Abstract
Background
High-quality care must be not only appropriate but also timely. We assessed time to initiation of adjuvant chemotherapy for breast cancer as well as factors associated with delay to help identify targets for future efforts to reduce unnecessary delays.
Methods
Using data from the National Comprehensive Cancer Network (NCCN) Outcomes Database, we assessed the time from pathological diagnosis to initiation of chemotherapy (TTC) among 6622 women with stage I to stage III breast cancer diagnosed from 2003 through 2009 and treated with adjuvant chemotherapy in nine NCCN centers. Multivariable models were constructed to examine factors associated with TTC. All statistical tests were two-sided.
Results
Mean TTC was 12.0 weeks overall and increased over the study period. A number of factors were associated with a longer TTC. The largest effects were associated with therapeutic factors, including immediate postmastectomy reconstruction (2.7 weeks; P < .001), re-excision (2.1 weeks; P < .001), and use of the 21-gene reverse-transcription polymerase chain reaction assay (2.2 weeks; P < .001). In comparison with white women, a longer TTC was observed among black (1.5 weeks; P < .001) and Hispanic (0.8 weeks; P < .001) women. For black women, the observed disparity was greater among women who transferred their care to the NCCN center after diagnosis (P interaction = .008) and among women with Medicare vs commercial insurance (P interaction < .001).
Conclusions
Most observed variation in TTC was related to use of appropriate therapeutic interventions. This suggests the importance of targeted efforts to minimize potentially preventable causes of delay, including inefficient transfers in care or prolonged appointment wait times.
A number of clinical trials demonstrating the benefit of adjuvant chemotherapy have been published over the past 20 years (1) and clinical practice guidelines recommend chemotherapy for many breast cancer patients following completion of definitive surgery to reduce the risk of recurrence (2). The optimal time interval between diagnosis and initiation of adjuvant chemotherapy is unclear. Long intervals between surgery and chemotherapy have been associated with poorer disease-specific outcomes (3–5), although null associations between time to chemotherapy (TTC) and outcomes have also been reported (6). No studies were identified that examined the impact of the diagnosis to chemotherapy interval on patient outcomes.
Currently, several professional societies endorse time-dependent quality measures. For example, one of the American Society of Clinical Oncology (ASCO)/National Comprehensive Cancer Network (NCCN) quality measures recommends adjuvant chemotherapy within 120 days of diagnosis for women aged less than 70 years with stage II or stage III hormone receptor–negative breast cancer (7). In reviewing concordance with this measure in NCCN centers, Hughes et al. (8) found that treatment for 87% of patients met the quality measure; however, 6% of patients (47% of nonconcordant patients) were nonconcordant because chemotherapy began more than 120 days after diagnosis.
In this analysis, we sought to examine the sociodemographic, clinical, and treatment factors associated with an increased TTC initiation at NCCN centers. Our goals were to characterize patients who might be at increased risk for delay and to identify potentially mutable factors contributing to delay.
Methods
Data Source
The analysis was conducted using data from the NCCN outcomes database (9). Data are ascertained through regular standardized medical record reviews and a patient survey administered at first presentation (10–13). Nine institutions participating in the NCCN database during the entire study period contributed data: City of Hope Comprehensive Cancer Center, Duarte, California; Dana-Farber/Brigham Women’s Cancer Center, Boston, Massachusetts; Fox Chase Cancer Center, Philadelphia, Pennsylvania; The University of Texas MD Anderson Cancer Center, Houston, Texas; H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida; Arthur G. James Cancer Hospital and Richard J. Solove Research Institute at The Ohio State University, Columbus, Ohio; Roswell Park Cancer Institute, Buffalo, New York; University of Michigan Comprehensive Cancer Center, Ann Arbor, Michigan; and UNMC Eppley Cancer Center at the Nebraska Medical Center, Omaha, Nebraska. Institutional review boards from participating centers approved all data collection, transmission, and storage protocols. At centers requiring patient consent, only consented patients are included in the database.
Cohort Selection
Overall, 19759 women with stage I to stage III unilateral breast cancer who presented between January 2003 and December 2009 were identified. Patients were sequentially excluded if they had less than 180 days of follow-up (n = 1624; 8%), neoadjuvant therapy (n = 3814; 21%), an unknown type or date of definitive surgery or biopsy (n = 250; 2%), no adjuvant chemotherapy (n = 6609; 47%), radiation therapy before adjuvant chemotherapy (n = 132; 2%), adjuvant chemotherapy at a non-NCCN institution (n = 1068; 15%), or adjuvant chemotherapy more than 32 weeks after diagnosis (n = 40; <1%). Patients initiating chemotherapy more than 32 weeks after diagnosis were excluded to avoid extreme outlier values skewing mean TTC in the parametric models. After all exclusions, 6222 patients who received adjuvant chemotherapy at the NCCN center were included in the analytical cohort (Table 1).
Table 1.
