT, MRI, PET, and NM categories with schemas published by Dinan et al. (7), the Healthcare Cost and Utilization Project (12), and the Accreditation Council for Graduate Medical Education (13); a complete list is shown in Supplementary Table 1, available online.
For the purpose of counting imaging procedures, we eliminated duplicate claims within each file by matching on patient identifier, date of procedure, CPT or HCPCS code, and provider (facility or physician identifier). We repeated this matching algorithm between files, cross-linking CPT/HCPCS to ICD-9 codes. Because outpatient claims are often missing date of service and may be submitted according to a billing cycle rather than the date of service, we extended the date criteria for this match to 7 days; over 80% of matches occurred on the same day. Among duplicates, we preferentially retained the claim billed with CPT or HCPCS codes over corresponding claims billed with ICD-9 codes because no PET-specific codes exist in the ICD-9 classification system; in the inpatient file (the only file to use the ICD-9 system), PET scans are classified under NM. Inpatient PET scans for which no corresponding claims were found in the physician file were therefore necessarily classified as NM procedures; however, only 1.1% of NM claims in our final dataset originated from the inpatient file. Finally, because a facility (inpatient or outpatient) may bill for any particular service in parallel with the providing physician, we also removed all claims with a technical component modifier, which indicates a facility-provided service.
Phases of Care
We adapted Yabroff’s (14) definitions of cancer care phases—the diagnostic phase, the continuing care phase, and the last month of life—to fit within the shortened lifespan of our patient population. Because SEER-Medicare provides only a month and year for date of diagnosis, we defined the “diagnostic phase” as a 60-day window centered around the month of diagnosis. The “continuing care phase” was defined as the time between the end of the diagnostic phase and the “last month of life” in those who died or between the diagnostic phase and the end of follow-up in those who did not. To make these phases mutually exclusive, trumping rules were applied to prioritize certain phases over others in patients who survived 90 days or less and thus could not contribute to every phase. Patients who died during the diagnostic period were excluded from all other phases, and patients who died within 30 days of the end of the diagnostic phase were included in the last month of life analyses but excluded from continuing care. When patients died less than 30 days from the beginning of a phase, we conservatively assumed that no further scans would have been performed had they lived for the entire month. For example, a patient who survived 75 days (15 days after the 60-day diagnostic period) and had five procedures (one after the diagnostic period) was categorized as having four scans during the diagnostic period (or two per month), zero during the continuing care phase, and one (rather than two) during the last month of life.
Statistical Analysis
To describe use in recent years, we counted the number of imaging procedures performed on patients diagnosed in the last five years of the study period (January 2002 to December 2006, “recent care” cohort; N = 55 253 patients), using claims submitted between January 2002 and December 2009, and calculated the proportion of all patients imaged and the per-patient per-month imaging rates, overall and stratified by procedure type, cancer type, and phase of care. In addition to reporting overall imaging rates during the last month of life, we also calculated rates among strata defined by survival (time between diagnosis and death), classified as 30–89 days, 90–179 days, 180–364 days, and 365 days or more.
To examine trends in imaging use over time, we expanded the cohort to include patients diagnosed between January 1995 and December 2006, as well as patients with stage I or II disease of the same four tumor types as their first or only cancer diagnosis (N = 192 429 patients). As the optimal rate of imaging is unknown for stage IV patients, we included this early-stage patient cohort to provide context and help inform the interpretation of the time trends data. We selected the early-stage cohort rather than the general population because its patient characteristics were similar (data not shown) and because imaging guidelines were issued for this population during the period of interest. However, because early-stage and stage IV disease differ dramatically in management, we did not seek to directly compare imaging use between the two. Rather, our intention was to examine time trends, such that each cohort’s recent use (ie, use in 2006, the last year of the study period) was judged only against its own historical control (ie, use in 1995, the starting year of the study period). As such, for each population, we present only the relative changes in the outcome (proportion of patients imaged or imaging tests per-patient per-month) between these two time points, rather than the absolute rates at any single time point or the absolute changes over time. We obtained 95% confidence intervals and two-sided P values for these trends over time, using logistic regression for the proportion of patients imaged and robust linear regression (15) for the number of procedures per patient. All P values less than .05 were considered statistically significant.
For analyses of the diagnostic and continuing care phases, patients were classified based on year of diagnosis. For analyses of the last month of life, patients were classified based on year of death. Within the stage IV cohort, time trends were further examined by imaging type. We performed a secondary analysis controlling for the demographic variables that changed in our stage IV cohort over time (cancer type; SEER registry location, categorized into Northwest, West, South, and Midwest; and Charlson index, as a measure of comorbidities); because the trends were similar, only the unadjusted results are presented here.
All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC). This study was reviewed by the Institutional Review Board at the Dana-Farber/Harvard Cancer Center.
