Abstract
Background
The risk of anal cancer is substantially increased in HIV-infected individuals. Thus, the HIV epidemic may have influenced the increasing anal cancer trends in the United States. We estimated the impact of the HIV epidemic on trends in anal cancer incidence in the United States during 1980–2005.
Methods
Data on anal cancer cases with and without AIDS were obtained from the HIV/AIDS Cancer Match Study. The number of HIV-infected anal cancer cases without AIDS was estimated from the number of anal cancers occurring before diagnosis of AIDS. The proportion of anal cancer cases with HIV infection in the general population was calculated. We estimated temporal trends in the incidence rates of anal cancer in the general population overall and after exclusion of HIV-infected cancer cases by calculating annual percent changes and 95% confidence intervals (CIs) using a Joinpoint log-linear model. All incidence rates were standardized to the 2000 US population by age, sex, and race.
Results
During 1980–2005, of the 20 533 estimated anal cancer cases, 1665 (8.1%) were HIV-infected. During 2001–2005, the proportion of anal cancer cases with HIV infection was the highest—1.2% (95% CI = 0.93 to 1.4%) among females and 28.4% (95% CI = 26.6 to 29.4%) among males. During 1980–2005, HIV infection did not have an impact on the trends in anal cancer among females (incidence rates increased by 3.3% [95% CI = 3.0 to 3.7%] annually overall, and by 3.3% [95% CI = 2.9 to 3.6%] annually without HIV-infected anal cancer cases) but had a strong impact on the trends in anal cancer among males (incidence rates increased by 3.4% [95% CI = 2.9 to 3.9%] annually overall, and by 1.7% [95% CI = 1.2 to 2.3%] annually without HIV infection).
Conclusion
During 1980–2005, the increasing anal cancer incidence rates in the United States were strongly influenced by the HIV epidemic in males but were independent of HIV infection in females.
Although anal cancer is rare in the United States, with an estimated 2250 men and 3980 women diagnosed in 2012, anal cancer incidence has increased steadily since as early as 1940 (1,2). Between 1975–1984 and 1995–2004, the US incidence rates increased by 57% in men (from 0.79 to 1.24 per 100 000 person-years) and by 41% in women (from 1.04 to 1.47 per 100 000 person-years) (3). The rates are consistently higher in women than in men, with the exception of those aged 20–49 years, among whom men have a higher incidence (3,4).
An estimated 84% of anal carcinomas are caused by human papillomavirus (HPV), with a large proportion positive for oncogenic HPV types 16 and 18 (5). The risk factors for anal cancer are primarily related to sexual behaviors, including number of lifetime sex partners, anal intercourse, genital warts, and male homosexual behavior, reflecting HPV acquisition (6).
It has been proposed that the HIV epidemic has contributed to the rising anal cancer incidence in the United States, given the substantially elevated anal cancer risk among HIV-infected individuals and the growth of the HIV-infected population (1,7–9). The risk of anal cancer, compared with the general population, is elevated 24-fold in HIV-infected women, 32-fold in HIV-infected men, and 52-fold in HIV-infected men who have sex with men (MSM) (10,11). Although the rates of Kaposi sarcoma and non-Hodgkin lymphoma (two other HIV-associated virus-related malignancies) declined with the introduction of highly active antiretroviral therapy (HAART) in 1996, the rates of anal cancer among HIV-infected individuals have increased over time (10,12,13).
The contribution of the increasing number of HIV-infected anal cancer cases to the anal cancer rates in the general population has not been previously assessed. Quantifying this contribution would help clarify the epidemiology and etiology of anal cancer. The aim of our study was to estimate the proportion of anal cancer cases with HIV infection in the United States and to assess trends in anal cancer incidence overall, and after exclusion of HIV-infected anal cancer cases.
Methods
Study Population and Ascertainment of Anal Cancer
The HIV/AIDS Cancer Match (HACM) Study (http://hivmatch.cancer.gov/) links 17 US population-based HIV/AIDS and cancer registries (Colorado; Connecticut; Florida; Illinois; Georgia; Maryland; Massachusetts; Michigan; New Jersey; Texas; California; New York, New York; Los Angeles, San Diego, and San Francisco, California; Seattle, Washington; and Washington, DC) and is funded by the Intramural Program of the National Cancer Institute (14). A probabilistic linkage algorithm was used to link HIV/AIDS and cancer registries, and only anonymized data are retained by investigators. All registry areas provided data on anal cancers occurring in people who developed AIDS. Additionally, six registries also ascertained anal cancers in HIV-infected individuals without an AIDS diagnosis (ie, HIV-only) for a more limited number of years (1991–2008). The HACM Study was approved by institutional review boards at participating registries.
Invasive anal cancers were ascertained through cancer registries participating in the HACM Study. Anal cancers were defined using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3), topography codes C210 (anus, not otherwise specified) and C211 (anal canal), and excluding poorly specified cancers, hematologic malignancies, and Kaposi sarcoma (15). Anal cancers were classified by histology as squamous cell carcinomas (ICD-O-3 codes 8050–8089), adenocarcinomas (ICD-O-3 codes 8140–8309), carcinomas not otherwise specified (ICD-O-3 code 8010), and other histological subtypes.