Distribution of the number and proportion of patients and unadjusted mean time to chemotherapy (TTC) of the sociodemographic, referral pattern, clinical, and therapeutic factors examined*
| Characteristics | No. (%) | Arthimetic mean TTC (SD) |
|---|---|---|
| Sociodemographic factors | ||
| Age at diagnosis, y | ||
| <40 | 793 (13) | 11.2 (4.4) |
| 40–54 | 3180 (51) | 12.0 (4.5) |
| 55–70 | 1994 (32) | 12.2 (4.5) |
| >70 | 255 (4) | 13.1 (4.3) |
| Race/ethnicity | ||
| White | 4848 (78) | 11.6 (4.2) |
| Hispanic | 462 (7) | 13.4 (5.1) |
| Black | 553 (9) | 13.9 (5.3) |
| Asian | 210 (3) | 12.0 (4.9) |
| Other | 149 (2) | 12.2 (4.4) |
| Community SES | ||
| High | 1950 (31) | 11.5 (4.2) |
| Intermediate | 2017 (32) | 11.8 (4.4) |
| Low | 1936 (31) | 12.7 (4.9) |
| Unknown | 319 (5) | 11.8 (4.4) |
| Insurance | ||
| Commercial | 4895 (79) | 11.6 (4.3) |
| Medicare | 679 (11) | 12.8 (4.6) |
| Medicaid | 476 (8) | 14.9 (5.3) |
| Other | 172 (3) | 11.4 (4.8) |
| Residential distance to institution | ||
| <30 miles | 3882 (62) | 12.0 (4.5) |
| 30–60 miles | 1045 (17) | 11.9 (4.5) |
| 61–120 miles | 676 (11) | 12.0 (4.4) |
| >120 miles | 453 (7) | 12.2 (4.8) |
| Unknown/foreign | 166 (3) | 10.8 (4.3) |
| Referral patterns | ||
| Diagnosing institution | ||
| NCCN | 1656 (27) | 11.0 (3.9) |
| Non-NCCN | 4566 (73) | 12.4 (4.6) |
| Clinical factors | ||
| Clinical tumor stage | ||
| cTis | 175 (3) | 13.9 (4.6) |
| cT1 | 3296 (53) | 11.9 (4.3) |
| cT2 | 1781 (29) | 12.0 (4.6) |
| cT3/4 | 194 (3) | 12.2 (4.8) |
| Unknown | 776 (12) | 12.0 (4.9) |
| Clinical node stage | ||
| cN0 | 4503 (72) | 12.1 (4.4) |
| cN1 or greater | 896 (14) | 11.8 (4.6) |
| Unknown | 823 (13) | 11.8 (4.9) |
| Pathological upstage | ||
| No | 2709 (43) | 11.9 (4.5) |
| Yes | 2340 (38) | 12.2 (4.4) |
| Unknown | 1173 (19) | 11.8 (4.8) |
| Lymphovascular invasion | ||
| No | 4091 (66) | 12.1 (4.5) |
| Yes | 2131 (34) | 11.7 (4.4) |
| High grade | ||
| No | 3535 (57) | 12.3 (4.6) |
| Yes | 2687 (43) | 11.6 (4.3) |
| ER/PR status | ||
| Negative | 1805 (29) | 11.5 (4.4) |
| Positive | 4417 (71) | 12.2 (4.5) |
| HER2 status | ||
| Negative | 4902 (79) | 12.0 (4.5) |
| Positive | 1320 (21) | 11.8 (4.4) |
| Charlson comorbidity score | ||
| 0 | 5018 (81) | 11.8 (4.4) |
| 1 | 797 (13) | 12.6 (4.6) |
| >1 | 407 (6) | 13.1 (4.9) |
| BMI | ||
| Underweight | 87 (1) | 11.8 (5.3) |
| Normal weight | 2200 (35) | 11.6 (4.3) |
| Overweight | 1836 (29) | 11.9 (4.4) |
| Obese | 1576 (25) | 12.5 (4.7) |
| Morbidly obese | 325 (5) | 12.8 (4.7) |
| Unknown | 198 (3) | 11.5 (4.1) |
| Therapeutic factors | ||
| Excisional procedures | ||
| 1 | 4117 (66) | 11.5 (4.3) |
| 2 | 1839 (30) | 12.4 (4.4) |
| >2 | 266 (4) | 16.2 (5.2) |
| Reconstruction before adjuvant therapy | ||
| No | 5106 (82) | 11.5 (4.3) |
| Yes | 1116 (18) | 14.3 (4.8) |
| Received ALND | ||
| No | 2727 (44) | 11.9 (4.3) |
| Yes | 3495 (56) | 12.1 (4.6) |
| Diagnostic breast ultrasound | ||
| No | 1636 (26) | 11.8 (4.7) |
| Yes | 4586 (74) | 12.1 (4.4) |
| Diagnostic breast MRI | ||
| No | 4974 (80) | 11.8 (4.5) |
| Yes | 1284 (20) | 12.6 (4.4) |
| 21-gene RT-PCR assay | ||
| No | 5751 (92) | 11.8 (4.4) |
| Yes | 471 (8) | 14.3 (4.7) |
* ALND = axillary lymph node dissection; BMI = body mass index; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; MRI = magnetic resonance imaging; NCCN = National Comprehensive Cancer Network; PR = progesterone receptor; RT-PCR = reverse-transcription polymerase chain reaction; SES = socioeconomic status.
Data Definitions
TTC was defined as the number of weeks between pathological diagnosis and first administration of adjuvant chemotherapy. Patient presentation date was defined as the date of initial clinic visit at the treating NCCN center.
Residential distance to the institution was computed as the great-circle distance between the centroid of the patient’s zip code and the main campus of the treating NCCN center. Community-level socioeconomic status was defined at the zip code level using year 2000 Census data reduced to a single variable by factor analysis (R 2 = 69%). Census elements examined include median household income (mean = $48865; factor score [fs] = 0.86); proportion single parent households (mean = 12%; fs = −0.86); proportion aged more than 25 years without a high school diploma (mean = 16%; fs = −0.82); proportion of households below the poverty level (mean = 10%; fs = −0.92); and proportion of vacant households (mean = 4%; fs = −0.69). The validity of composite measures of area-level socioeconomic status has been evaluated previously (14).