Results
Characteristics of Patients
Table 1 presents the characteristics of the 100 594 patients who were diagnosed with stage IV breast, colorectal, lung, or prostate cancer between January 1995 and December 2006. The “recent care” cohort included 55 253 patients diagnosed with stage IV cancer between January 2002 and December 2006. Of these, 39 721 patients survived more than 30 days after the diagnostic phase and were therefore included in the continuing care phase analyses. A total of 43 366 patients had a death date within the study period (January 1995 to December 2009) and were therefore included in the last month of life analyses.
Table 1.
Characteristics of stage IV cancer patients*
| Characteristic | Patients (N=100 594) |
| Cancer site, No. (%) Breast Colorectal Lung Prostate | 6157 (6.1) 21 596 (21.5) 61 344 (61.0) 11 497 (11.4) |
| Age, median (IQR), y | 75.3 (70.5–80.6) |
| Sex, No. (%) Male Female | 56 139 (55.8) 44 455 (44.2) |
| Race, No. (%) White Black Asian Hispanic Other | 84 886 (84.4) 9474 (9.4) 2620 (2.6) 1384 (1.4) 2229 (2.2) |
| Marital status, No. (%) Single Married Separated/divorced Widowed Unknown | 8348 (8.3) 52 348 (52.0) 8259 (8.2) 28 147 (28.0) 3492 (3.5) |
| County of residency, No. (%) Urban Rural | 91 086 (90.6) 9506 (9.5) |
| Census tract income in US dollars, median (IQR) | $44 332 ($33 512–$59 266) |
| SEER region†, No. (%) Northeast Midwest West South | 21 218 (21.1) 20 826 (20.7) 42 356 (42.1) 16 194 (16.1) |
| Charlson score‡, No. (%) 0 1 2 3+ | 55 088 (58.7) 22 910 (24.4) 8602 (9.2) 7249 (7.7) |
* Patients in National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER)-Medicare, diagnosed with stage IV breast, colorectal, lung, or prostate cancer between January 1995 and December 2006.
† Northeast = Connecticut, New Jersey; Midwest = Detroit, Iowa; West = San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles, greater California; South = Atlanta, rural Georgia, Kentucky, Louisiana.
‡ The Charlson score predicts 10-year mortality based upon the presence of 22 comorbid conditions. The scores correspond to predicted 10-year mortality rates as follows: 0 = 1%; 1 = 4%; 2 = 10%; 3+ = greater than 23%.
Recent Use of High-Cost Imaging Among Stage IV Patients
Imaging use among the “recent care” cohort (diagnosed between January 2002 and December 2006) of stage IV patients is shown in Table 2. In this population, 52 985 of 55 253 (95.9%) stage IV patients underwent at least one high-cost imaging procedure (CT, MRI, PET, or NM scan) between diagnosis and death or the end of follow-up (Table 2). This proportion was similar across all four tumor types. On average, patients underwent almost 10 scans (mean [SD] = 9.79 [9.77] scans; median [10th–90th percentile] = 7 [2–21] scans) or more than 1 scan per month (mean [SD] = 1.38 [1.24] scans; median [10th–90th percentile] = 1.07 [0.19–2.95] scans), during the course of their illness (Table 3). Although many of these procedures occurred during the diagnostic phase (mean [SD] = 3.71 [2.61] scans; median [10th–90th percentile] = 3 [1–7] scans), the majority did not. Three-quarters (75.3%) of patients were scanned during the continuing care phase; on average, including patients who had no tests, patients were scanned every 6 weeks (mean [SD] = 0.68 [0.81] scan per month; median [10th–90th percentile] = 0.47 [0–1.64] scans). More than one-third (34.3%) of the patients underwent at least one high-cost imaging procedure in their last month of life. Imaging use during this phase varied with survival time, suggesting that imaging use was sensitive to prognosis. The proportion of patients imaged was higher in those who survived 3–12 months (90–179 days: 40.3%, 180–364 days: 39.4%) than in those with either shorter (30–89 days: 30.7%) or longer (≥365 days: 29.6%) survival times (data not shown).
Table 2.