Statistical Analysis
We estimated the number of anal cancers in HIV-infected people during 1980–2005 as the sum of incident anal cancers in people with AIDS and incident anal cancers in people with HIV-only. Incident anal cancers in people with AIDS were identified by the cancer registries, matched with the AIDS registries, and occurred at or after AIDS diagnosis. To estimate the number of incident anal cancers in people with HIV-only for registries without HIV-only data, we applied weights to (ie, upweighted) prevalent anal cancers in people with AIDS (cancers that matched with HIV/AIDS registries and occurred within 5 years before AIDS diagnosis). This adjustment was necessary because prevalent anal cancers in people with AIDS only included anal cancer cases occurring in HIV-infected individuals who developed AIDS; thus, the number of prevalent cancers in people with AIDS underestimates the number of anal cancers in people with HIV-only.
For this upweighting, we computed weights using the following steps: 1) with data from the six HIV-only registries, we estimated the probability that anal cancer cases with HIV-only developed AIDS in the periods 0–1, 1–2, and 2–5 years after anal cancer diagnosis (93 cancers with 164 person-years of follow-up), and this probability Pi (i = 1, 2, and 3 for the three periods 0–1, 1–2, and 2–5 years, respectively) was estimated using competing risk methods, treating death as a competing event (the estimated probabilities of AIDS were P1 = .495, P2 = .059, P3 = .101); 2) we computed the variance (Vi) of the inverse of this probability Pi for each time point, using the delta method; 3) we computed standardized weights as Wi = 1/Pi * [(1/Vi)/(1/V1 + 1/V2 + 1/V3)], and for those registries that did not provide HIV-only data, we then multiplied the prevalent anal cancers occurring 0–1, 1–2, and 2–5 years before AIDS diagnosis, by the corresponding weight Wi.
The number of anal cancer cases in the general population, population with AIDS, and population with HIV-only were summed in categories defined by single calendar year (1980–2005), attained age (0–19, 20–49, 50–69 and ≥70 years old), sex, race (white, black, other or unknown), and registry. We previously estimated the sensitivity of the match between HIV/AIDS and cancer registries in the HACM Study to be 81.1% (16). To correct for imperfect sensitivity, we divided the estimated number of HIV-infected anal cancer cases by 0.811 in each stratum defined by the cross-classification of the above characteristics to estimate the corrected number of HIV-infected anal cancer cases. To estimate the corrected number of HIV-uninfected anal cancer cases, we subtracted the corrected number of HIV-infected anal cancer cases from the total number of anal cancer cases in each stratum.
We estimated the proportion of anal cancer cases in the general population with HIV infection, stratified by sex, age, race, and anal cancer histology. We have focused on presenting proportions from 2001–2005, because this is the most recent time period. We estimated the following general population incidence rates stratified by sex, age, and race: 1) overall, 2) anal cancer cases without AIDS, and 3) anal cancer cases without HIV infection. For males, we also estimated incidence rates without HIV-infected MSM and without all HIV-infected males. Incidence rates were standardized to the 2000 US population by age, sex, and race (17) and were calculated per 100 000 person-years.
For all proportions and incidence rates, a bootstrap procedure with 500 bootstrap iterations was used to estimate standard errors incorporating variation from all sources, including uncertainty in the weights. In the first step of the bootstrap, we recomputed weights by resampling anal cancers from the prospective HIV data with replacement. In the second step, we used a parametric bootstrap, assuming that counts followed a Poisson distribution, to generate the total number of anal cancer cases and the number of incident and prevalent anal cancer cases with AIDS. We then combined the bootstrapped weights with the new counts and computed incidence rates and proportions for each bootstrap iteration using the same procedures as in the main analysis. We utilized the replicates to estimate 95% confidence intervals based on the quantiles from the bootstrap distribution of the estimates. Temporal trends in incidence were estimated in a log-linear model using Joinpoint (version 3.5.1) (18,19). Annual percent change was defined as 100 * [exp(β)−1], where β is the slope of the trend provided by Joinpoint (18). All statistical tests were two-sided, and all remaining analyses were carried out using SAS version 9.3 (SAS, Cary, NC).
To assess the sensitivity of our results to the weighting procedure used to estimate anal cancer cases with HIV-only, we compared trends in anal cancer based on data from the 17 HACM registries (including upweighted anal cancers for people with HIV-only) to trends in anal cancer observed using the more limited data from six registries that had actual counts of HIV-only anal cancer cases.
Results
Characteristics of Anal Cancer Cases
During 1980–2005, a total of 20 533 anal cancers occurred in 2.3 billion person-years of follow-up in the general population from the US regions included in the HACM Study. An estimated 1665 (8.1%) anal cancer cases were HIV-infected, of which 1185 cancer cases had AIDS and 479 cancer cases had HIV-only; 18 868 anal cancer cases did not have HIV infection (Table 1). HIV-uninfected anal cancer cases were older than HIV-infected anal cancer cases (age ≥50 years, 81.0% vs 25.8%) and were predominantly females (64.9%), whereas the vast majority of HIV-infected anal cancer cases were males (94.7%). Although most of the HIV-uninfected and HIV-infected anal cancer cases were whites, a larger proportion of HIV-infected cancer cases were blacks (29.4% HIV-infected vs 9.7% HIV-uninfected). Among the HIV-infected anal cancer cases, 83.5% were MSM. Eighty-six percent of anal cancer in HIV-infected individuals and 71.4% of anal cancers in HIV-uninfected individuals were squamous cell carcinomas.