Both clinical and pathologic staging are recorded according to American Joint Committee on Cancer TNM (tumor size, lymph nodes affected, metastases) criteria (15). We defined pathologic upstaging as an increase in stage between clinical and pathologic evaluations. Comorbidity was measured using the Charlson comorbidity index (16). Body mass index was calculated from the patient’s height and weight at first visit and classified as underweight (<18.5 km/m2), normal weight (18.5 to <25.0kg/m2), overweight (25.0 to <30.0kg/m2), obese (30.0 to 40kg/m2), or morbidly obese (>40kg/m2).
Breast ultrasound or magnetic resonance image was considered diagnostic if performed between 90 days before diagnosis and initial excision. Biopsy was classified as needle (fine needle aspiration or core needle biopsy) or surgical biopsy (excisional or incisional procedure).
Surgery was classified as breast conserving surgery or mastectomy, definitive surgery was defined as the last excision performed on the ipsilateral breast before adjuvant chemotherapy, and number of excisions was defined as the number of ipsilateral excisional procedures performed on separate days before initiation of adjuvant chemotherapy. Excisional procedures include surgical biopsies and therapeutic excisions because of the difficulty in distinguishing procedures performed with a diagnostic vs therapeutic intent. Location of diagnosis was defined as the location (either NCCN center or outside institution) where the diagnostic biopsy was performed. At matrix centers, patients referred to the cancer center after diagnosis elsewhere in the health system were classified as diagnosed outside.
Statistical Analysis
All analyses utilized SAS version 9.2 (SAS Inc, Cary, NC). An alpha of 0.05 denoted statistical significance. All tests were two-sided. Spearman correlation (r s) and paired t tests were used to compare subsets of the TTC interval. Linear contrast was used to assess changes in mean TTC by year of presentation. Probability ratios (PRs) were computed to assess the association between proportions.
Analysis of covariance was used to compare the association between each independent variable and TTC. Results are reported as the adjusted estimates of the difference between each level of the regression variable and the reference level (denoted as ΔTTC). Interactions between variables were evaluated for statistical significance by including interaction terms in the model. Results of three analysis of covariance models are presented in Table 2. The unadjusted model includes only the independent variable. The institution-adjusted model contains ΔTTC values adjusted for the treating NCCN institution. The multivariable adjusted analysis of covariance model includes adjustment for all factors in Table 2 plus institution and type of diagnostic biopsy and initial surgery. A fourth multivariable model was constructed to evaluate colinearity between surgical factors (Table 3). In this model, initial surgical strategy, number of excisions, and receipt of reconstruction were combined into a single composite variable.
Table 2.
Full modeling results detailing change in time to chemotherapy (ΔTTC) measured in weeks for each factor compared with the noted reference group*
| Unadjusted | Institution-adjusted | Multivariable-adjusted | |
|---|---|---|---|
| Characteristics | ΔTTC (95% CI) | ΔTTC (95% CI) | ΔTTC (95% CI) |
| Sociodemographic factors | |||
| Age at diagnosis, y | |||
| <40 | −0.8 (−1.3 to −0.4)§ | −0.8 (−1.2 to −0.3)§ | −0.9 (−1.3 to −0.5)§ |
| 40–54 | Referent | Referent | Referent |
| 55–70 | +0.2 (−0.1 to +0.5) | +0.2 (−0.1 to +0.5) | +0.3 (0.0 to +0.6)† |
| >70 | +1.1 (+0.4 to +1.9)§ | +0.9 (+0.2 to +1.7)‡ | +1.0 (+0.2 to +1.8)‡ |
| Race/ethnicity | |||
| White | Referent | Referent | Referent |