High-Cost Imaging in the “Recent Care” Cohort (diagnosed between January 2002 and December 2006) of Stage IV Patients*
| Procedure type | Type of stage IV cancer, % | |||||
| Phase of care† | Breast | Colorectal | Lung | Prostate | All types | |
| All phases | n=3295 | n=11 106 | N=35 410 | n=5442 | N=55 253 | |
| CT | 92.7 | 94.1 | 95.0 | 85.3 | 93.7 | |
| MRI | 52.6 | 25.3 | 52.7 | 45.2 | 46.4 | |
| PET‡ | 26.8 | 22.6 | 32.5 | 5.5 | 27. | |
| NM | 80.6 | 33.3 | 57.4 | 86.8 | 56.9 | |
| All | 97.0 | 95.1 | 96.2 | 94.6 | 95.9 | |
| Diagnostic phase | n=3295 | n=11 106 | N=35 410 | n=5442 | N=55 253 | |
| CT | 73.6% | 85.4% | 90.5% | 54.4% | 84.9% | |
| MRI | 28.0% | 9.5% | 36.6% | 14.9% | 28.5% | |
| PET‡ | 11.5% | 7.4% | 23.6% | 1.2% | 17.4% | |
| NM | 61.6% | 17.8% | 44.3% | 66.9% | 42.2% | |
| All | 85.6% | 87.7% | 93.6% | 77.4% | 90.4% | |
| Continuing care phase | n=2667 | n=8291 | N=23 637 | n=5126 | N=39 721 | |
| CT | 73.1% | 69.6% | 67.9% | 67.3% | 68.5% | |
| MRI | 41.9% | 21.8% | 36.9% | 37.5% | 34.2% | |
| PET‡ | 24.7% | 24.0% | 20.9% | 4.8% | 19.7% | |
| NM | 58.9% | 22.7% | 30.1% | 62.2% | 34.6% | |
| All | 81.8% | 72.9% | 74.1% | 81.1% | 75.3% | |
| Last month of life | n=2472 | n=8904 | N=28 357 | n=3633 | N=43 366 | |
| CT | 26.3% | 25.9% | 31.1% | 25.7% | 29.3% | |
| MRI | 7.5% | 3.9% | 11.1% | 6.7% | 9.1% | |
| PET‡ | 0.9% | 0.8% | 1.3% | 0.2% | 1.1% | |
| NM | 7.5% | 3.6% | 6.3% | 8.3% | 6.0% | |
| All | 31.2% | 28.6% | 36.8% | 30.6% | 34.3% | |
*High-cost imaging procedures (CT, MRI, PET, and NM) have been defined by the Medicare Payment Advisory Commission. CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography; NM = nuclear medicine.
†Diagnostic Phase = 60 days centered around SEER-Medicare-provided month of diagnosis; Continuing Care Phase = time between Diagnostic Phase and Last Month of Life; Last Month of Life = 30 days before death. Trumping rules were applied such that patients who died during the diagnostic phase were excluded from all other phases, and patients who died within 30 days of the end of the diagnostic phase were included in the last month of life analyses but excluded from continuing care.
‡A small number of PET scans may be misclassified as NM studies because the International Statistical Classification of Diseases and Related Health Problems, Version 9 (ICD-9) system does not contain separate PET codes.
Table 3.
High-Cost Imaging Use Per-Patient and Per Patient Per Month in the “Recent Care” Cohort (diagnosed between January 2002 and December 2006) of Stage IV Patients*
| Phase of care (no. of patients)† | Procedure type* | Over entire phase | Per month | ||
| Mean (SD) | Median (10th–90th percentile) | Mean (SD) | Median (10th–90th percentile) | ||
| All phases of care (N=55 253) | CT | 6.81 (7.41) | 4 (1–15) | 0.97 (0.91) | 0.75 (0.09–2.11) |
| MRI | 1.23 (2.16) | 0 (0–4) | 0.18 (0.37) | 0 (0–0.57) | |
| PET‡ | 0.51 (1.22) | 0 (0–1) | 0.05 (0.12) | 0 (0–0.18) | |
| NM | 1.25 (1.89) | 1 (0–3) | 0.18 (0.31) | 0.06 (0–0.50) | |
| All | 9.79 (9.77) | 7 (2–21) | 1.38 (1.24) | 1.07 (0.19–2.95) | |
| Diagnostic phase (N=55 253) | CT | 2.47 (1.83) | 2 (0–5) | 1.24 (0.92) | 1 (0–2.50) |
| MRI | 0.47 (0.93) | 0 (1–2) | 0.23 (0.47) | 0 (0–1) | |
| PET‡ | 0.19 (0.44) | 0 (0–1) | 0.10 (0.22) | 0 (0–0.50) | |
| NM | 0.58 (0.87) | 0 (0–1) | 0.29 (0.43) | 0 (0–0.50) | |
| All | 3.71 (2.61) | 3 (1–7) | 1.85 (1.30) | 1.50 (0.50–3.50) | |
| Continuing care phase (N=39 721) | CT | 5.34 (7.80) | 3 (0–15) | 0.47 (0.61) | 0.28 (0–1.20) |
| MRI | 0.91 (2.00) | 0 (0–3) | 0.10 (0.27) | 0 (0–0.31) | |
| PET‡ | 0.43 (1.24) | 0 (0–1) | 0.03 (0.11) | 0 (0–0.11) | |
| NM | 0.85 (1.81) | 0 (0–3) | 0.07 (0.19) | 0 (0–0.21) | |
| All | 7.54 (10.07) | 4 (0–20) | 0.68 (0.81) | 0.47 (0–1.64) | |
| Last month of life (N=43 366) | CT | 0.63 (1.21) | 0 (0–2) | 0.63 (1.21) | 0 (0–2) |
| MRI | 0.14 (0.52) | 0 (0–0) | 0.14 (0.52) | 0 (0–0) | |
| PET‡ | 0.01 (0.11) | 0 (0–0) | 0.01 (0.11) | 0 (0–0) | |
| NM | 0.07 (0.32) | 0 (0–0) | 0.07 (0.32) | 0 (0–0) | |
| All | 0.85 (1.53) | 0 (0–3) | 0.85 (1.53) | 0 (0–3) | |
* High-cost imaging procedures (CT, MRI, PET, and NM) have been defined by the Medicare Payment Advisory Commission. CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography; NM = nuclear medicine.