Table 1.
Characteristics of anal cancer cases in the general population by HIV status between 1980 and 2005*
| Characteristics | HIV-uninfected | HIV-infected† |
|---|---|---|
| No. (%) | No. (%) | |
| Total | 18 868 (100) | 1665 (100) |
| Sex | ||
| Male | 6626 (35.1) | 1577 (94.7) |
| Female | 12243 (64.9) | 87 (5.2) |
| Age at cancer diagnosis, y | ||
| <20 | 8 (0.04) | 0 (0) |
| 20–49 | 3589 (19.0) | 1235 (74.2) |
| 50–69 | 8520 (45.2) | 421 (25.3) |
| >70 | 6751 (35.8) | 9 (0.5) |
| Race | ||
| White | 16 579 (87.9) | 1150 (69.1) |
| Black | 1836 (9.7) | 489 (29.4) |
| Other | 453 (2.4) | 26 (1.6) |
| AIDS diagnosis | ||
| Yes | — | 1185 (71.2) |
| No | — | 479 (28.8) |
| MSM | ||
| Yes | — | 1390 (83.5) |
| No | — | 275 (16.5) |
| Histologic subtype‡ | ||
| Squamous cell carcinoma | 13 465 (71.4) | 1438 (86.4) |
| Adenocarcinoma | 1952 (10.3) | 17 (1.0) |
| Carcinoma, NOS | 344 (1.8) | 20 (1.2) |
| Other | 3107 (16.5) | 190 (11.4) |
* Data from the HIV/AIDS Cancer Match Study, which links 17 US population-based HIV/AIDS and cancer registries during 1980–2005. MSM = men who have sex with men; NOS = not otherwise specified; — = not applicable.
† As the number of HIV-infected anal cancer cases is an estimate, due to rounding the sum of the subgroups may not add up to the total (n = 1665).
‡ Histology was classified using International Classification of Diseases for Oncology (ICD-O-3) codes: squamous cell carcinomas (ICD-O-3 codes 8050–8089), adenocarcinomas (8140–8309), carcinomas not otherwise specified (8010), and other histological subtypes.
Proportions of Anal Cancer Cases With HIV Infection
We estimated the proportions of anal cancer cases with HIV infection stratified by sex, age group, race, and histology. The proportions increased steadily from 1980–1984 to 2001–2005, rising from 1.1% (95% CI = 0.02% to 2.7%) to 28.4% (95% CI = 26.6% to 29.4%) among males, and from 0% to 1.2% (95% CI = 0.93% to 1.4%) among females. Results are presented separately by sex throughout, because the proportion of anal cancer cases with HIV infection was much higher among males than females. During 2001–2005, younger age groups had a larger proportion of anal cancer cases with HIV infection compared with older age groups, among both males (age 20–49 years, 58.0% [95% CI = 52.9% to 60.7%]; age 50–69 years, 18.8% [95% CI = 16.8% to 20.7%]; age ≥70 years, 1.0% [95% CI = 0.59% to 1.6%]) and females (age 20–49 years, 3.7% [95% CI = 3.0% to 4.3%]; age 50–69 years, 0.90% [95% CI = 0.55% to 1.4%]; age ≥70 years, 0%) (Table 2). Among blacks, a larger proportion of anal cancer cases was estimated to be HIV-infected (males, 60.3% [95% CI = 52.2% to 64.4%]; females 6.4% [95% CI = 4.7% to 8.3%]) compared with whites (males, 23.4% [95% CI = 22.0% to 24.6%]; females, 0.65% [95% CI = 0.50% to 0.85%]). When stratified by histology, a larger proportion of squamous cell carcinoma cases were HIV-infected (males, 31.3% [95% CI = 32.2% to 35.6%]; females, 1.2% [95% CI = 1.1% to 1.7%]) compared with adenocarcinoma cases (males, 2.7% [95% CI = 0.84% to 4.6%]; females, 0%).
Table 2.
