Abstract
Background
Basal cell carcinoma (BCC) is the most common malignancy in the United States. Ionizing radiation is an established risk factor in certain populations, including cancer survivors. We quantified the association between ionizing radiation dose and the risk of BCC in childhood cancer survivors.
Methods
Participants in the Childhood Cancer Survivor Study who reported a BCC (case subjects, n = 199) were matched on age and length of follow-up to three study participants who had not developed a BCC (control subjects, n = 597). The radiation-absorbed dose (in Gy) to the BCC location was calculated based on individual radiotherapy records using a custom-designed dosimetry program. Conditional logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between demographic and treatment factors, therapeutic radiation dose, and surrogate markers of sun sensitivity (skin and hair color) and the risk of BCC. A linear dose–response model was fitted to evaluate the excess odds ratio per Gy of radiation dose.
Results
Among case subjects, 83% developed BCC between the ages of 20 and 39 years. Radiation therapy, either alone or in combination with chemotherapy, was associated with an increased risk of BCC compared with no chemotherapy or radiation. The odds ratio for subjects who received 35 Gy or more to the skin site vs no radiation therapy was 39.8 (95% CI = 8.6 to 185). Results were consistent with a linear dose–response relationship, with an excess odds ratio per Gy of 1.09 (95% CI = 0.49 to 2.64). No other treatment variables were statistically significantly associated with an increased risk of BCC.
Conclusions
Radiation doses to the skin of more than 1 Gy are associated with an increased risk of BCC.
In the United States, an estimated 14 000 children younger than 20 years are diagnosed annually with a first primary cancer (1). The decline in cancer mortality due to advances in cancer treatment over the past 30 years has resulted in a large and increasing population of long-term cancer survivors; currently, there are more than 352 000 pediatric cancer survivors in the United States (2). These individuals are at risk for many late effects of the therapy they received for their initial cancer, including subsequent primary malignancies. Previous research within the Childhood Cancer Survivor Study (CCSS) demonstrated an excess risk of subsequent primary neoplasms for childhood survivors of all primary diagnoses compared to the general population (3). In addition, as childhood cancer survivors progress through adulthood, this excess risk of subsequent neoplasms continues to increase with a cumulative incidence at 30 years of 7.9% (95% confidence interval [CI] = 7.2% to 8.5%) for second malignant neoplasms (excluding nonmelanoma skin cancer [NMSC]) and 9.1% (95% CI = 8.1% to 10.1%) for NMSC (3).
Skin cancer is the most common malignancy in the United States, and basal cell carcinomas (BCCs) account for 75% of the 1 million NMSCs diagnosed annually (4,5). Although 95% of BCCs are cured with surgery, the cosmetic outcome is often unsatisfactory and sometimes disfiguring (6). The predominant risk factor for NMSC is sun exposure (7). Exposure to ionizing radiation is also an established risk factor for NMSC (8–10). For example, survivors of the atomic bombing of Hiroshima and Nagasaki (8,10) and patients treated for tinea capitis with radiation (9,11) were found to be at increased risk of developing NMSCs, of which 50% and 98%, respectively, were BCCs. In a study of stem cell transplant recipients, those who underwent a conditioning regimen that included total body irradiation had an absolute excess risk of 24 cases of BCC per 10 000 person-years compared with those who did not receive total body irradiation prior to transplant (12).
A previous analysis of 213 CCSS participants who developed NMSC found that white race, a family history of skin cancer, longer follow-up, history of radiation therapy, and older age at initial cancer diagnosis were associated with an increased risk of BCC (13); however, the radiation dose–response relationship was not quantified. This study was designed to quantify the risks of BCC associated with therapeutic ionizing radiation doses within the CCSS. We also evaluated contributions of other factors, such as chemotherapy exposure, sun sensitivity, and sex, to the risk of BCC.
Methods
Study Population
This study was conducted as part of the CCSS, a multicenter National Institutes of Health–funded retrospective cohort study of childhood cancer survivors established in 1994 (14,15). Eligibility criteria for the CCSS are: 1) diagnosis of leukemia, central nervous system cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, kidney tumor, neuroblastoma, soft tissue sarcoma, or bone cancer; 2) age at diagnosis younger than 21 years; 3) diagnosis and initial treatment occurred between January 1, 1970, and December 31, 1986, at one of 26 participating institutions in the United States and Canada; and, 4) survival of more than 5 years after diagnosis. The details of the study design and descriptions of the cohort have been published previously (15). The CCSS protocol and contact documents were reviewed and approved by the institutional review boards at each participating site.
Collection of Treatment Information and Follow-up Surveys
Of the 20 276 childhood cancer survivors who met the eligibility criteria, 14 370 (or their next of kin for those known to have died) completed the self-administered baseline questionnaire in 1996. Participants were sent a consent form to sign and return with the survey. According to institutional review board regulations, response to questions on the baseline survey was considered implied consent.
The questionnaire elicited information on demographic characteristics, personal health habits, family history of cancer, and the development of subsequent malignancies. In 2001 and 2003, subsequent follow-up surveys were mailed to cohort members who responded to the baseline questionnaire. Occurrence of NMSC was ascertained through self-report via the baseline questionnaire and the 2001 follow-up survey. For each reported NMSC, we attempted to obtain pathological confirmation by coordination with the CCSS Biopathology Center at Nationwide Children’s Hospital (Columbus, OH). The 2003 follow-up survey solicited information on sun sensitivity using questions about skin color and hair color. An ancillary survey distributed with the 2003 follow-up survey obtained specific information about the type of skin cancer, their anatomical location, and the date of physician-conducted skin examinations from survivors who reported NMSCs on the 2001 follow-up survey. The surveys are available at http://ccss.stjude.org.
The medical records for 12 858 participants who gave consent and who completed the baseline questionnaire were abstracted using a standard protocol. Details of the medical record abstraction methods have been published previously (15), and abstraction forms are available at http://ccss.stjude.org. Information was collected on chemotherapy, including specific agents, cumulative doses, and dates and routes of administration. Exposures to anthracyclines and alkylating agents were expressed as scores that were determined as follows: for each individual agent, we determined the tertiles of the cumulative dose for all subjects who received the agent (expressed as 0 [no agent], 1 [lowest tertile], 2, or 3 [highest tertile]). The tertiles were then summed for all of the agents in each of the two chemotherapy categories to provide scores that were used in the regression model. Radiation therapy records were photocopied and sent to the CCSS Radiation Dosimetry Center at The University of Texas M.D. Anderson Cancer Center where they were reviewed and abstracted. Dates of radiation therapy and information on beam energy, field location and size, use of radiation field blocks, age at treatment, and total dose from each field were obtained for all case and control subjects.