| Hispanic | +1.8 (+1.2 to +2.4)§ | +1.3 (+0.7 to +1.9)§ | +0.8 (+0.3 to +1.4)§ |
| Black | +2.3 (+1.7 to +2.8)§ | +2.0 (+1.4 to +2.5)§ | +1.5 (+1.0 to +2.0)§ |
| Asian | +0.3 (−0.5 to +1.2) | −0.1 (−0.9 to +0.8) | +0.4 (−0.3 to +1.2) |
| Other | +0.7 (−0.3 to +1.7) | +0.7 (−0.3 to +1.6) | +0.4 (−0.4 to +1.3) |
| Community SES | |||
| High | Referent | Referent | Referent |
| Intermediate | +0.3 (0.0 to +0.7) | +0.4 (0.0 to +0.8)† | +0.3 (0.0 to +0.7)† |
| Low | +1.2 (+0.8 to +1.6)§ | +1.1 (+0.8 to +1.5)§ | +0.6 (+0.2 to +1.0)§ |
| Unknown | +0.3 (−0.4 to +1.0) | +0.1 (−0.6 to +0.8) | +0.6 (−0.2 to +1.5) |
| Insurance | |||
| Commercial | Referent | Referent | Referent |
| Medicare | +1.2 (+0.7 to +1.7)§ | +1.1 (+0.7 to +1.6)§ | +0.7 (+0.2 to +1.2)‡ |
| Medicaid | +3.2 (+2.7 to +3.8)§ | +3.1 (+2.6 to +3.7)§ | +2.8 (+2.3 to +3.3)§ |
| Other | −0.2 (−1.1 to +0.6) | +0.1 (−0.8 to +1.0) | +0.4 (−0.4 to +1.2) |
| Residential distance to institution | |||
| <30 miles | Referent | Referent | Referent |
| 30–60 miles | −0.1 (−0.6 to +0.3) | +0.2 (−0.2 to +0.6) | +0.1 (−0.2 to +0.5) |
| 61–120 miles | −0.1 (−0.6 to +0.4) | +0.4 (−0.1 to +0.9) | +0.3 (−0.2 to +0.8) |
| >120 miles | +0.2 (−0.4 to +0.8) | −0.2 (−0.8 to +0.5) | −0.3 (−0.9 to +0.3) |
| Unknown/foreign | −1.3 (−2.2 to −0.3)‡ | −1.0 (−1.9 to 0.0) | −1.3 (−2.5 to −0.0)† |
| Referral patterns | |||
| Diagnosing institution | |||
| NCCN | Referent | Referent | Referent |
| Non-NCCN | +1.4 (+1.1 to +1.6)§ | +0.9 (+0.7 to +1.2)§ | +1.1 (+0.9 to +1.3)§ |
| Clinical factors | |||
| Clinical tumor stage | |||
| cTis | +2.1 (+1.1 to +3.0)§ | +2.3 (+1.3 to +3.2)§ | +1.7 (+0.8 to +2.6)§ |
| cT1 | Referent | Referent | Referent |
| cT2 | +0.2 (−0.2 to +0.5) | +0.1 (−0.2 to +0.5) | 0.0 (−0.3 to +0.3) |
| cT3/4 | +0.3 (−0.5 to +1.3) | +0.3 (−0.6 to +1.1) | 0.0 (−0.8 to +0.9) |
| Unknown | +0.2 (−0.3 to +0.6) | +0.2 (−0.3 to +0.6) | +0.5 (−0.2 to +1.3) |
| Clinical node stage | |||
| cN0 | Referent | Referent | Referent |
| cN1 or greater | −0.3 (−0.7 to +0.1) | −0.3 (−0.7 to 0.0) | −0.4 (−0.8 to 0.0)† |
| Unknown | −0.3 (−0.7 to +0.1) | −0.4 (−0.8 to −0.1)† | −0.5 (−1.2 to +0.1) |
| Pathological upstage | |||
| No | Referent | Referent | Referent |
| Yes | +0.2 (−0.1 to +0.5) | +0.2 (−0.1 to +0.5) | −0.2 (−0.5 to +0.1) |
| Unknown | −0.2 (−0.5 to +0.2) | −0.1 (−0.5 to +0.2) | −0.4 (−1.2 to +0.4) |
| Lymphovascular invasion | |||
| No | Referent | Referent | Referent |
| Yes | −0.4 (−0.6 to −0.2)‡ | −0.4 (−0.6 to −0.1)‡ | −0.4 (−0.6 to −0.2)§ |
| High grade | |||
| No | Referent | Referent | Referent |
| Yes | −0.6 (−0.9 to −0.4)§ | −0.6 (−0.9 to −0.4)§ | −0.3 (−0.5 to 0.0)† |
| ER/PR status | |||
| Negative | Referent | Referent | Referent |
| Positive | +0.7 (+0.5 to +1.0)§ | +0.7 (+0.4 to +0.9)§ | +0.2 (−0.0 to +0.4) |
| HER2 status | |||
| Negative | Referent | Referent | Referent |
| Positive | −0.3 (−0.5 to +0.0) | −0.2 (−0.5 to +0.0) | 0.0 (−0.2 to +0.2) |
| Charlson comorbidity score | |||
| 0 | Referent | Referent | Referent |
| 1 | +0.8 (+0.4 to +1.2)§ | +0.6 (+0.2 to +1.0)§ | +0.4 (+0.0 to +0.7)† |
| >1 | +1.3 (+0.7 to +1.8)§ | +1.2 (+0.6 to +1.7)§ | +0.8 (+0.4 to +1.3)§ |
| BMI | |||
| Underweight | +0.2 (−1.2 to +1.6) | +0.2 (−1.1 to +1.6) | −0.2 (−1.4 to +1.0) |
| Normal weight | Referent | Referent | Referent |
| Overweight | +0.3 (−0.1 to +0.7) | +0.3 (−0.1 to +0.7) | +0.2 (−0.2 to +0.5) |
| Obese | +0.8 (+0.4 to +1.3)§ | +0.8 (+0.4 to +1.3)§ | +0.6 (+0.2 to +1.0)§ |
| Morbidly obese | +1.2 (+0.4 to +2.0)§ | +1.3 (+0.6 to +2.1)§ | +1.3 (+0.7 to +2.0)§ |
| Unknown | −0.1 (−1.0 to +0.9) | +0.5 (−0.4 to +1.5) | +0.3 (−0.5 to +1.2) |
| Therapeutic factors | |||
| Excisional procedures | |||
| 1 | Referent | Referent | Referent |
| 2 | +0.8 (+0.5 to +1.1)§ | +0.8 (+0.5 to +1.0)§ | +2.1 (+1.7 to +2.4)§ |