Proportion of anal cancer cases in the general population during 2001–2005 estimated to have HIV infection*
| Males | Females | |||
|---|---|---|---|---|
| Category | No. of cancers | HIV-infected, % of total (95% CI) | No. of cancers | HIV-infected, % of total (95% CI) |
| Total | 2705 | 28.4 (26.6 to 29.4) | 3864 | 1.2 (0.93 to 1.4) |
| Age at cancer diagnosis, y† | ||||
| 20–49 | 937 | 58.0 (52.9 to 60.7) | 777 | 3.7 (3.0 to 4.3) |
| 50–69 | 1164 | 18.8 (16.8 to 20.7) | 1779 | 0.90 (0.55 to 1.4) |
| >70 | 603 | 1.0 (0.59 to 1.6) | 1307 | 0 |
| White race | 2257 | 23.4 (22.0 to 24.6) | 3398 | 0.65 (0.50 to 0.85) |
| White race, by age, y | ||||
| 20–49 | 714 | 51.4 (47.0 to 55.4) | 653 | 1.9 (1.6 to 2.0) |
| 50–69 | 1000 | 15.7 (14.0 to 17.4) | 1566 | 0.63 (0.31 to 1.1) |
| >70 | 543 | 0.91 (0.44 to 1.6) | 1179 | 0 |
| Black race | 377 | 60.3 (52.2 to 64.4) | 363 | 6.4 (4.7 to 8.3) |
| Black race, by age, y | ||||
| 20–49 | 200 | 84.1 (71.8 to 89.1) | 99 | 17.2 (11.8 to 21.9) |
| 50–69 | 131 | 44.3 (35.6 to 53.8) | 172 | 3.6 (2.0 to 6.0) |
| >70 | 46 | 2.7 (2.1 to 3.7) | 91 | 0 |
| Squamous cell carcinoma‡ | 838 | 31.3 (32.2 to 35.6) | 3141 | 1.2 (1.1 to 1.7) |
| Squamous cell carcinoma, by age, y | ||||
| 20–49 | 928 | 56.6 (57.8 to 65.8) | 673 | 3.8 (3.7 to 4.9) |
| 50–69 | 377 | 20.7 (20.3 to 23.7) | 1512 | 0.74 (0.58 to 1.2) |
| >70 | 262 | 0.98 (0.72 to 1.9) | 960 | 0 |
| Adenocarcinoma‡ | 262 | 2.7 (0.84 to 4.6) | 246 | 0 |
| Adenocarcinoma, by age, y | ||||
| 20–49 | 41 | 8.5 (2.5 to 14.3) | 28 | 0 |
| 50–69 | 108 | 3.4 (0 to 8.5) | 78 | 0 |
| >70 | 113 | 0 | 140 | 0 |
* Proportions of anal cancer cases with HIV infection were estimated by dividing the number of HIV-infected anal cancer cases by the total number of anal cancer cases. CI = confidence interval.
† Results are not presented for the age 0–19 year group, as none of the eight anal cancer cases in this age group were HIV-infected. However, this age group was included in the total estimate and the overall estimates for whites, blacks, squamous cell carcinoma, and adenocarcinoma.
‡ Histology was classified using International Classification of Diseases for Oncology (ICD-O-3) codes: squamous cell carcinomas (ICD-O-3 codes 8050–8089), adenocarcinomas (8140–8309), carcinomas not otherwise specified (8010), and other histological subtypes.
Trends in Incidence Rates of Anal Cancer in the General Population and in HIV-Uninfected Individuals
We estimated the incidence rates of anal cancer and annual percent changes in incidence rates in both males and females during 1980–2005, stratified by sex, age group, and race; the annual percent change corresponds to the slope of the incidence rate curve (Figure 1 and Table 3). During 1980–1984 and 2001–2005, anal cancer incidence rates increased from 0.44 to 0.93 per 100 000 person-years in males and from 0.68 to 1.29 per 100 000 person-years in females. Among males, HIV-infected anal cancer cases strongly influenced the trends in anal cancer in the general population. During 1980–2005, anal cancer incidence rates in males increased by 3.4% (95% CI = 2.9% to 3.9%) annually overall (0.37 to 1.06 per 100 000 person-years), but after exclusion of HIV-infected anal cancer cases, the rates only increased by 1.7% (95% CI = 1.2% to 2.3%) annually (0.37 to 0.77 per 100 000 person-years) (Table 3 and Figure 1, A). Among females, the incidence rates increased by 3.3% (95% CI = 3.0% to 3.7%) annually overall (0.64 to 1.52 per 100 000 person-years), which remained unchanged after exclusion of HIV-infected anal cancer cases, showing an increase of 3.3% (95% CI = 2.9% to 3.6%) annually (0.64 to 1.49 per 100 000 person-years) (Table 3 and Figure 1, B).
Figure 1.