Case and Control Subjects
Potentially eligible case subjects included 257 childhood cancer survivors in the CCSS cohort who reported an occurrence of NMSC. Of these, 19 subjects self-reported a BCC specifically but did not have pathological confirmation and were included in this study. Forty-two subjects were excluded because they did not have a documented BCC on either the pathology report or the self-report from the ancillary survey, and three subjects were excluded because they had Gorlin syndrome, an autosomal dominant syndrome in which patients typically develop multiple BCCs (16). Case subjects who did not have treatment information available in the medical record (n = 13) were also excluded from this analysis, leaving 199 case subjects who were eligible for inclusion in this nested case–control study.
Each case subject was matched to three control subjects from the CCSS cohort; control subjects were defined as childhood cancer survivors with available medical records who had not developed a BCC by the same follow-up time after BCC diagnosis for the case subject. Control subjects were matched to case subjects on age at original cancer diagnosis (within 5 years) and length of follow-up. The follow-up period was defined as time (in days) from the date of CCSS cohort entry (≥5 years after childhood cancer diagnosis) to the date of the first BCC diagnosis (for case subjects), death, or completion of the last questionnaire, whichever occurred first. For the 128 case subjects who were diagnosed with multiple BCCs during the study period, we used the first BCC that was diagnosed for analysis and control subject selection. For case subject with synchronous BCC diagnoses (N = 55), defined as multiple occurrences within 60 days, we randomly selected one BCC for analysis.
Radiation Dosimetry
For patients undergoing radiation therapy, all parts of their skin will be exposed to some radiation, either from the primary radiation beam (where doses will typically be the highest) or from scattered radiation. The dose to the skin is substantially different from the dose prescribed to the primary tumor. Although the skin dose within the primary radiation field can be 50% or more of the tumor dose, it can also be as low as 5% or less, depending on treatment parameters, such as energy and field size (18). Outside the treatment field, the skin dose is typically less than a few percent of the tumor dose, but can exceed 10% of the tumor dose close to the field edge.
For this study, we used the radiation dose to the patient’s skin surface at the location of the BCC (or the matched location for the control subjects). Because skin surface doses are not available in patient records and are not easily determined from treatment doses, we determined the dose for each patient with the use of a dosimetry program designed specifically to estimate dose to the skin (18). This dosimetry program was previously validated using thermoluminescent dosimeters (TLD–100, LiF powder) on anthropomorphic phantoms. On average, the accuracy of the dosimetry program for calculating the skin dose was ±22% (18).
The dose to the site of the BCC for each treatment field was calculated based on the treatment dose, field size, beam energy, and beam modifiers. The specific approach used to determine the skin dose varied with location of the BCC relative to the treatment field and the surface containing the BCC. In the basis of the dosimetry system and the precision of the BCC location, the BCC site was classified as being: 1) in the field; 2) on the edge of the field; 3) under a treatment block; 4) near the field (≤3cm from the field edge); or 5) outside of the field (>3cm from the field edge). The surface containing the BCC was identified as an anterior, lateral, or posterior surface. For each case subject and their matched control subjects, we summed the doses from all fields to give a total dose to the BCC site. Dosimetry information was assigned one quality score that was based on the completeness of the radiotherapy record, ranging from 1 (complete record) to 4 (no record), and a second quality score that was based on the precision of the BCC location, ranging from 1 (very certain) to 4 (completely uncertain).
Statistical Methods
We used conditional logistic regression to calculate odds ratios (ORs) and 95% confidence intervals for the associations between demographic, clinical, and treatment variables and the risk of BCC. We first evaluated univariate models for each covariate and then developed multivariable regression models to estimate associations between treatment and BCC adjusted for confounding variables. Variables that were statistically significantly different between case subjects and control subjects at the .05 level were retained, as were covariates that were confounders (ie, those that altered the odds ratio by 10% or more). Due to the high collinearity between the original childhood cancer diagnosis and radiation variables, diagnosis was dichotomized as Hodgkin lymphoma vs other cancers. All models were adjusted a priori for sex and race (non–Hispanic white vs other).
We developed additional models to determine the most appropriate shape of the relationship between radiation dose and the risk of BCC (Supplementary Table 1, available online). We used nonlinear optimization procedures to maximize the log likelihood and to generate model parameter estimates (19). We fit a variety of models, including linear, quadratic, and exponential terms, to examine the possibility of an increasing dose response at low to moderate doses and a decreasing dose response at high doses due to cell killing. In an additional attempt to detect a leveling of risk with increasing dose, we fit a series of models using plateaus that began between 20 and 50 Gy, tested at 1-Gy increments, with 40 Gy resulting in lowest model deviance. A restricted cubic spline with three knots also was included as a flexibly shaped statistical model. All nonlinear models were adjusted for covariates in the baseline model (ie, sex, race [non–Hispanic white vs other], year of first primary diagnosis [1970–1973, 1974–1977, 1978–1981, or 1982–1986], and diagnosis of Hodgkin lymphoma [yes or no]).
Statistical comparisons between nested dose–response models were based on likelihood ratio tests, and the Akaike information criterion (20) was used for nonnested comparisons. All nonlinear models were superior to the categorical model but similar to each other in terms of the Akaike information criterion. None of the models that allowed for upward or downward curvature improved the fit compared with that of the linear model, based on the likelihood ratio test; therefore, we chose the linear dose–response model because it gave the best fit and was the most parsimonious, and we based all further modeling on it. The linear model corresponds to excess odds ratio (EOR) = β × D, in which excess odds ratio is the odds ratio minus 1; D is radiation dose in Gy; and β is the excess odds ratio for BCC per Gy.
We assessed the modifying effects of covariates on the radiation dose response by testing each demographic and chemotherapy covariate in separate models using an exponential term in the model of form: EOR = β × D × exp(γY), where Y represents potential modifying covariate and γ represents the change in the EOR for BCC per Gy due to different levels of the covariate(s) represented by Y. The covariate was also added to the baseline model to ensure that the modification effect of the covariate was clearly distinguished from any main effect of the covariate. If a covariate appeared to modify the radiation effect, additional models were assessed within the strata of that covariate. The models were based on 171 case subjects with complete information on radiation treatment, sex, race, year of diagnosis, and procarbazine dose and their matched control subjects. Because of missing data, not all case subjects were matched to three control subjects for the analysis (29 case subjects had two matched control subjects, and five case subjects had one matched control subject). In a subanalysis that included the 162 case patients and matched control subjects who also completed the 2003 follow-up survey, we assessed the sun sensitivity variables (hair color and skin color) as possible modifiers of the radiation dose response.
We could not assess the effect of anatomical location of the BCC on the radiation dose response in these models because the radiation dose for each control subject was computed at the same anatomical location as the BCC in the matched case patient. However, we conducted modeling of the linear dose response by stratifying on BCC location, using head or face vs other locations.
All P values are two-sided and were considered statistically significant at an alpha of .05.