| >2 | +4.6 (+4.0 to +5.3)§ | +4.4 (+3.8 to +5.1)§ | +5.7 (+5.0 to +6.3)§ |
| Reconstruction before adjuvant therapy | |||
| No | Referent | Referent | Referent |
| Yes | +2.8 (+2.5 to +3.1)§ | +2.7 to (+2.4 to +2.9)§ | +2.7 (+2.4 to +3.0)§ |
| Received ALND | |||
| No | Referent | Referent | Referent |
| Yes | +0.2 (0.0 to +0.5)† | +0.4 (+0.2 to +0.6)§ | +0.5 (+0.2 to +0.7)§ |
| Diagnostic breast ultrasound | |||
| No | Referent | Referent | Referent |
| Yes | +0.2 (−0.0 to +0.5) | −0.1 (−0.4 to +0.1) | +0.2 (−0.0 to +0.4) |
| Diagnostic breast MRI | |||
| No | Referent | Referent | Referent |
| Yes | +0.8 (+0.5 to +1.0)§ | +0.8 (+0.5 to +1.1)§ | +0.8 (+0.5 to +1.0)§ |
| 21-gene RT-PCR assay | |||
| No | Referent | Referent | Referent |
| Yes | +2.5 (+2.1 to +2.9)§ | +2.5 (+2.1 to +2.9)§ | +2.2 (+1.9 to +2.6)§ |
* Institution-adjusted ΔTTC are adjusted for only treating institution. Multivariable ΔTTC are adjusted for all factors listed plus type of diagnostic biopsy, type of initial surgery, and treating institution. The sign denotes where the reported ΔTTC is longer (+) or shorter (−) than the noted reference group. ALND = axillary lymph node dissection; BMI = body mass index; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; MRI = magnetic resonance imaging; NCCN = National Comprehensive Cancer Network; PR = progesterone receptor; RT-PCR = reverse-transcription polymerase chain reaction; SES = socioeconomic status.
† P < .05.
‡ P < .01.
§ P < .001.
Table 3.
Change in time to chemotherapy (TTC) by surgical pathway*
| Surgical pathway | No. (%) | Mean (SD) | ΔTTC (95% CI) |
|---|---|---|---|
| Mastectomy: no reconstruction | 1166 (19) | 11.4 (4.3) | 0.5 (+0.0 to +1.0)† |
| Mastectomy: with reconstruction | 784 (13) | 13.8 (4.6) | 3.0 (+2.5 to +3.6)‡ |
| Needle biopsy: BCS: no re-excision | 1973 (32) | 11.0 (3.9) | Referent |
| Needle biopsy: BCS: BCS re-excision | 550 (9) | 13.1 (4.1) | +2.1 (+1.5 to +2.7)‡ |
| Needle biopsy: BCS: mastectomy re-excision: no reconstruction | 159 (3) | 14.5 (4.7) | +3.5 (+2.5 to +4.5)‡ |
| Needle biopsy: BCS: mastectomy re-excision: with reconstruction | 78 (1) | 17.8 (4.9) | +6.5 (+5.0 to +7.9)‡ |
| Surgical biopsy: BCS: no re-excision | 211 (3) | 9.2 (4.5) | −1.7 (−2.6 to −0.7)‡ |
| Surgical biopsy: BCS: BCS re-excision | 661 (11) | 11.5 (4.4) | +0.7 (+0.1 to +1.2)† |
| Surgical biopsy: BCS: mastectomy re-excision: no reconstruction | 397 (6) | 12.0 (4.6) | +1.0 (+0.3 to +1.8)‡ |
| Surgical biopsy: BCS: mastectomy re-excision: with reconstruction | 243 (4) | 14.7 (4.9) | +3.7 (+2.8 to +4.6)‡ |
* Pathways were constructed by combining diagnostic biopsy, type of initial surgery, receipt and type of re-excision, and use of reconstruction. Mean is the unadjusted arithmetic mean. ΔTTC data are adjusted for institution and all factors listed in Table 2 except those factors included in the composite surgical pathway. The sign denotes where the reported ΔTTC is longer (+) or shorter (−) than the noted reference group. BCS = breast-conserving surgery.
† P < .05.
‡ P < .001.
Results
Characteristics of patients included in the analysis are shown in Table 1. Arithmetic mean TTC was 12.0 weeks (SD = 4.5). Median TTC was 11.3 weeks (Figure 1). The mean interval between diagnosis and definitive surgery was 5.6 weeks (SD = 3.6) and between definitive surgery and chemotherapy was 6.3 weeks (SD = 2.9; P < .001). Mean TTC increased monotonically over the study period (Figure 2), from 10.8 weeks in 2003 to 13.3 weeks in 2009 (P trend < .001).
Figure 1.
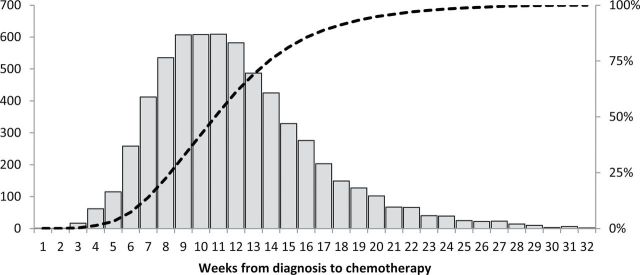
Distribution of weeks from diagnosis to chemotherapy. Bars refer to patient numbers on the y axis on the left. The dashed line refers to the cumulative percentage of all patients on the y axis on the right.
Figure 2.