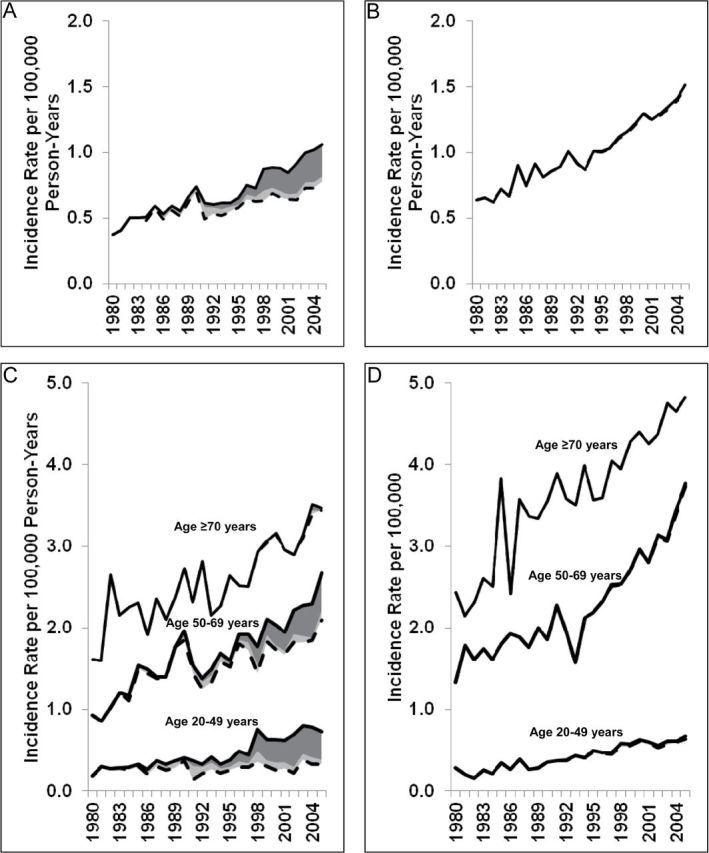
Anal cancer incidence in the general population with and without HIV-infected anal cancer cases, by age and sex, between 1980 and 2005. The solid lines represent the overall incidence rates of anal cancers in the general population; the dashed lines represent the incidence rates for anal cancers without HIV-infected anal cancer cases; the dark gray shaded areas represent anal cancer cases with AIDS, and the light gray shaded areas represent anal cancer cases with HIV-only. A) Incidence rates in males. B) Incidence rates in females. C) Incidence rates in males, stratified by age group. D) Incidence rates in females, stratified by age group.
Table 3.
Trends in anal cancer incidence in the general population during 1980–2005
| Category | Annual percent change*, (95% CI), based on all anal cancer cases | Annual percent change* (95% CI), with exclusion of HIV-infected anal cancer cases |
|---|---|---|
| Females | ||
| Total† | 3.3 (3.0 to 3.7) | 3.3 (2.9 to 3.6) |
| 20–49 y | 4.5 (3.8 to 5.3) | 4.3 (3.6 to 5.1) |
| 50–69 y | 3.6 (3.0 to 4.1) | 3.5 (3.0 to 4.0) |
| >70 y | 2.4 (1.8 to 2.9) | 2.4 (1.8 to 2.9) |
| Males | ||
| Total† | 3.4 (2.9 to 3.9) | 1.7 (1.2 to 2.3) |
| 20–49 y | 5.2 (4.2 to 6.2) | 0.7 (−0.4 to 1.7) |
| 50–69 y | 3.1 (2.5 to 3.7) | 2.0 (1.3 to 2.7) |
| >70 y | 2.2 (1.6 to 2.8) | 2.1 (1.5 to 2.7) |
| White | 3.4 (2.8 to 3.9) | 2.0 (1.5 to 2.6) |
| Black | 4.3 (3.2 to 5.4) | −0.6 (−2.0 to 0.8) |
* Annual percent changes in anal cancer incidence rates were estimated using Joinpoint software. CI = confidence interval.
† Results are not presented for those aged 0–19 years because there were too few anal cancer cases (n = 8) in this age group to assess trends over time. However, this age group is included in the total and race-specific incidence rates. Results are presented stratified by race for males only, because HIV-infected anal cancer cases had little impact on anal cancer incidence rates in females.
Anal cancer incidence was very low among people aged 0–19 years; this age group had no HIV-infected anal cancer cases. However, HIV-infected anal cancer cases strongly influenced trends in anal cancer incidence rates in males aged 20-49 years; incidence rates increased by 5.2% (95% CI = 4.2% to 6.2%) annually overall, but no statistically significant increase was observed after excluding HIV-infected anal cancer cases (annual increase: 0.7% (95% CI = −0.4% to 1.7%) (Table 3 and Figure 1, C). Likewise, among males aged 50–69 years, the increase in the incidence rates declined from 3.1% (95% CI = 2.5% to 3.7%) annually overall to 2.0% (95% CI = 1.3% to 2.7%) annually when the HIV-infected anal cancer cases were excluded. In contrast, trends in anal cancer changed very little among females aged 20–49 years and 50–69 years when HIV-infected anal cancer cases were excluded (Table 3 and Figure 1, D). Among males and females aged 70 years or older, anal cancer incidence rates increased over time, with only nine HIV-infected anal cancer cases among males and none among females.
The rates of anal cancer in the general population were higher among females than males in each 10-year age group 50 years or older (Figure 2, A). In contrast, rates were similar among females and males aged 40–49 years (1.09 vs 1.07 per 100 000 person-years) and lower in females than males aged 20–29 years (0.03 vs 0.05 per 100 000 person-years) and 30–39 years (0.25 vs 0.41 per 100 000 person-years). After excluding HIV-infected anal cancer cases, rates were the same in females and males aged 20–29 years (0.02 vs 0.02 per 100 000 person-years) and uniformly higher in females than males in older age groups (aged 30–39 years: 0.23 vs 0.17 per 100 000 person-years; aged 40–49 years: 1.08 vs 0.64 per 100 000 person-years) (Figure 2, B).
Figure 2.