Results
Table 1 displays characteristics of the 199 case subjects and 597 matched control subjects. The majority of case and control subjects reported their race as white. The median age at BCC diagnosis was 31 years (range = 11–46 years), and 83% of case subjects were diagnosed with their first BCC between the ages of 20 and 39 years. The median time from first primary cancer diagnosis to BCC diagnosis was 18.2 years (range 5.2–29.6 years). The distribution of first primary cancers was comparable in case and control subjects with two exceptions: 50% of case subjects had an initial diagnosis of Hodgkin lymphoma compared with 24% of control subjects, and only 10% of case subjects had an initial diagnosis of Wilms tumor, soft tissue sarcoma, bone cancer, or neuroblastoma compared with 32% of control subjects. In a multivariable analysis that adjusted for sex, race, year of diagnosis, initial diagnosis of Hodgkin lymphoma, and linear radiation dose response, we found that earlier calendar year of the first primary diagnosis was associated with statistically significantly reduced risk of BCC (OR for 1970–1973 vs 1982–1986 = 0.4, 95% CI = 0.2 to 0.9), whereas later calendar years were not (OR for 1974–1977 vs 1982–1986 = 0.6, 95% CI = 0.3 to 1.2; OR for 1978–1981 vs 1982–1986 = 0.6, 95% CI = 0.3 to 1.3). A first primary diagnosis of Hodgkin lymphoma was associated with a non–statistically significant increased risk of developing a BCC compared with a first primary diagnosis of bone, kidney, or soft tissue cancer or neuroblastoma (OR = 2.2, 95% CI = 1.0 to 4.9). Case and control subjects had similar distributions of skin color and hair color as reported on the follow-up 2003 survey. In multivariable analyses, hair color and skin color—markers of sensitivity to UV radiation—were associated with the risk of BCC. Compared with black or dark brown, light or medium brown hair was associated with a statistically significant increased risk of BCC (OR = 3.1, 95% CI = 1.5 to 6.4), and blond hair and red hair were associated with non–statistically significant increased risks (blond hair: OR = 2.6, 95% CI = 1.0 to 6.9; red hair: OR = 1.6, 95% CI = 0.6 to 4.6). Compared with light tan, brown, olive, or dark brown skin color, very light brown, freckled skin color was associated with a statistically significantly increased risk of BCC (OR = 2.7, 95% CI = 1.3 to 5.6), and white skin color was associated with a non–statistically significant increased risk (OR = 1.8, 95% CI = 0.9 to 3.7). Eye color was not statistically significantly associated with the risk of BCC (data not shown).
Table 1.
Demographic and clinical characteristics of BCC case subjects and control subjects, the Childhood Cancer Survivor Study*
| Characteristics | Case subjects (n = 199), N (%) | Control subjects (n = 597), N (%) | OR† (95% CI) | OR‡ (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 106 (53.3) | 285 (47.7) | 1.0 (referent) | – | ||||
| Female | 93 (46.7) | 312 (52.3) | 0.9 (0.6 to 1.5) | – | ||||
| Race | ||||||||
| White | 182 (91.5) | 533 (89.3) | 1.8 (0.8 to 4.3) | – | ||||
| Other | 17 (8.0) | 64 (10.7) | 1.0 (referent) | – | ||||
| First primary cancer diagnosis | ||||||||
| Wilms tumor, soft tissue sarcoma, bone cancer, or neuroblastoma | 20 (10.1) | 191 (32.0) | – | 1.0 (referent) | ||||
| Central nervous system cancer | 17 (8.5) | 59 (9.9) | – | 2.3 (0.9 to 5.9) | ||||
| Hodgkin lymphoma | 100 (50.3) | 144 (24.1) | – | 2.2 (1.0 to 4.9) | ||||
| Leukemia | 48 (24.1) | 151 (25.3) | – | 1.5 (0.7 to 3.2) | ||||
| Non–Hodgkin lymphoma | 14 (7.0) | 52 (8.7) | – | 1.8 (0.7 to 4.8) | ||||
| Year of first primary cancer diagnosis | ||||||||
| 1970–1973 | 59 (29.6) | 196 (32.8) | 0.4 (0.2 to 0.9)§ | – | ||||
| 1974–1977 | 65 (32.7) | 186 (31.2) | 0.6 (0.3 to 1.2) | – | ||||
| 1978–1981 | 44 (22.1) | 145 (24.3) | 0.6 (0.3 to 1.3) | – | ||||
| 1982–1986 | 31 (15.6) | 70 (11.7) | 1.0 (referent) | – | ||||
| Age at first primary cancer diagnosis||, y | ||||||||
| 0–4 | 32 (16.1) | 96 (16.1) | – | – | ||||
| 5–9 | 28 (14.1) | 84 (14.1) | – | – | ||||
| 10–14 | 47 (23.6) | 141 (23.6) | – | – | ||||
| 15–20 | 92 (46.2) | 276 (46.2) | – | – | ||||
| Age at BCC diagnosis, y | ||||||||
| 10–19 | 13 (6.5) | 48 (8.0) | – | – | ||||
| 20–29 | 61 (30.7) | 197 (33.0) | – | – | ||||
| 30–39 | 105 (52.8) | 297 (49.7) | – | – | ||||
| 40–50 | 20 (10.1) | 55 (9.2) | – | – | ||||
| Time from first primary cancer diagnosis to BCC diagnosis||, y | ||||||||
| 5–14 | 54 (27.1) | – | – | – | ||||
| 15–19 | 76 (38.2) | – | – | – | ||||
| 20–24 | 44 (22.1) | – | – | – | ||||
| 25–29 | 25 (12.6) | – | – | – | ||||
| Location of BCC | ||||||||
| Abdomen | 9 (4.5) | – | – | – | ||||
| Arm | 2 (1.0) | – | – | – | ||||
| Back | 41 (20.6) | – | – | – | ||||
| Chest | 42 (21.1) | – | – | – | ||||
| Head or face | 69 (34.7) | – | – | – | ||||
| Leg | 3 (1.5) | – | – | – | ||||
| Neck | 29 (14.6) | – | – | – | ||||
| Pelvis | 3 (1.5) | – | – | – | ||||
| Unknown | 1 (0.5) | – | – | – | ||||
| Skin color¶ | ||||||||
| Pale or milky white | 91 (48.4) | 200 (41.7) | 1.8 (0.9 to 3.7) | – | ||||
| Very light brown, sometimes freckles | 67 (35.6) | 148 (30.8) | 2.7 (1.3 to 5.6) | – | ||||
| Light tan, brown, olive, or dark brown | 30 (16.0) | 126 (26.3) | 1.0 (referent) | – | ||||
| Unknown | 0 (0) | 6 (1.3) | – | – | ||||
| Hair color¶ | ||||||||
| Light blond or blond | 19 (10.1) | 50 (10.5) | 2.6 (1.0 to 6.9) | – | ||||
| Strawberry blond, red, or red-brown | 16 (8.5) | 42 (8.7) | 1.6 (0.6 to 4.6) | – | ||||
| Light brown or medium brown | 121 (64.4) | 265 (55.2) | 3.1 (1.5 to 6.4) | – | ||||
| Dark brown or black | 31 (16.5) | 116 (24.2) | 1.0 (referent) | – | ||||
| Unknown | 1 (0.5) | 7 (1.5) | – | – | ||||
*OR = odds ratio; CI = confidence interval; BCC = basal cell carcinoma; – = not applicable.