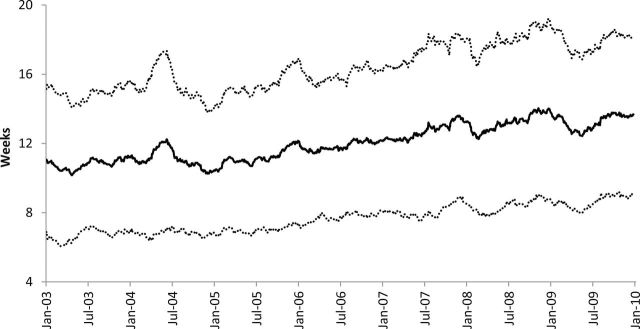
Weeks from diagnosis to chemotherapy by date of presentation to the National Comprehensive Cancer Network center. The solid black line refers to the 90-day simple moving average (SMA). Dashed lines refer to the SMA +/– 1 SD.
Sociodemographic Characteristics and Referral Patterns
A number of patient sociodemographic characteristics were associated with longer TTC (Table 2). Increasing age, decreasing community-level socioeconomic status, and Medicare or Medicaid insurance were associated with increased TTC in adjusted analyses. Compared with white women, black (1.5 weeks; P < .001) and Hispanic (0.8 weeks; P < .001) women experienced a longer TTC.
Examining referral patterns, 73% of patients had their diagnostic procedure performed before presentation to the NCCN center (ie, diagnosed outside). These patients experienced a TTC that was 1.1 week longer than those diagnosed at an NCCN center, adjusting for other factors (Table 2).
To better understand potential moderators of the association between race/ethnicity and TTC, we assessed for interactions between those variables and community-level socioeconomic status, diagnosis at an outside center, and insurance type. Statistically significant interactions were observed between race/ethnicity and both insurance type (P < .001) and diagnosing institution (P = .008). No interaction was observed between community-level socioeconomic status and race/ethnicity (P = .79).
In comparison with white patients, black patients with Medicare (ΔTTC = +2.6 weeks, 95% confidence interval [CI] = +1.0 to +4.2, P < .001) experienced a twofold longer ΔTTC than patients with commercial insurance (ΔTTC = +1.1 weeks, 95% CI = +0.2 to +1.9, P < .001). Differences between white and black (ΔTTC = +1.0 weeks, 95% CI = −0.7 to +2.8, P = .85) women with Medicaid were not statistically significant. Among the subset of patients with Medicare (n = 623), black women were more likely to be aged less than 65 years (PR = 2.1, 95% CI = 1.6 to 2.9, P < .001) and without supplemental insurance (PR = 2.1, 95% CI = 1.4 to 3.1, P = .002) than white women.
Referral after diagnosis also appeared to disproportionately impact black women. Relative to white women, black women diagnosed at an outside center before presentation at the NCCN center (ΔTTC = +2.6 weeks, 95% CI = +1.4 to +3.8, P < .001) experienced a twofold greater disparity in TTC compared with black women diagnosed at the NCCN center (ΔTTC = +1.4 weeks, 95% CI = 0.0 to +2.7, P = .03).
Clinical Characteristics
TTC increased with a greater number of comorbid conditions and increasing body mass index, controlling for other factors (Table 2). In contrast, tumor characteristics had little effect except that patients with a clinical diagnosis of noninvasive disease (ie, cTis) and subsequent pathological diagnosis of invasive disease experienced an adjusted delay in TTC of 1.7 weeks.
Diagnostic and Therapeutic Interventions
A number of therapeutic factors were associated with substantial effects on TTC (Table 2). Compared with a single excision, having a second excision added 2.1 weeks (P < .001) and having a third excision added 5.7 weeks in TTC after adjustment for other factors including biopsy type. Postmastectomy reconstruction was associated with an additional 2.7 weeks (P < .001), and axillary lymph node dissection was associated with an additional 0.5 weeks. Diagnostic breast magnetic resonance image increased TTC by 0.8 weeks, whereas breast ultrasound had no statistically significant effect. The diagnostic test with the largest impact was the 21-gene reverse-transcription polymerase chain reaction (RT-PCR) assay, which was associated with a 2.2 week increase in TTC in the adjusted analysis (P < .001). All these differences were highly statistically significant after controlling for other factors.
In the model that replaced the individual components of surgical care with a composite surgical management pathway (Table 3), mastectomy without reconstruction was associated with an adjusted TTC that was 0.5 week longer than that of a needle biopsy followed by breast-conserving surgery without re-excision. Receipt of a surgical biopsy followed by breast-conserving surgery (ΔTTC = +0.7) or mastectomy without reconstruction (ΔTTC = +1.0) were also associated with a longer TTC relative to needle biopsy followed by a single breast-conserving surgery excision.