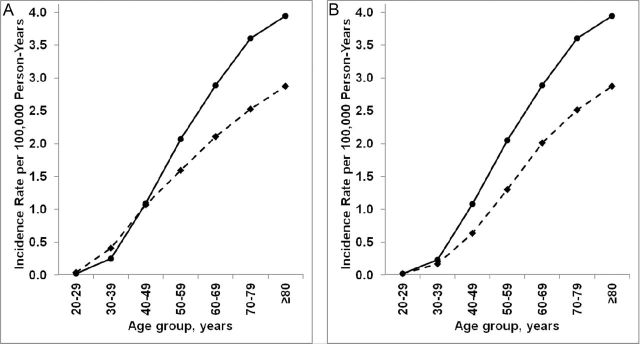
Anal cancer incidence in the general population in males and females by age group. Solid lines represent rates in females and dashed lines represent rates in males. A) Incidence rates in males and females overall. B) Incidence rates in males and females in the absence of HIV-infected anal cancer cases.
We further stratified incidence rates of anal cancer in males by race and MSM status. Among white males, rates increased by 3.4% (95% CI = 2.8% to 3.9%) annually overall and by 2.0% (95% CI = 1.5% to 2.6%) annually after excluding HIV-infected anal cancer cases; among black males, rates increased by 4.3% (95% CI = 3.2% to 5.4%) annually overall but were constant after excluding HIV-infected cases (Table 3, and Supplementary Figure 1, A and B, available online). Also, HIV-infected MSM contributed substantially to the number of male anal cancer cases (Supplementary Figure 1, C, available online).
Sensitivity Analysis
In a sensitivity analysis, we compared trends in incidence rates of anal cancer based on data from the 17 HACM registries (including upweighted cancers for people with HIV-only) to trends observed using the more limited data from six registries that had actual counts of HIV-only cases. The proportion of anal cancer cases that were HIV-infected and the incidence rates of anal cancer after excluding HIV-infected anal cancer cases were similar (1.9% of female and 28.8% of male anal cancer cases with HIV infection; Supplementary Figure 2, A–D, available online).
Discussion
Using population-based HIV and cancer registry data from 17 US areas, we estimated that 28% of males with anal cancer during 2001–2005 were HIV-infected, and that the temporal increase in male anal cancer rates during 1980–2005 was strongly influenced by the contribution of HIV-infected cancer cases. Furthermore, the vast majority of these HIV-infected males were MSM. In contrast, HIV had little impact on trends among US females, and the rates increased consistently over time in females, even in the absence of HIV-infected cancer cases.
Anal cancer is rare in the US general population but the fourth most common cancer in HIV-infected people, following non-Hodgkin lymphoma, Kaposi sarcoma, and lung cancer (9). In the United States, the number of anal cancers occurring in HIV-infected individuals has increased over time because of the growth and aging of the HIV-infected population (9). The elevated risk of anal cancer in HIV-infected individuals is partly because of the high prevalence of anal HPV infection (20,21). HIV and HPV have a shared mode of transmission through sexual intercourse. MSM represent the largest HIV risk group in the United States, comprising more than half of all people living with HIV infection in 2008 (22). Furthermore, among HIV-infected individuals, low CD4 cell counts are associated with increased risk of anal cancer (13,23). Thus, HIV-associated immunosuppression may contribute directly to development of anal cancer by impairing cell-mediated immune control of HPV (24). Though HAART partially restores immune function, HIV therapy does not lead to the regression of anal cancer precursor lesions (25), and anal cancer incidence has increased in the HAART era (10,12).
In this context, we note that not all anal cancers in HIV-infected people would be directly attributable to HIV infection. For example, as MSM are at increased risk of anal cancer, and most HIV-infected anal cancer cases occurred among MSM, we cannot say that HIV-infected anal cancer cases would not have occurred in the absence of HIV infection. However, HIV-infected MSM have a higher prevalence of abnormal anal cytology, and a 10-fold increased risk of anal squamous cell carcinoma, compared with HIV-uninfected MSM (26–28). Thus, it is likely that a fraction of anal cancers in HIV-infected MSM (and HIV-infected people overall) are caused by coinfection with HIV and HPV.
Notably, the contribution of HIV-infected anal cancer cases to general population estimates of anal cancer was essentially limited to males, and in the absence of HIV-infected cases, anal cancer incidence was uniformly higher across age groups among females than males. The small proportion of HIV-infected anal cancer cases among females partly results from lower HIV prevalence among US women compared with men (0.2% vs 0.5%) (22). The sex differences in incidence rates may be explained by differences in exposure to anal HPV infection. In 2009, 35% of US women aged 50–59 years, but only 10% of men, reported ever engaging in receptive anal sex (29). Further, anal HPV prevalence appears to be higher in women, approaching the prevalence observed in MSM (30,31). The cause of the increasing anal cancer incidence among women over time is unclear but may be due to an increase in the number of sexual partners or in the practice of receptive anal intercourse (4). Further, this increase is consistent with rising trends in other HPV-related cancers, including oropharyngeal cancer, vulvar cancer, and cervical adenocarcinoma, perhaps reflecting an increasing prevalence of infection with oncogenic HPV types (32–34).