†Multivariable analysis; model adjusted for sex, race, year of diagnosis, radiation dose, and Hodgkin lymphoma diagnosis.
‡Multivariable analysis; model adjusted for sex, race, year of diagnosis, and radiation dose.
§P trend = .20 (two-sided).
||Indicates matching variable.
¶Sun sensitivity descriptors as listed in the 2003 follow-up survey. Number of respondents to 2003 survey: 188 case subjects, 480 control subjects. Each sun sensitivity variable was assessed in a separate risk model.
The anatomical location of the BCC aligned with the first primary cancer that was diagnosed. For example, the majority of BCCs in survivors of Hodgkin lymphoma (80%) were located on the back, chest, or neck, which were the most common radiation treatment fields; BCCs of the head and face (n = 69) occurred more frequently in survivors of leukemia (67%) and central nervous system tumors (76%), which are diagnoses that typically receive brain irradiation, than in survivors of Hodgkin lymphoma (12%), who typically do not receive brain irradiation (data not shown).
Table 2 presents the treatment characteristics of case and control subjects and their associations with the risk of BCC from models that adjusted for confounders. Radiation therapy, either alone or in combination with chemotherapy, was associated with an increased risk of BCC compared with no chemotherapy or radiation. Among case subjects, the average radiation dose to the skin site was16.0 Gy; the average radiation dose to the corresponding site for matched control subjects was 5.9 Gy. The multivariable model for radiation dose included the first primary cancer diagnosis (dichotomized to Hodgkin lymphoma vs other), sex, race, and year of diagnosis of the first primary cancer. Radiation doses of 1 Gy or higher were associated with an increased risk of BCC. The linear dose–response model demonstrated an increase in the excess odds ratio per Gy of 1.09 (95% CI = 0.49 to 2.64). The odds ratio for subjects who received 35 Gy or more to the skin site relative to those who received no radiation therapy was 39.8 (95% CI = 8.6 to 185). With regard to BCC proximity to the radiation treatment field, the highest risks of BCC were for locations within or on the edge of the treatment field. In addition, we found that the odds ratio for BCC was increased for locations that were outside but near <3cm) the treatment field (OR = 3.1, 95% CI = 1.1 to 9.2) as well as for locations that had been under a treatment block (OR = 5.6, 95% CI = 1.7 to 18.5) compared with that in subjects who did not receive radiation therapy. Although these locations were shielded from the primary beam, they nevertheless received some scattered radiation dose. No other treatment variables were associated with an increased risk of BCC.
Table 2 .
Treatment characteristics of BCC case subjects and control subjects*
| Characteristics | Case subjects (n = 199), N (%) | Control subjects (n = 597), N (%) | OR† (95% CI) | OR‡ (95% CI) | OR§ (95% CI) | P trend|| | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiation and/ or chemotherapy | – | |||||||||||||
| No chemotherapy or radiation | 4 (2.0) | 65 (10.9) | 1.0 (referent) | |||||||||||
| Radiation only | 59 (29.6) | 119 (19.9) | 4.3 (1.4 to 13.3) | |||||||||||
| Chemotherapy only | 7 (3.5) | 113 (18.9) | 0.7 (0.2 to 2.7) | |||||||||||
| Radiation and chemotherapy | 129 (64.8) | 295 (49.4) | 4.1 (1.4 to 11.6) | |||||||||||
| Unknown | 0 (0.0) | 5 (0.8) | – | |||||||||||
| Radiation dose to BCC site, Gy | <.001 | |||||||||||||
| None | 11 (5.5) | 178 (29.8) | 1.0 (referent) | |||||||||||
| 0.01–0.9 | 10 (5.0) | 141 (23.6) | 1.1 (0.4 to 2.8) | |||||||||||
| 1–4.9 | 18 (9.0) | 64 (10.7) | 3.6 (1.4 to 9.1) | |||||||||||
| 5–14.9 | 55 (27.6) | 83 (13.9) | 11.7 (4.9 to 27.9) | |||||||||||
| 15–24.9 | 45 (22.6) | 56 (9.4) | 14.9 (6.0 to 37.3) | |||||||||||
| 25–34.9 | 26 (13.1) | 26 (4.4) | 22.2 (7.5 to 65.8) | |||||||||||
| 35–63.3 | 14 (7.0) | 10 (1.7) | 39.8 (8.6 to 185) | |||||||||||
| Unknown | 20 (10.1) | 39 (6.5) | – | |||||||||||
| Proximity of BCC to treatment field¶ | <.001 | |||||||||||||
| No radiation | 11 (5.5) | 178 (29.8) | 1.0 (referent) | |||||||||||
| Outside treatment field (≥3cm) | 22 (11.1) | 178 (29.8) | 1.5 (0.7 to 3.5) | |||||||||||
| Near treatment field (<3cm) | 10 (5.0) | 39 (6.5) | 3.1 (1.1 to 9.2) | |||||||||||
| Under block | 16 (8.0) | 24 (4.0) | 5.6 (1.7 to 18.5) | |||||||||||
| On the edge of treatment field | 23 (11.6) | 30 (5.0) | 12.5 (4.3 to 36.5) | |||||||||||
| In treatment field | 98 (49.2) | 111 (18.6) | 14.5 (6.3 to 33.2) | |||||||||||
| Not enough information | 19 (9.5) | 37 (6.2) | – | |||||||||||
| Any alkylating agent: | – | |||||||||||||
| No | 90 (45.2) | 314 (52.6) | 1.0 (referent) | |||||||||||
| Yes | 109 (54.8) | 280 (46.9) | 1.1 (0.7 to 1.8) | |||||||||||
| Unknown | 0 (0.0) | 3 (0.5) | – | |||||||||||
| Dose of procarbazine, mg/m2 | .76 | |||||||||||||
| None | 146 (73.4) | 517 (86.6) | 1.0 (referent) | |||||||||||
| 1–3999 | 12 (6.0) | 10 (1.7) | 1.6 (0.5 to 4.5) | |||||||||||
| ≥4000 | 30 (15.1) | 46 (7.7) | 0.9 (0.4 to 1.8) | |||||||||||
| Unknown | 11 (5.5) | 24 (4.0) | – | |||||||||||
| Dose of IV cyclophosphamide, mg/m2 | .23 | |||||||||||||
| None | 145 (72.9) | 384 (64.3) | 1.0 (referent) | |||||||||||
| 1–3999 | 21 (10.6) | 62 (10.4) | 0.9 (0.5 to 1.8) | |||||||||||