† Diagnostic Phase = 60-days centered around SEER-Medicare-provided month of diagnosis, Continuing Care Phase = time between Diagnostic Phase and Last Month of Life, Last Month of Life = 30 days before death. Trumping rules were applied such that patients who died during the Diagnostic Phase were excluded from all other phases, and patients who died within 30 days of the end of the diagnostic phase were included in the last month of life analyses but excluded from continuing care.
‡ A small number of PET scans may be misclassified as NM studies because the International Statistical Classification of Diseases and Related Health Problems, Version 9 (ICD-9) system does not contain separate PET codes.
The most commonly performed high-cost imaging procedure was CT. Approximately 93.7% of patients had at least one CT; most had multiple CT scans (mean [SD] = 6.81 [7.41] scans; median [10th–90th percentile] = 4 [1–15] scans) and nearly one scan per month (mean [SD] = 0.97 [0.91] scan; median [10th–90th percentile] = 0.75 [0.09–2.11] scans). A substantial proportion of patients used NM (56.9% of patients), MRI (46.4% of patients), and PET (27.5% of patients). The use of different imaging modalities varied with cancer type. Relative to other patients, those with colorectal cancer were less likely to be imaged with MRI or NM studies. Prostate cancer patients rarely underwent PET scans, but a much higher proportion had NM studies than in other disease groups.
Trends in High-Cost Imaging Use Between January 1995 and December 2006
Throughout the study period (January 1995 to December 2009), nearly all stage IV patients had at least one high-cost imaging procedure, and the proportion of patients imaged at least once during the course of their cancer care (all phases) increased statistically significantly between January 1995 and December 2006 (relative increase = 4.6%, 95% CI = 3.7% to 5.6%) (Figure 1, A, left panel). A statistically significant increase in the rate of scanning per-patient per-month was observed (relative increase = 57.1%, 95% CI = 51.8% to 62.5%) (Figure 1, A, right panel). Similar increases in the proportion of stage IV patients imaged and rate of scanning per-patient per-month were seen in the diagnostic phase (Figure 1, B), continuing care phase (Figure 1, C), and the last month of life (Figure 1, D).