Among males, blacks with anal cancer had more than double the prevalence of HIV infection compared with whites, likely reflecting the higher prevalence of HIV infection in black men compared with white men in the United States (1.7% vs 0.3%) (22). The proportion of anal cancer cases in HIV-infected males was far greater for squamous cell carcinomas than for adenocarcinomas (31.3% vs 2.7%), which may be because of the difference in HPV prevalence reported in anal squamous cell carcinomas (78%) and adenocarcinomas (43%) (35).
Two cancer prevention strategies have been suggested for reducing the burden of anal cancer: HPV vaccination and anal Papanicolaou (Pap) testing. Widespread vaccination against HPV 16 and -18 will reduce the burden of HPV-associated cancers, including anal cancer. The available bivalent and quadrivalent HPV vaccines are highly efficacious in preventing anal HPV infection and anal cancer precursor lesions due to HPV 16 and -18 (36,37). The US Advisory Committee on Immunization Practices has recommended HPV vaccination for adolescent and young women since 2007 (38) and recently endorsed vaccination of boys aged 11–12 years (39). However, vaccine uptake remains low, and because most anal cancers in the general population occur among those aged 60 years or older, any benefits of HPV vaccination on anal cancer rates will not be observed for decades (40). Among HIV-infected individuals, who develop anal cancer at a younger age (41), the HPV vaccine is safe and highly immunogenic, but efficacy studies are still needed (42).
Based on the success of the Pap test for cervical cancer screening, use of a similar Pap test for detection of anal cancer precursors could potentially reduce anal cancer incidence. Anal cancer screening may be cost-effective in HIV-infected and HIV-uninfected MSM (43,44), and New York State guidelines recommend anal Pap testing for certain HIV-infected individuals (45). However, anal Pap testing has not been shown to reduce anal cancer incidence or mortality (46), and a recent study concluded that more information is needed about the natural history of anal cancer and the progression rates of high-grade anal intraepithelial lesions before anal cancer screening in high-risk groups should be implemented (28).
The main strength of our study was the availability of population-based data on anal cancer and AIDS diagnoses from 17 regions of the United States over the entire course of the HIV epidemic. The size of our study allowed us to assess incidence trends despite the rarity of anal cancer.
This study had a few limitations. The main weakness of our study was the lack of complete data on anal cancer cases with HIV-only. However, we addressed this issue by upweighting prevalent anal cancers in people with AIDS to represent cancers in people with HIV-only. These upweighted results were quite similar to results based on more limited prospective data following HIV diagnosis. We also note that the HACM Study includes US areas with higher-than-average HIV prevalence; so the proportion of anal cancer cases with HIV infection may be higher that of the entire country. Finally, 21% of HIV-infected people in the United States are undiagnosed and are not captured in HIV/AIDS registries, which would lead to an underestimate of the proportion of HIV-infected anal cancer cases (47).
In conclusion, a large proportion of US males with anal cancer in recent years, particularly younger and black males, were HIV-infected. Measures that would effectively prevent anal cancer in HIV-infected males could markedly reduce anal cancer rates at the population level. In contrast, very few females with anal cancer were HIV-infected, and more research is needed to understand causes of rising anal cancer incidence in females.
Funding
This study was funded by the Intramural Research Program of the National Cancer Institute.
Notes
For providing data for the HIV/AIDS Cancer Match (HACM) Study, the authors thank the HIV/AIDS and cancer registry staff in the states of Colorado, Connecticut, Florida, Illinois, Georgia, Maryland, Massachusetts, Michigan, New Jersey, Texas, and California; and the metropolitan areas of New York, New York; Los Angeles, San Diego, and San Francisco, California; Seattle, Washington; and Washington, DC. We also thank Tim McNeel of Information Management Systems (Silver Spring, MD), who was compensated for database management.
The sponsor reviewed and approved final submission but did not have a role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or the decision to submit for publication.