| 4000–9999 | 12 (6.0) | 70 (11.7) | 0.5 (0.2 to 1.3) | |||||||||||
| 10 000–19 999 | 10 (5.0) | 49 (8.2) | 0.5 (0.2 to 1.4) | |||||||||||
| 20 000–39 999 | 4 (2.0) | 9 (1.5) | 1.7 (0.2 to 12.5) | |||||||||||
| Unknown | 7 (3.5) | 23 (3.9) | – | |||||||||||
| Dose of oral cyclophosphamide, mg/m2 | .69 | |||||||||||||
| None | 185 (93.0) | 552 (92.5) | 1.0 (referent) | |||||||||||
| 1–3999 | 5 (2.5) | 7 (1.2) | 1.3 (0.3 to 5.0) | |||||||||||
| ≥4000 | 5 (2.5) | 30 (5.1) | 0.7 (0.2 to 2.2) | |||||||||||
| Unknown | 4 (2.0) | 8 (1.3) | – | |||||||||||
| Alkylating agent score # | .62 | |||||||||||||
| 0 | 90 (45.2) | 314 (52.6) | 1.0 (referent) | |||||||||||
| 1 | 24 (12.1) | 91 (15.2) | 1.0 (0.5 to 2.0) | |||||||||||
| 2 | 28 (14.1) | 71 (11.9) | 1.5 (0.8 to 3.1) | |||||||||||
| 3 | 31 (15.6) | 71 (11.9) | 0.7 (0.3 to 1.3) | |||||||||||
| Unknown | 26 (13.1) | 50 (8.4) | – | |||||||||||
| Any anthracycline | – | |||||||||||||
| No | 145 (72.9) | 405 (67.8) | 1.0 (referent) | |||||||||||
| Yes | 54 (27.1) | 189 (31.7) | 1.3 (0.8 to 2.1) | |||||||||||
| Unknown | 0 (0.0) | 3 (0.5) | – | |||||||||||
| Anthracycline score # | .63 | |||||||||||||
| 0 | 145 (72.9) | 405 (67.8) | 1.0 (referent) | |||||||||||
| 1 | 19 (9.5) | 40 (6.7) | 2.3 (1.0 to 5.3) | |||||||||||
| 2 | 18 (9.0) | 57 (9.5) | 1.3 (0.6 to 2.8) | |||||||||||
| 3 | 12 (6.0) | 74 (12.4) | 0.9 (0.4 to 2.4) | |||||||||||
| Unknown | 5 (2.5) | 21 (3.5) | – | |||||||||||
| Prednisone | – | |||||||||||||
| No | 94 (47.2) | 345 (57.8) | 1.0 (referent) | |||||||||||
| Yes | 105 (52.8) | 249 (41.7) | 1.2 (0.7 to 1.8) | |||||||||||
| Unknown | 0 (0.0) | 3 (0.5) | – | |||||||||||
| Second cancer prior to BCC** | – | |||||||||||||
| No | 187 (94.0) | 569 (95.3) | 1.0 (referent) | |||||||||||
| Yes | 12 (6.0) | 28 (4.7) | 1.4 (0.5 to 3.6) | |||||||||||
*OR = odds ratio; CI = confidence interval; BCC = basal cell carcinoma; IV = intravenous; – = not applicable.
†Multivariable analysis; model adjusted for sex, race, and Hodgkin lymphoma diagnosis.
‡Multivariable analysis; model adjusted for sex, race, Hodgkin lymphoma diagnosis, and year of first cancer diagnosis.
§Multivariable analysis; model adjusted for sex, race, radiation dose, and Hodgkin lymphoma diagnosis.
||P trend based on one-sided χ2 test with listed covariate included as continuous increasing variable.
¶The proximity of the radiation field for the case subject was set to the proximity of the radiation field that had the highest contribution to the BCC site or matched location in the control subject.
#Score corresponds to tertiles of the cumulative dose for all listed agents. Alkylating agents include carmustine, busulfan, lomustine, chlorambucil, cyclophosphamide, dacarbazine, ifosfamide, melphalan, nitrogen mustard, procarbazine, and thiotepa; anthracyclines include daunorubicin, doxorubicin, and idarubicin.
**Among case subjects: melanoma (n = 4), breast cancer (n = 3), central nervous system cancer (n = 1), thyroid cancer (n = 1), bone cancer (n = 1), lymphoma (n = 1), and squamous cell carcinoma (n = 1). Among control subjects: melanoma (n = 1), breast cancer (n = 10), central nervous system cancer (n = 1), thyroid cancer (n = 5), lymphoma (n = 5), and soft tissue sarcoma (n = 6).
The radiation doses described here primarily encompassed treatment the subjects received within 5 years after their first primary cancer diagnosis. Although the CCSS does capture information on subsequent malignancies that could result in additional radiation treatment, it does not generally capture the treatment for these cancers. However, survivors who reported being diagnosed with a second cancer before the BCC diagnosis did not show an increased risk of BCC compared with survivors with no second cancer prior to BCC (OR = 1.4, 95% CI = 0.5 to 3.6) (Table 2).
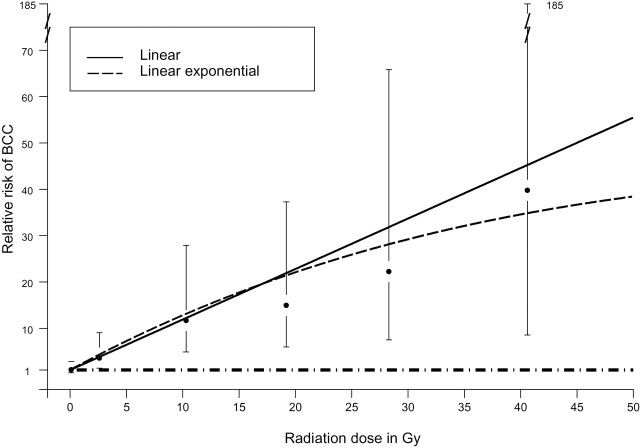
To better understand the role of radiation in BCC risk, we assessed several nonlinear multivariable models. Figure 1 demonstrates the fit of linear and linear exponential dose–response models to the odds ratio estimates for BCC based on odds ratios from the model using the previously described categories of radiation to the BCC sites shown in Table 2. The goodness-of-fit comparisons between nonlinear multivariable dose–response models for radiation dose are presented in Supplementary Table 1 (available online). Likelihood ratio tests for nested models indicated no statistically significant improvement in fit for models with quadratic or exponential terms compared with that of the linear model (eg, P = .47 for linear exponential vs linear model), thus supporting the use of the linear model as the most parsimonious model and the basis for testing effect modification.
Figure 1.
Dose–response models for ionizing radiation to the skin surface. Black circles represent odds ratios from categorical model (error bars represent 95% confidence intervals) for the median dose for the category; median (category): 0.14 Gy (0.01–0.9 Gy), 2.6 Gy (1.0–4.9 Gy), 10.3 Gy (5.0–14.9 Gy), 19.2 Gy (15.0–24.9 Gy), 28.4 Gy (25.0–34.9 Gy), and 40.6 Gy (35.0–63.3 Gy). Linear model: relative risk = 1 + 1.09 × dose; linear exponential: relative risk = 1 + 1.32 × dose × exp(−0.0114 × dose). The horizontal dashed line corresponds to a relative risk of 1.0.