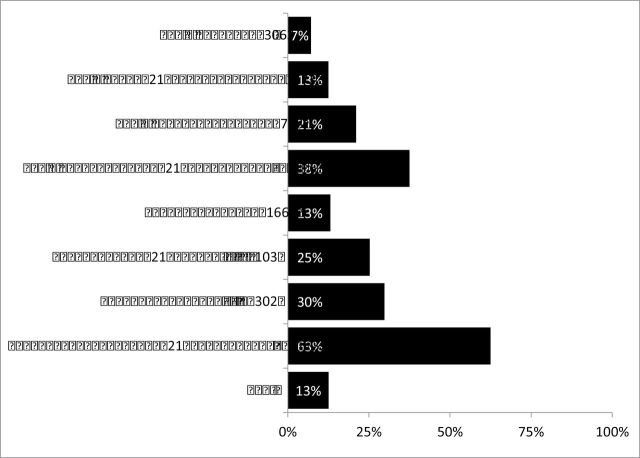
A total of 13% of patients received chemotherapy more than 120 days after diagnosis (Figure 3). Considerable variability was observed based on individual therapeutic pathways. The addition of the 21-gene RT-PCR assay to a single excision nearly doubled (PR = 1.7, 95% CI = 1.2 to 2.4, P = .001) the probability of initiating chemotherapy more than 120 days after diagnosis, and reconstruction tripled (PR = 2.9, 95% CI = 2.4 to 3.6, P < .001) the probability of initiating chemotherapy more than 120 days after diagnosis. This increased to a 3.5 to 4 times greater likelihood of a TTC of more than 120 days when re-excision was combined with receipt of the 21-gene RT-PCR assay (PR = 3.5, 95% CI = 2.5 to 5.0, P < .001) or reconstruction (PR = 4.2, 95% CI = 3.4 to 4.2, P < .001) compared with single excision alone.
Figure 3.
Proportion of patients receiving chemotherapy more than 120 days after diagnosis by composite therapeutic pathway. Ex = excision; Recon = reconstruction.
Discussion
In a large, multi-institutional cohort of women with breast cancer, time from diagnosis to initiation of adjuvant chemotherapy was approximately 12 weeks. This interval increased steadily from 10.8 to 13.3 weeks between 2003 and 2009. The largest effects were associated with diagnostic and therapeutic interventions, including immediate postmastectomy reconstruction, receipt of re-excision, and use of the 21-gene RT-PCR assay. In addition, we found that structural or systems factors, including insurance type and patient referral patterns, appeared to disproportionately impact TTC for black women.
There are few published reports examining timing in the initiation of adjuvant chemotherapy for breast cancer patients. A study of time to any adjuvant therapy from two regional centers in Nova Scotia reported that, in 2003, the mean time to surgery was 3 weeks for all patients and time from surgery to chemotherapy was 7 weeks (17). This is similar to the TTC of 10.8 weeks observed at the beginning of our study period, despite differences in health-care delivery between the United States and Canada. An analysis of data from the National Cancer Database found that time from definitive surgery to chemotherapy in the United States was 6 weeks, which is, again, consistent with our findings. The National Cancer Database study also reported that black and Hispanic women were at increased risk for long delays (18). Our analysis adds to this literature by examining trends over time, evaluating detailed diagnostic, therapeutic, patient-related, and system-related factors and by assessing factors that may be moderating disparities in time to treatment.
Our analysis has a number of strengths, including a large sample size from multiple institutions and access to rich clinical and treatment data. Further, this analysis examines chemotherapy timing from diagnosis rather than surgery, permitting us to assess the impacts surgical patterns of care have on chemotherapy timing. This analysis also has several limitations. Most important, this is a study of patterns of care in tertiary care centers, and the results may not be generalizable to other settings. Further, this analysis excluded patients with a TTC greater than or equal to 32 weeks, which limits the generalizability of these findings to patients with exceedingly long delays in chemotherapy. The exclusion of patients who received neoadjuvant therapy and patients who omitted chemotherapy limits our ability to assess the impact of clinical factors that are strongly associated with those patterns of care (19) A formal evaluation of the relationship between therapy timing and the appropriate use of chemotherapy was not conducted, although individual clinical parameters were either unrelated or minimally associated with delays in chemotherapy. Lastly, these data only consider time to first dose of chemotherapy, so they do not speak to choice of regimen or treatment completion once initiated.
Interestingly, the factors most strongly associated with a longer TTC included several that may represent higher quality care (re-excision to achieve clear margins or immediate reconstruction) or technological advances in care (21-gene RT-PCR assay). This finding highlights how advances along one dimension of care may negatively affect performance in other aspects of care, and it makes it all the more important to work toward minimizing potentially preventable causes of delay, including inefficient transfers in care or prolonged appointment wait times. Further, these data suggest specific interventions that might be effective in shortening time to treatment. For example, if 21-gene RT-PCR assay testing were expedited for patients who were farther from diagnosis, it could help reduce the number of patients in an institution initiating chemotherapy more than 120 days after diagnosis.
Our finding that TTC is often prolonged by appropriate diagnostic and therapeutic interventions has implications for performance measures that include time to treatment thresholds. For example, the ASCO/NCCN quality measures recommend chemotherapy for stage II and stage III patients with hormone receptor–negative cancer be initiated within 120 days of diagnosis (7). The 120-day threshold was selected as a “reasonable estimate of the time required to deliver the preceding components of therapy that would not jeopardize outcome” (7). The measure, as written, places equal weight on nonconcordance due to either the complete omission of therapy or receipt of delayed chemotherapy. This lack of distinction is problematic in that there is high-level evidence from randomized trials that suggests that selected patients benefit from chemotherapy (1,19), whereas there is currently limited evidence about the detriment attributable to delayed chemotherapy (3–6). Further, our data suggest that widespread adoption of the ASCO/NCCN breast cancer quality measures for adjuvant chemotherapy as an accountability measure could create misaligned incentives for physicians to alter their treatment recommendations—for example, by omitting or deferring reconstruction for selected patients or refusing to care for patients who wish to transfer their care well after diagnosis (20). Attention to these issues would be required to minimize any adverse effects of considering time to treatment as an accountability measure.