Figure 1.
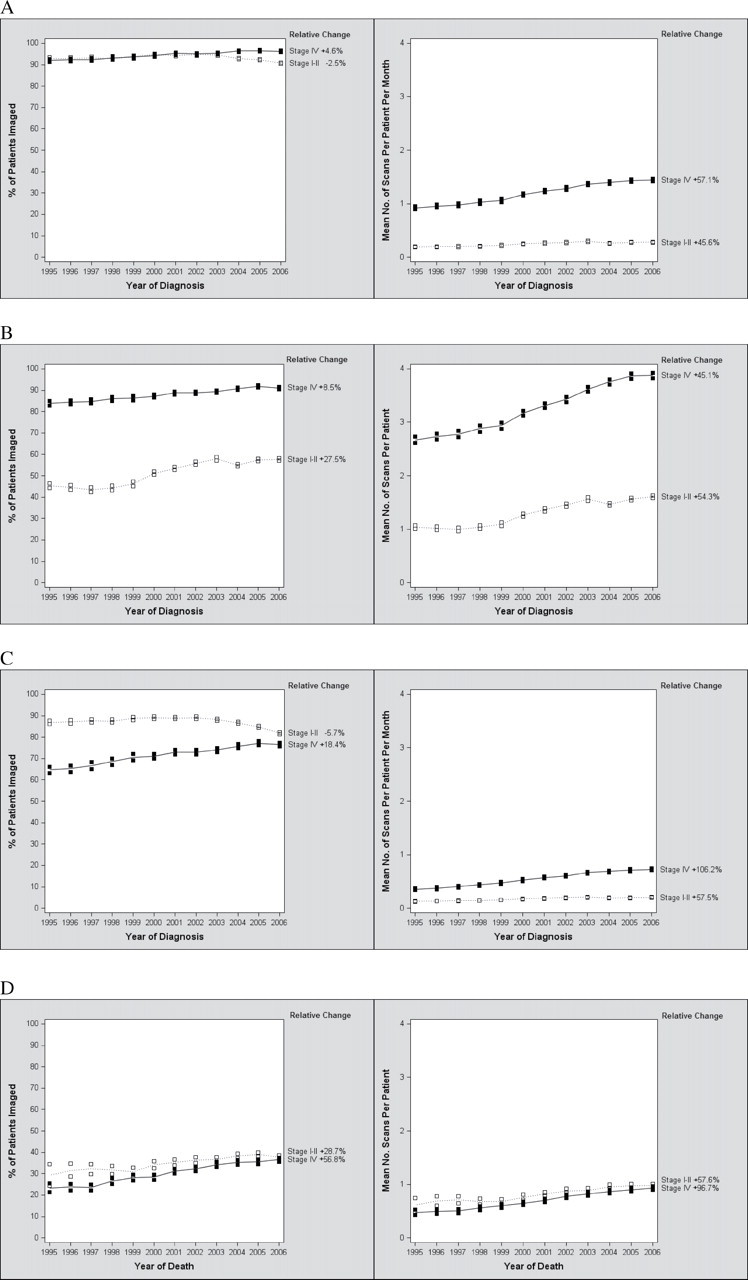
Time trends (1995–2006) in high-cost imaging for early-stage (stages I and II) and stage IV cancer patients. Relative changes (%) in high-cost imaging (CT, MRI, PET, and NM) use within each cohort between January 1995 and December 2006 are shown on the right side of each graph. Each row represents a different phase of care. A) All phases of care. B) Diagnostic phase. C) Continuing care phase. D) Last month of life. For analyses of the diagnostic and continuing care phases (A, B, and C), patients were classified based on year of diagnosis. For analyses of the last month of life (D), patients were classified based on year of death. In (A) and (C), the mean number of scans per patient was normalized per month of survival. CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography; NM = nuclear medicine; + = relative increase; − = relative decrease.
The subanalysis of the time trends by specific procedure type demonstrated an increase in the proportion of stage IV patients using PET (and PET-CT) scans between January 1995 and December 2006 (from 0.6% to 36.8%) (Figure 2, A, left panel). The use of CT and MRI during this period increased as well, in terms of both proportion of patients scanned (CT, relative increase = 8.1%, 95% CI = 6.9% to 9.4%; MRI, relative increase = 65.2%, 95% CI = 57.7% to 73.0%) (Figure 2, A, left panel) and the rate of scanning per-patient per-month (CT, relative increase = 55.7%, 95% CI = 50.1% to 61.4%; MRI, relative increase = 132.4%, 95% CI = 113.1% to 153.4%) (Figure 2, A, right panel). The use of NM procedures decreased slightly, in terms of both proportion of patients scanned (relative decrease = −13.5%, 95% CI = −16.0% to −11.0%) (Figure 2, A, left panel) and the rate of scanning per-patient per-month (relative decrease = −12.3%, 95% CI = −17.2% to −7.1%). Time trends were similar in all phases of care: diagnostic (Figure 2, B), continuing care (Figure 2, C), and last month of life (Figure 2, D). The differences (increase or decrease) in rates between 1995 and 2006 were highly statistically significant (P < .001) for every procedure type in every phase of care, whether measured by proportion of patients imaged or by number of scans per patient.
Figure 2.