In models stratified on anatomical location, with adjustment for sex, race, year of diagnosis, and Hodgkin lymphoma, the linear radiation dose response was stronger among those with BCC on the head or face than among those with BCC in other anatomical locations as reflected by the excess odds ratio (head or face EOR per Gy = 2.7 [95% CI = 0.68 to 15.10], P < .001; other locations EOR per Gy = 0.69 [95% CI = 0.23 to 2.20], P < .001).
Finally, we assessed the potential modifying effects of demographic (age at diagnosis, year of cancer diagnosis, type of first cancer [Hodgkin lymphoma vs other diagnosis], sex, and race) and chemotherapy covariates on the radiation dose response (Table 3). Year of diagnosis of the first primary cancer (1970–1973 vs 1974–1986) was the only statistically significant effect modifier (P = .02). The linear dose response was stronger among those diagnosed in 1974–1986 (EOR = 3.15, 95% CI = 0.89 to 16.59, P < .001) than among those diagnosed in 1970–1973 (EOR = 0.34, 95% CI = 0.05 to 1.63, P = .0013) after adjustment for sex, race, and Hodgkin lymphoma.
Table 3.
Assessment of possible effect modifiers for BCC radiation dose response*
| Effect modifier | Risk estimate† (γ) | P‡ | ||
| Sex, female vs male§ | −0.69 | .29 | ||
| Age at diagnosis, 0–9 y vs 10–20 y|| | −0.31 | .72 | ||
| Race, white vs other | N/A | N/A | ||
| Second cancer prior to BCC vs no second cancer | N/A | N/A | ||
| Dose of procarbazine 1–3999mg/m2 vs none | −3.79 | .09 | ||
| ≥4000mg/m2 vs none | −2.12 | |||
| Any oral cyclophosphamide||¶ vs none | −0.66 | .35 | ||
| Dose of intravenous cyclophosphamide|| 1–3999mg/m2 vs none | −1.02 | .53 | ||
| ≥4000mg/m2 vs none | −0.75 | |||
| Latency, ≥20 y after diagnosis vs <20 y|| | −1.30 | .11 | ||
| Year of diagnosis, 1970–1973 vs 1974–1986 | −1.73 | .02 | ||
| Hodgkin lymphoma diagnosis vs other diagnoses | −1.54 | .14 | ||
| Any prednisone vs none | −0.04 | .96 | ||
| Any anthracycline vs none | 0.04 | .95 | ||
| Anthracycline score# ¶ 1 vs 0 | −0.03 | .96 | ||
| 2 vs 0 | −0.55 | |||
| 3 vs 0 | 0.18 | |||
| Any alkylating agent vs none | −0.92 | .16 | ||
| Alkylating agent score#¶ 1 vs 0 | −0.55 | .30 | ||
| 2 vs 0 | −1.09 | |||
| 3 vs 0 | −1.84 | |||
| Hair color (3 models)** Blond vs other | −0.39 | .69 | ||
| Brown vs other | 0.25 | .73 | ||
| Red vs other | 0.14 | .92 | ||
| Skin color (2 models) ** Light brown vs other | −0.79 | .26 | ||
| White vs other | 0.02 | .98 |
*Each modifier was assessed in a separate risk model. BCC = basal cell carcinoma; N/A = nonestimable model.
†Risk = exp(ΣαX + δY) [1 + β × radiation × exp(ΣγY) ], where Y = potential modifier, X = baseline covariates sex, race, year of diagnosis, and Hodgkin lymphoma diagnosis, and γ = change in the excess OR for BCC per Gy.
‡P value from likelihood ratio test (two-sided) comparing model with listed covariate to baseline model with covariate, sex, race, year of diagnosis, Hodgkin lymphoma diagnosis, and linear radiation dose response.
§Baseline model does not include race.
||Model would not converge with modifier also included in the baseline model.
¶Due to missing chemotherapy information, only 158 case subjects were used in modeling.
#Tertiles of the cumulative dose for all agents within each of the two chemotherapy categories.
**Modeling included 162 case subjects who responded to the follow-up 2003 survey.
Discussion
Childhood cancer survivors are at risk of subsequent primary malignancies as a result of the therapy they receive to treat the primary illness, and nonmelanoma skin cancers account for 58% of all reported subsequent neoplasms (3). This study provides new information on the role of ionizing radiation in the development of BCCs by defining the dose-dependent relationship between the amount of radiation to the skin and subsequent risk of developing a BCC. For subjects who received a dose of 35 Gy or more to the skin from radiation therapy, the likelihood of developing BCC was approximately 40 times that of survivors who were not treated with radiation. Furthermore, in multivariable analysis, radiation therapy was the only treatment-related exposure associated with risk of BCC. Other markers of skin sensitivity, such as hair and skin color, were associated with an increased risk of BCC development.
Both ionizing and UV radiation are associated with development of BCC. Data from other studies suggest that the basal layer of the epidermis, where 70% of highly proliferating stem cells are located, is the most radiosensitive part of the skin tissue [reviewed in (21)]. A study of Japanese atomic bomb survivors (8) showed that survivors exposed to ionizing radiation in the bombing had a higher risk of developing BCC compared with those who were not exposed, with an excess relative risk per Sv of 1.8 (90% CI = 0.8 to 3.3). Subsequent analysis of this cohort based on additional follow-up demonstrated an excess relative risk per Gy of 0.17 for subjects exposed to less than 1 Gy of radiation compared with an excess relative risk per Gy of 1.2 for those exposed to greater than 1 Gy of radiation (10). The incidence of BCCs increased with time after initial exposure, and more than 75% of BCCs were attributed to atomic bomb exposure. It is unknown how similar ionizing radiation would have affected a lighter-skinned population, given that Asians have a lower risk of BCC compared with whites. For example, the incidence of BCC in China and Japan is approximately 10 per 100 000 persons (22), whereas the incidence among whites in the United States by age 70 years is 1 in 5 (23). Differences in skin tones make it difficult to quantify the risk of BCC that would occur had the atomic bombs affected a white population; however, it is reasonable to assume that the risk persists, and is potentially greater, in populations with lower skin melanin content.