In examining nontherapeutic factors associated with TTC prolongation, patients who transferred their care to an NCCN center after diagnosis elsewhere experienced a 1-week delay in TTC. The underlying mechanism is unclear, but it may result from system-level factors such as delay in patient referrals (including self-referrals), transfer of health records, or repetition of diagnostic studies. Although it is reassuring that the effect of such transfers on time to treatment was modest, this is a potentially mutable contribution to overall delay that might be reduced further with attention to effective coordination within and across health systems. Delay associated with transitioning care after diagnosis was not uniformly experienced; black women who transferred care experienced a much longer relative TTC than white women.
Race also interacted with insurance type; black women with Medicare experienced a greater relative disparity in TTC than black women with a commercial payer. Without detailed ethnographic data on how individual women transitioned through the process, we cannot definitively characterize the specific mechanisms that led to delay. However, these data provide some indirect evidence. Black women in our cohort were less likely to have supplemental insurance, and those on Medicare were more likely to be aged less than 65 years at diagnosis, which suggests that higher rates of disability may have played a role.
Patient survival outcomes associated with a prolongation in adjuvant chemotherapy were not evaluated because of limited follow-up in this cohort. We intentionally selected a cohort diagnosed relatively recently to characterize current patterns of care and factors associated with delay. Future studies of patients diagnosed earlier with longer follow-up are needed to better assess the association of delay with breast cancer outcomes.
These data detail the relationship between a patient’s therapeutic path and timing in initiation of adjuvant chemotherapy. As a result, with only the knowledge that a patient experienced a long interval in time between diagnosis and chemotherapy initiation, a definite conclusion that the patient received inefficient or poor-quality care cannot be made. However, system-level level factors related to transferring care and insurance issues were also found to be associated with a prolongation in TTC. This latter point highlights that opportunities for improving the efficiency of care delivery do exist despite the observed confounding of therapeutic factors. A better understanding of the root cause of these system-level factors will be critical to developing interventions designed to alleviate observed disparities and improve access to care.
Funding
The National Comprehensive Cancer Network provides financial and material support for the development of the NCCN Outcomes Database, which is the source of data used in the current analysis. Data collection was funded in part by the National Cancer Institute (grant P50 CA89393 to Dana-Farber Cancer Institute).
The design of the study, analysis and interpretation of the data, and writing and submission of the manuscript were all conducted independently of the National Comprehensive Cancer Network. Collection of data used in this study was conducted as part of the general operation of the National Comprehensive Cancer Network Outcomes Database Project.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365(9472):1687–1717 [DOI] [PubMed] [Google Scholar]
- 2. Carlson RW, Allred DC, Anderson BP, et al. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer.. v1. Fort Washington, PA: National Comprehensive Cancer Network; 2009 [Google Scholar]
- 3. Colleoni M, Bonetti M, Coates AS, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J Clin Oncol. 2000; 18(3):584–590 [DOI] [PubMed] [Google Scholar]
- 4. Hershman DL, Wang X, McBride R, et al. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006; 99(3):313–321 [DOI] [PubMed] [Google Scholar]
- 5. Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006; 24(30):4888–4894 [DOI] [PubMed] [Google Scholar]
- 6. Cold S, During M, Ewertz M, et al. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer. 2005; 93(6):627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network quality measures. J Clin Oncol. 2008; 26(21):3631–3637 [DOI] [PubMed] [Google Scholar]
- 8. Hughes ME, Ottesen R, Niland JC, et al. Quality of breast cancer care in NCCN centers as assessed by the ASCO/NCCN quality measures: overall performance and reasons for nonconcordance. J Clin Oncol. 2009; 27(15s):6506 [Google Scholar]
- 9. Weeks JC. Outcomes assessment in the NCCN. Oncology (Williston Park). 1997; 11(11A):137–140 [PubMed] [Google Scholar]
- 10. Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: a study of the National Comprehensive Cancer Network. Ann Surg. 2006; 243(2): 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edge SB, Niland JC, Bookman MA, et al. Emergence of sentinel node biopsy in breast cancer as standard-of-care in academic comprehensive cancer centers. J Natl Cancer Inst. 2003; 95(20):1514–1521 [DOI] [PubMed] [Google Scholar]
- 12. Niland JC. NCCN Internet-based data system for the conduct of outcomes research. Oncology (Williston Park). 1998; 12(11A):142–146 [PubMed] [Google Scholar]
- 13. Weeks J. Outcomes assessment in the NCCN: 1998 update. National Comprehensive Cancer Network. Oncology (Williston Park). 1999; 13(5A): 69–71 [PubMed] [Google Scholar]
- 14. Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002; 156(5): 471–482 [DOI] [PubMed] [Google Scholar]
- 15. Greene FL. American Joint Committee on Cancer, American Cancer Society. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 6thed. New York: Springer; 2002; [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. . J Chronic Dis. 1987; 40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 17. Rayson D, Saint-Jacques N, Younis T, et al. Comparison of elapsed times from breast cancer detection to first adjuvant therapy in Nova Scotia in 1999/2000 and 2003/04. CMAJ. 2007; 176(3):327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fedewa SA, Ward EM, Stewart AK, et al. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. . J Clin Oncol. 2010;;28(27):4135–4141. [DOI] [PubMed] [Google Scholar]
- 19. Carlson RW, Allred DC, Anderson BP, et al. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. v2. Fort Washington, PA: National Comprehensive Cancer Network; 2011
- 20. Werner RM, Asch DA. The unintended consequences of publicly reporting quality information. JAMA. 2005; 293(10):1239–1244 [DOI] [PubMed] [Google Scholar]