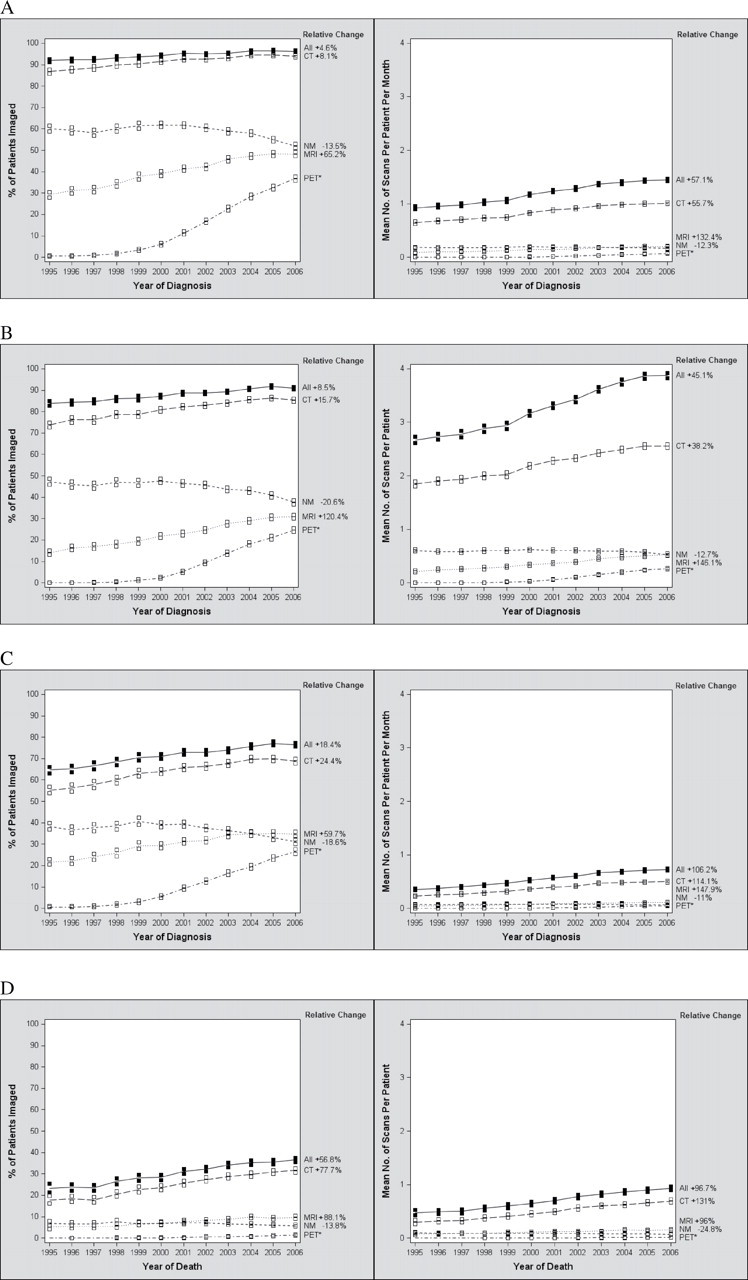
Time trends (1995–2006) in high-cost imaging modalities in stage IV cancer patients. Relative changes (%) in high-cost imaging procedures (CT, MRI, PET, or NM) between January 1995 and December 2006 are shown on the right side of each graph. Each row represents a different phase of care. A) All phases of care. B) Diagnostic phase. C) Continuing care phase. D) Last month of life. For (A), (B), (C), and (D), the left panels show the time trends in the proportion of patients imaged, analyzed using logistic regression method, and the right panels show the time trends in the number of procedures per patient, analyzed using robust linear regression method. Boxes represent 95% confidence intervals for use in each year. For analyses of the diagnostic and continuing care phases (A, B, and C), patients were classified based on year of diagnosis. For analyses of the last month of life (D), patients were classified based on year of death. In (A) and (C), the mean number of scans per patient is normalized per month of survival. Within each cohort, all two-sided P values for time trends from 1995 to 2006 were statistically significant (P trend < .001). CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography; NM = nuclear medicine; + = relative increase; − = relative decrease. *No relative increase is presented for PET scans given that the utilization rate approached 0 at the beginning of the study period.
The proportion of early-stage (stages I and II) patients undergoing at least one high-cost imaging procedure decreased overall (relative decrease = −2.5%, 95% CI = −3.2% to −1.9%) between 1995 and 2006 (Figure 1, A, left panel). The average number of scans per-person per-month increased, but at a lower rate (relative increase = 45.6%, 95% CI = 39.1% to 52.4%) than in the stage IV cohort (Figure 1, A, right panel). The diagnostic phase was the only phase in which the use of imaging grew more rapidly among early-stage patients than stage IV patients; the rate of increase was faster for early-stage patients than stage IV patients during the diagnostic phase in terms of both proportion of patients scanned (Figure 1, B, left panel) and the number of scans per patient (Figure 1, B, right panel). After this phase, the rates of imaging in stage I and II patients either declined or increased at a slower rate than in their stage IV counterparts (Figure 1, C and D).
Discussion
In a nationally representative cohort of patients with stage IV breast, colorectal, lung, and prostate cancer, we found that essentially all patients undergo frequent high-cost imaging procedures, including CT, MRI, PET, and NM, as defined by MedPAC. Imaging occurs throughout the continuum of these patients’ cancer care, and rates of imaging have steadily increased during the study period, from January 1995 to December 2006.
Unlike previous studies (7), the inclusion of SEER-provided clinical data allowed us to examine the delivery of care prospectively from diagnosis of stage IV cancer until death or the end of follow-up (3 years after diagnosis); because survival is understood to be limited in stage IV disease, our observations cannot be attributed to an inability to anticipate death, which is often a limitation of retrospectively designed end-of-life studies (16). We also build upon previous work by defining clinically relevant phases of care, as described by others in the study of cancer (14), and characterizing use of imaging within each. It may be argued that the last month of life is not definitively identifiable in a prospective manner. Despite this fact, our findings that utilization varies with both phase of care and length of survival seem to indicate that patient and physician decision-making is influenced by perceived survival. Indeed, physicians’ predictions of survival are highly correlated with actual survival (Spearman rank correlation [r 2] = 0.6, P < .001) in terminally ill cancer patients (17).