Other evidence of the risk of BCC development in subjects exposed to ionizing radiation comes from children treated for tinea capitis. In a cohort study of children who were given x-ray treatment for tinea capitis (mean scalp dose = 4.3 Gy), the relative risk of BCC after 50 years of follow-up was 3.6 (95% CI = 2.3 to 5.9), and the estimated rate ratio per Gy for head and neck BCCs among whites was 1.6 (95% CI = 1.3 to 2.1), with an excess absolute risk that was 10 times higher in whites than in blacks (11). Similarly, a study of Israeli children treated with radiation for tinea capitis detected a relative risk per Gy of 1.7 with a mean scalp dose of 6.8 Gy (9). These results are similar to the estimated relative risk per Gy of 2.7 we report for head and face BCCs. The authors of the first study (11) also demonstrated an increased risk of BCC in irradiated individuals who were susceptible to UV radiation (ie, those with light complexion, severe sunburning, and North European ancestry). We found that host characteristics that might indicate sun sensitivity, such as hair color and skin color, were associated with an increased risk of BCC in childhood cancer survivors. Additional investigation is needed to determine whether the effect of radiation is strong enough to mask the effect of exposure to UV radiation from sunlight or whether it may simply reflect the fact that our study population is younger than the average age of BCC development in the United States and Canada (24,25).
We found that a linear dose–response model best described the association between radiation dose and the risk of developing BCC. By contrast, studies of secondary leukemia and thyroid malignancies demonstrated a plateau effect or downturn in risk at very high radiation doses (26–28). These studies have suggested that high doses of radiation result in cell death, preventing later malignant transformation. Our results suggest that the risk of skin cancer is not similarly abated at higher doses of radiation.
An unexpected finding of this study was the reduced risk of BCCs in earlier calendar years of initial cancer diagnosis, particularly because cohort members in the earlier year-of-diagnosis group were older. It is possible that other factors that are related to differences in treatment eras (and not taken into consideration in this analysis) are associated with the risk of BCC. It also is possible that this was a chance finding, given the large number of tests that were performed.
Because of the design of this cohort study, several factors need to be considered when interpreting the findings. First, 15% of the survivors who were eligible for the underlying cohort study could not be located and were lost to follow-up, and, of those found, 15% declined to participate. Comparisons of available cancer-related characteristics between participants and nonparticipants showed no statistically significant differences (29). Second, 18 case subjects died after reporting a BCC; however, BCC was not related to their causes of death. Finally, in this group of survivors, one case subject and four control subjects developed a subsequent cancer during the 5 years prior to study entry, for which radiation records were included in this analysis. In total, 12 case subjects and 28 control subjects developed a subsequent cancer prior to BCC diagnosis (or during corresponding time for control subjects); these BCCs could potentially represent radiotherapy exposures (ie, treatment for the subsequent cancers) that were not accounted for in the analyses. However, when development of a subsequent cancer was included in analysis, the estimates of excess odds ratio per Gy did not change markedly, suggesting additional treatment for these subsequent cancers had a minimal impact on the risk of BCC.
This study has limitations that should be considered when interpreting results. This was a retrospective study, and a large proportion of the data was self-reported. It is possible that survivors underreported the number of skin cancers, particularly cancers that occurred longest ago, or they may have incorrectly located the skin cancers on the self-report diagram. More importantly, survivors might not have received a clinical skin examination to confirm the occurrence of a skin cancer. In addition, we were unable to examine the effect of chemotherapy on the development of BCCs in survivors who received either low doses (<1 Gy) or no radiation exposure because of small numbers in these groups.
As the survival of those treated for childhood malignancies continues to improve, it is important to understand and educate survivors about treatment-related late effects, such as subsequent malignancies. Fewer than 30% of childhood cancer survivors seek appropriate medical care, either because they are not aware of their initial diagnosis or, more frequently, because they do not know the risks associated with the therapy they received (30,31). Given that any radiotherapy treatment for childhood cancer will confer some skin doses greater than 1 Gy (18), all white pediatric patients who have undergone radiotherapy should be considered as having an elevated risk of developing BCC. In addition, because more than 80% of the BCCs in this cohort occurred between the ages of 20 and 39 years, early and regular dermatological examinations in childhood cancer survivors who received radiation therapy are indicated to facilitate the timely identification of BCCs. It is hoped that such early identification will lead to reduced scarring resulting from surgical treatment, thereby mitigating an important source of anxiety for survivors. An understanding of the radiation dose-dependent nature of BCC risk may facilitate the development of improved surveillance and treatment guidelines for physicians who care for cancer survivors.
Appendix
The CCSS is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived five or more years after diagnosis of childhood cancer.
CCSS is a retrospectively ascertained cohort of 20 346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4000 siblings of survivors who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant # U24 CA55727) awarded to St Jude Children’s Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14 000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss.
CCSS Institutions and Investigators
| St Jude Children’s Research Hospital, Memphis, TN | Leslie L. Robison*,†, Melissa Hudson†,‡, Greg T. Armstrong†, Daniel M. Green†, Kevin R. Krull†, Kiri Ness† | |
| Children’s Healthcare of Atlanta/ Emory University, Atlanta, GA | Lillian Meacham‡, Ann Mertens† | |
| Children’s Hospitals and Clinics of Minnesota Minneapolis, St Paul, MN | Joanna Perkins‡ | |
| Seattle Children’s Hospital, Seattle, WA | Scott Baker‡, Eric Chow† | |
| Children’s Hospital, Denver, CO | Brian Greffe‡ | |
| Children’s Hospital Los Angeles, CA | Kathy Ruccione‡ | |
| Children’s Hospital, Oklahoma City, OK | John Mulvihill‡,† | |
| Children’s Hospital of Orange County, Orange, CA | Leonard Sender‡ | |
| Children’s Hospital of Philadelphia, Philadelphia, PA | Jill Ginsberg‡, Anna Meadows† | |
| Children’s Hospital of Pittsburgh, Pittsburgh, PA | Jean Tersak‡ | |
| Children’s National Medical Center, Washington, DC | Sadhna Shankar‡, Roger Packer† | |
| Cincinnati Children’s Hospital Medical Center, Cincinnati, OH | Stella Davies†,‡ | |
| City of Hope Medical Center, Los Angeles, CA | Smita Bhatia†,‡ | |
| Cook Children’s Medical Center, Ft. Worth, TX | Paul Bowman‡ | |
| Dana-Farber Cancer Institute/ Children’s Hospital, Boston, MA | Lisa Diller†,‡ | |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring†,‡ | |
| Hospital for Sick Children, Toronto, ON | Mark Greenberg‡, Paul C. Nathan†,‡ | |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez‡ | |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar†,‡, Kevin Oeffinger† | |
| National Cancer Institute, Bethesda, MD | Roy Wu†, Nita Seibel†, Preetha Rajaraman†, Peter Inskip†, Julia Rowland† | |
| Nationwide Children’s Hospital, Columbus, Ohio | Laura Martin‡, Sue Hammond† | |
| Northwestern University, Chicago, IL | Kimberley Dilley‡ | |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik‡ | |
| Roswell Park Cancer Institute, Buffalo, NY | Denise Rokitka‡ | |
| St Louis Children’s Hospital, St Louis, MO | Robert Hayashi‡ | |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina‡, Sarah S. Donaldson† | |
| Texas Children’s Hospital, Houston, TX | Zoann Dreyer‡ | |
| University of Alabama, Birmingham, AL | Kimberly Whelan‡ | |
| University of Alberta, Edmonton, AB | Yutaka Yasui†,‡ | |
| University of California–Los Angeles, CA | Jacqueline Casillas‡, Lonnie Zeltzer† | |
| University of California–San Francisco, CA | Robert Goldsby‡ | |
| University of Chicago, Chicago, IL | Tara Henderson‡ | |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson‡ | |
| University of Minnesota, Minneapolis, MN | Daniel Mulrooney‡, Joseph Neglia† | |
| University of Southern California, Los Angeles, CA | Dennis Deapen†,‡ | |
| University of Texas Southwestern Medical Center, Dallas, TX | Daniel C. Bowers‡ | |
| University of Texas MD Anderson Cancer Center, Houston, TX | Louise Strong†,‡, Marilyn Stovall† |
*Project Principal Investigator (U24 CA55727).