References
- 1. Melbye M, Rabkin C, Frisch M, Biggar RJ. Changing patterns of anal cancer incidence in the United States, 1940-1989 Am J Epidemiol. 1994. 139(8):772–780 [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. Cancer Facts and Figures, 2012 Atlanta: American Cancer Society; 2012. [Google Scholar]
- 3. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age Cancer Epidemiol Biomarkers Prev. 2009. 18(4):1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000 Cancer. 2004. 101(2):281–288 [DOI] [PubMed] [Google Scholar]
- 5. DeVuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis Int J Cancer. 2009. 124(7):1626–1636 [DOI] [PubMed] [Google Scholar]
- 6. Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer Cancer. 2004. 101(2):270–280 [DOI] [PubMed] [Google Scholar]
- 7. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis Lancet. 2007. 370(9581):59–67 [DOI] [PubMed] [Google Scholar]
- 8. Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic J Acquir Immune Defic Syndr. 2005. 40(4):451–455 [DOI] [PubMed] [Google Scholar]
- 9. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States J Natl Cancer Inst. 2011. 103(9):753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS J Natl Cancer Inst. 2009. 101(16):1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals J Acquir Immune Defic Syndr. 2009. 52(5):611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003 Ann Intern Med. 2008. 148(10):728–736 [DOI] [PubMed] [Google Scholar]
- 13. Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America Clin Infect Dis. 2012. 54(7):1026–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults JAMA. 2001. 285(13):1736–1745 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. International Classification of Diseases for Oncology 3rded. Geneva: World Health Organization; 2000. [Google Scholar]
- 16. Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007 JAMA. 2011. 305(14):1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Surveillance, Epidemiology, and End Results [SEER] program SEER*Stat Database: Populations - Total U.S. (1969-2007) <Single Ages to 85+> - Linked to County Attributes - Total U.S., 1969-2007 Counties. 2011 National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch released November 2009. [Google Scholar]
- 18. Statistical Research and Applications Branch NCI. Joinpoint Regression Program, Version 3.5.1. 2011 [Google Scholar]
- 19. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates Stat Med. 2000. 19(3):335–351 [DOI] [PubMed] [Google Scholar]
- 20. Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men J Infect Dis. 1998. 177(2):361–367 [DOI] [PubMed] [Google Scholar]
- 21. Hessol NA, Holly EA, Efird JT, et al. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women AIDS. 2009. 23(1):59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. HIV Surveillance Report, 2009; vol.21 August 29, 2011.. [Google Scholar]
- 23. Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study Lancet Oncol. 2009. 10(12):1152–1159 [DOI] [PubMed] [Google Scholar]
- 24. Palefsky JM. Human papillomavirus infection and anogenital neoplasia in human immunodeficiency virus-positive men and women J Natl Cancer Inst Monogr. 1998;. 23 15–20 [DOI] [PubMed] [Google Scholar]
- 25. Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men AIDS. 2005. 19(13):1407–1414 [DOI] [PubMed] [Google Scholar]
- 26. D'Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study J Acquir Immune Defic Syndr. 2008. 48(4):491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li AH, Phanuphak N, Sahasrabuddhe VV, et al. Anal squamous intraepithelial lesions among HIV positive and HIV negative men who have sex with men in Thailand Sex Transm Infect. 2009. 85(7):503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis Lancet Oncol. 2012. 13(5):487–500 [DOI] [PubMed] [Google Scholar]
- 29. Herbenick D, Reece M, Schick V, Sanders SA, Dodge B, Fortenberry JD. Sexual behavior in the United States: results from a national probability sample of men and women ages 14-94 J Sex Med. 2010. 7(suppl 5):255–265 [DOI] [PubMed] [Google Scholar]
- 30. Goodman MT, Shvetsov YB, McDuffie K, et al. Acquisition of anal human papillomavirus (HPV) infection in women: the Hawaii HPV Cohort study J Infect Dis. 2008. 197(7):957–966 [DOI] [PubMed] [Google Scholar]
- 31. Nyitray AG, Carvalho da Silva RJ, Baggio ML, et al. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study J Infect Dis. 2011. 203(1):49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States J Clin Oncol. 2011. 29(32):4294–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000 Cancer. 2004. 100(5):1035–1044 [DOI] [PubMed] [Google Scholar]
- 34. Bodelon C, Madeleine MM, Voigt LF, Weiss NS. Is the incidence of invasive vulvar cancer increasing in the United States? Cancer Causes Control. 2009. 20(9):1779–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions Int J Cancer. 2009. 124(10):2375–2383 [DOI] [PubMed] [Google Scholar]
- 36. Kreimer AR, Gonzalez P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial Lancet Oncol. 2011. 12(9):862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia N Engl J Med. 2011. 365(17):1576–1585 [DOI] [PubMed] [Google Scholar]
- 38. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007. 56(RR-2):1–24 [PubMed] [Google Scholar]
- 39.Dunne EF, Markowitz LE, Chesson H, et al. Recommendations on the Use of Quadrivalent Human Papillomavirus Vaccine in Males - Advisory Committee on Immunization Practices (ACIP), 2011MMWR Morb Mortal Wkly Rep.2011. 60 1705–1708 [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009Morb Mortal Wkly Rep.2010. 59(32):1018–1023 [PubMed] [Google Scholar]
- 41. Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States Ann Intern Med. 2010. 153(7):452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men J Infect Dis. 2010. 202(8):1246–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men JAMA. 1999. 281(19):1822–1829 [DOI] [PubMed] [Google Scholar]
- 44. Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Palefsky JM. Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men Am J Med. 2000. 108(8):634–641 [DOI] [PubMed] [Google Scholar]
- 45. New York State Department of Health. New York State Department of Health AIDS Institute Clinical Guidelines. Section V: Anal Dysplasia and Cancer; Albany, NY: New York State Department of Health AIDS Institute; 2007. [Google Scholar]
- 46. Katz KA, Clarke CA, Bernstein KT, Katz MH, Klausner JD. Is there a proven link between anal cancer screening and reduced morbidity or mortality? Ann Intern Med. 2009. 150(4):283–284 [DOI] [PubMed] [Google Scholar]
- 47. Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006 J Acquir Immune Defic Syndr. 2010. 53(5):619–624 [DOI] [PubMed] [Google Scholar]