Our study has a few limitations. As previously discussed, no ICD-9 codes exist for PET scans, thus all PET claims in the inpatient file without a corresponding claim in the physician file were necessarily misclassified as NM studies. However, the effect was likely minimal, as only 1.1% of NM claims in our final dataset originated from the inpatient file. Additionally, it is not possible to determine the intent behind the procedures ordered, a known limitation of using administrative data that impacts interpretability with regards to appropriateness of high-cost imaging use. If one assumes that the rationale behind the majority of imaging during the diagnostic period is staging, the higher absolute rate of scanning in the stage IV population may reflect the ability of advanced imaging to upstage cancer diagnoses. Beyond diagnosis, scans in stage IV patients likely represent a mixture of acute symptom management, evaluations of disease progression, and assessments of treatment effect. Because scans help clinicians determine whether a change in (or cessation of) treatment is indicated, the expanding use of advanced imaging in stage IV disease is likely a manifestation of the increasing number and types of treatment options available to these patients (6,18).
Although imaging can be important in guiding such decisions, the impact of imaging on care is intimately tied to the effectiveness of the care it drives. Studies suggest that patients with cancers unresponsive to chemotherapy continue to receive it (19) and the benefit of successive lines of chemotherapy is small, even in typically responsive tumors (20–22). For the four tumor types we studied, survival in incident stage IV disease has changed modestly if at all over the past one or two decades (23–26); although treatment may be changing (and imaging with it), the natural history of metastatic disease is not. As systemic treatments evolve, the role of imaging must be continuously evaluated.
Further research is needed to better characterize the appropriate role of advanced imaging in the setting of metastatic disease and thus to support physicians in the evidence-based care of these patients. Currently, few guidelines attempt to define the appropriate role of imaging in patients with advanced disease, and those that do largely reflect expert opinion. Routine use of imaging is recommended only in patients with colorectal cancer metastatic to the liver or lung; in such patients, the National Comprehensive Cancer Network (NCCN) advises a CT or MRI every 2 months after initiating chemotherapy to reevaluate for resectability, followed by every 2–3 months during palliative chemotherapy if conversion is not accomplished (27,28). In stage IV breast (29,30), lung (31), and prostate cancer (32), the guidelines either do not specify parameters for testing or advocate use only as indicated by symptomatology.
Despite these recommendations, we found that the vast majority of patients with breast, lung, and prostate cancer undergo imaging after diagnosis at rates similar to (or higher than) that of the colorectal subset. Such discretionary decision-making, ie, the “gray” area where recommendations are equivocal or nonexistent, is known to drive higher healthcare spending (33). Indeed, we observed a great deal of variability in the use of high-cost imaging, with patients in the top 10% receiving at least one scan per month during continuing care and at least three in their last month of life. Overuse is explicitly addressed only once in the guidelines—in a recommendation against PET monitoring for stage IV colorectal disease (27,28). Nevertheless, like their breast and lung cancer counterparts, for whom no such directives exist, nearly one in four colorectal cancer patients had a PET scan during continuing care.
In contrast, over the period we studied, national guidelines defined a circumscribed role for imaging in the surveillance of patients with early-stage breast, lung, colorectal, and prostate cancer after treatment, in response to evolving evidence of its limited utility in these settings. Routine advanced imaging is now recommended only in lung (34,35) and select colon cancer patients (those who are at high risk for recurrence and are candidates for resection) (27,28,36). Correspondingly, in our cohort of patients with stage I and II disease, the frequency and intensity of imaging outside of the diagnostic period declined over time.
Finally, as the increased national focus on comparative- and cost-effectiveness research and patient-centered outcomes reaches end-of-life care, quality of life must be among the primary outcomes assessed. Imaging, although it often leads to (appropriate) palliative measures, may also distract patients from focusing on achievable end-of-life goals, require them to spend more of their limited time in medical care settings, and/or provoke anxiety (37). These procedures represent a costly, yet underappreciated and understudied aspect of care in this vulnerable population. As our approach to their care evolves, it will be important to define the role of high-cost imaging to ensure that the maximum value, in terms of both societal resources and patient quality of life, is achieved.
Funding
National Institutes of Health (RC2CA148185-01 to JCW and 2T32DK00754-12 to Richard Hodin, Department of Surgery, Massachusetts General Hospital, Boston, MA.
Notes
The authors are solely responsible for the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors have no conflicts of interest to disclose.
References
- 1. Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life Health Serv Res. 2010;45(2):565–5–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medpac. A Data Book: Healthcare Spending and the Medicare Program Washington, DC: MPAC; 2010.