†Member of CCSS Steering Committee.
‡Institutional Principal Investigator.
Funding
This work was supported by Lance Armstrong Foundation (to ACM), the National Cancer Institute grant (CA 55727 to LLR) and the National Cancer Institute subgrant (5U2415 to MS), the National Cancer Institute/National Institutes of Health contract (N02 CP-2010-00-15 to MS), and the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Notes
Other investigators and institutions participating in the CCSS are listed in the Appendix. The study sponsor had no role in the study design, analysis or interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
References
- 1. USCSW Group United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK. (eds). SEER Cancer Statistics Review, 1975-2008 National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- 3. Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study J Natl Cancer Inst. 2010;102(14):1083–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGuire JFGN, Dyson S. Nonmelanoma skin cancer of the head and neck I: histopathology and clinical behavior Am J Otol. 2009;30(2):121–133 [DOI] [PubMed] [Google Scholar]
- 5. U.S. National Institutes of Health, 2010 Skin Cancer http://www.cancer.gov/cancertopics/types/skin Accessed October32011..
- 6. Walling HWFS, Geraminejad PA, Whitaker DC, Arpey CJ. Aggressive basal cell carcinoma: presentation, pathogenesis, and management Cancer Metastasis Rev. 2004;23(3-4):389– 402 [DOI] [PubMed] [Google Scholar]
- 7. Situm M, Buljan M, Bulat V, Mihic LL, Bolanca Z, Simic D. The role of UV radiation in the development of basal cell carcinoma Coll Antropol. 2008;32(2):167–170 [PubMed] [Google Scholar]
- 8. Ron E, Preston DL, Kishikawa M, et al. Skin tumor risk among atomic-bomb survivors in Japan Cancer Causes Control. 1998;9(4):393– 401 [DOI] [PubMed] [Google Scholar]
- 9. Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice JD., Jr. Radiation-induced skin carcinomas of the head and neck Radiat Res. 1991;125(3):318–325 [PubMed] [Google Scholar]
- 10. Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998 Radiat Res. 2007;168(1):1– 64 [DOI] [PubMed] [Google Scholar]
- 11. Shore RE, Moseson M, Xue X, Tse Y, Harley N, Pasternack BS. Skin cancer after X-ray treatment for scalp ringworm Radiat Res. 2002;157(4):410–418 [DOI] [PubMed] [Google Scholar]
- 12. Schwartz JL, Kopecky KJ, Mathes RW, Leisenring WM, Friedman DL, Deeg HJ. Basal cell skin cancer after total-body irradiation and hematopoietic cell transplantation Radiat Res. 2009;171(2):155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perkins JL, Liu Y, Mitby PA, et al. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: a report from the childhood cancer survivor study J Clin Oncol. 2005;23(16):3733–3741 [DOI] [PubMed] [Google Scholar]
- 14. Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research J Clin Oncol. 2009; 27(14):2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project Med Pediatr Oncol. 2002;38(4):229–239 [DOI] [PubMed] [Google Scholar]
- 16. Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphan J Rare Dis. 2008; 3:32– 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ICRP Publication 26 Recommendations of the International Commission on Radiological Protection Oxford, UK; 1977. [Google Scholar]
- 18. Kry SF, Smith SA, Weathers R, Stovall M. Skin dose during radiotherapy: a summary and general estimation technique J Appl Clin Med Phys. 2012;13(3):20– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langholz B, Richardson DB. Fitting general relative risk models for survival time and matched case-control analysis Am J Epidemiol. 2010;171(3):377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akaike H. A new look at the statistical model identification IEEE Transactions on Automatic Control 1974;19(6):716– 723 [Google Scholar]
- 21. Timares L, Katiyar SK, Elmets CA. DNA damage, apoptosis and Langerhans cells–Activators of UV-induced immune tolerance Photochem Photobiol. 2008;84(2):422–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim GK, Del Rosso JQ, Bellew S. Skin cancer in Asians: part 1: nonmelanoma skin cancer J Clin Aesthet Dermatol. 2009;2:39– 42 [PMC free article] [PubMed] [Google Scholar]
- 23. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model Arch Dermatol. 2010;146(3):279–282 [DOI] [PubMed] [Google Scholar]
- 24. Demers AA, Nugent Z, Mihalcioiu C, et al. Trends of nonmelanoma skin cancer from 1960 through 2000 in a Canadian population J Am Acad Dermatol. 2005;53(2):320– 328 [DOI] [PubMed] [Google Scholar]
- 25. Jung GW, Metelitsa AI, Dover DC, Salopek TG. Trends in incidence of nonmelanoma skin cancers in Alberta, Canada, 1988–2007 Br J Derm. 2010;163(1):146– 154 [DOI] [PubMed] [Google Scholar]
- 26. Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study Lancet. 2005;365(9476):2014–2023 [DOI] [PubMed] [Google Scholar]
- 27. Curtis RE, Boice JD, JR., Stovall M, et al. Relationship of leukemia risk to radiation dose following cancer of the uterine corpus J Natl Cancer Inst. 1994;86(17):1315–1324 [DOI] [PubMed] [Google Scholar]
- 28. Boice JD,, Jr, Blettner M, Kleinerman RA, et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix J Natl Cancer Inst. 1987;79(6):1295– 1311 [PubMed] [Google Scholar]
- 29. Leisenring WM, Mertens AC, Armstrong GT, Stovall MA, Neglia JP, Lanctot JQ, Boice JD,, Jr, Whitton JA, Yasui Y. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study J Clin Oncol. 2009; 27(14):2319–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oeffinger KC, Wallace WH. Barriers to follow-up care of survivors in the United States and the United Kingdom Ped Blood Cancer. 2006;46(2):135–142 [DOI] [PubMed] [Google Scholar]
- 31. Nathan PC, Greenberg ML, Ness KK,, et al. Medical care in long-term survivors of childhood cancer: A Report from the Childhood Cancer Survivor Study J Clin Oncol. 2008; 26(27):4401– 4409 [DOI] [PMC free article] [PubMed] [Google Scholar]