- 3.NCI. Cancer Trends Progress Report—2009/2010 Update Bethesda, MD: NIH, DHHS; 2010. [Google Scholar]
- 4. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020 J Natl Cancer Inst. 2011;103(2):117–1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care J Clin Oncol. 2009;27(23):3868–38–74 [DOI] [PubMed] [Google Scholar]
- 6. Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life J Clin Oncol. 2004;22(2):315–3–21 [DOI] [PubMed] [Google Scholar]
- 7. Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006 JAMA. 2010;303(16):1625–16–31 [DOI] [PubMed] [Google Scholar]
- 8. Baker LC, Atlas SW, Afendulis CC. Expanded use of imaging technology and the challenge of measuring value Health Aff (Millwood) 2008;27(6):1467–14–78 [DOI] [PubMed] [Google Scholar]
- 9.Medpac. Report to Congress: Aligning Incentives in Medicare Washington, DC: MPAC; 2010.
- 10. NCI . About the SEER Program http://seer.cancer.gov/about/overview.html
- 11. NCI SEER-Medicare Fact Sheet. Health NIo, ed. Bethesda, MD: U.S. National Institutes of Health (NIH); 2009. [Google Scholar]
- 12. HCUP . HCUP CCS-Services and Procedures http://www.hcup-us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp#questions.A
- 13. ACGME Case Log CPT/ICD-9 List. http://www.acgme.org/ residentdatacollection/documentation/codelist.asp Accessed July 8, 2010.
- 14. Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States J Natl Cancer Inst 2008;100(9):630–6–41 [DOI] [PubMed] [Google Scholar]
- 15. Rousseeuw PJ Leroy AM Robust Regression and Outlier Detection New York, NY: Wiley; 1987.
- 16. Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest JAMA. 2004;292(22):2765–27–70 [DOI] [PubMed] [Google Scholar]
- 17. Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients BMJ. 2003;327(7408):195–19–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer J Clin Oncol. 2006;24(21):3490–349–6 [DOI] [PubMed] [Google Scholar]
- 19. Emanuel EJ, Young-Xu Y, Levinsky NG, et al. Chemotherapy use among Medicare beneficiaries at the end of life Ann Intern Med. 2003;138(8):639–6–43 [DOI] [PubMed] [Google Scholar]
- 20. Roche H, Vahdat LT. Treatment of metastatic breast cancer: second line and beyond Ann Oncol. 2011;22(5):1000–10–10 [DOI] [PubMed] [Google Scholar]
- 21. Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study J Clin Oncol. 2004;22(2):229–2–37 [DOI] [PubMed] [Google Scholar]
- 22. Massarelli E, Andre F, Liu DD, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer Lung Cancer. 2003;39(1):55––61 [DOI] [PubMed] [Google Scholar]
- 23. Morgensztern D, Waqar S, Subramanian J, et al. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005 J Thorac Oncol. 2009;4(12):1524–152–9 [DOI] [PubMed] [Google Scholar]
- 24. Cetin K, Beebe-Dimmer JL, Fryzek JP, et al. Recent time trends in the epidemiology of stage IV prostate cancer in the United States: analysis of data from the Surveillance, Epidemiology, and End Results Program Urology. 2010;75(6):1396–1–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang K, Korn JR, Lee DW, et al. Factors associated with improved survival among older colorectal cancer patients in the US: a population-based analysis BMC Cancer. 2009;9:227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer J Clin Oncol. 2008;26(30):4891–489–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NCCN NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Fort Washington, PA: NCCN. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.Accessed February 25, 2011
- 28. NCCN NCCN Clinical Practice Guidelines in Oncology: Rectal Cancerhttp://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.Accessed February 25, 2011
- 29. NCCN NCCN Clinical Practice Guidelines in Oncology: Breast Cancerhttp://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.Accessed March 25, 2011
- 30. Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting J Clin Oncol. 2006;24(31):5091–509–7 [DOI] [PubMed] [Google Scholar]
- 31. Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003 J Clin Oncol. 2004;22(2):330–3–53 [DOI] [PubMed] [Google Scholar]
- 32. NCCN NCCN Clinical Practice Guidelines in Oncology: Prostate Cancerhttp://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.Accessed June 21, 2011.
- 33. Sirovich B, Gallagher PM, Wennberg DE, et al. Discretionary decision making by primary care physicians and the cost of U.S. health care Health Aff (Millwood). 2008;27(3):813–8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. NCCN NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancerhttp://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.AccessedJanuary 7, 2011
- 35. NCCN NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancerhttp://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.Accessed June 23, 2011
- 36. Desch CE, Benson AB III, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline J Clin Oncol. 2005;23(33):8512–851–9 [DOI] [PubMed] [Google Scholar]
- 37. Loprinzi CL, Hayes D, Smith T. Doc, shouldn’t we be getting some tests? J Clin Oncol 2003;21(suppl 9):108s––111s [DOI] [PubMed] [Google Scholar]


