Abstract
Background
Studies have suggested that the 5-year survival of women with ovarian cancer and a BRCA1 or BRCA2 mutation is better than expected. We sought to evaluate the impact of carrying a BRCA1 or BRCA2 mutation on long-term survival of women after a diagnosis of invasive ovarian cancer.
Methods
One thousand six hundred twenty-six unselected women diagnosed with invasive ovarian cancer in Ontario, Canada, or in Tampa, Florida, between 1995 and 2004 were followed for a mean of 6.9 years (range = 0.3 to 15.7 years). Mutation screening for BRCA1 and BRCA2 revealed mutations in 218 women (13.4%). Left-truncated survival analysis was conducted to estimate ovarian cancer–specific survival at various time points after diagnosis for women with and without mutations.
Results
In the 3-year period after diagnosis, the presence of a BRCA1 or BRCA2 mutation was associated with a better prognosis (adjusted hazard ratio = 0.68, 95% confidence interval [CI] = 0.48 to 0.98; P = .03), but at 10 years after diagnosis, the hazard ratio was 1.00 (95% CI = 0.83 to 1.22; P = .90). Among women with serous ovarian cancers, 27.4% of women who were BRCA1 mutation carriers, 27.7% of women who were BRCA2 carriers, and 27.1% of women who were noncarriers were alive at 12 years past diagnosis.
Conclusion
For women with invasive ovarian cancer, the short-term survival advantage of carrying a BRCA1 or BRCA2 mutation does not lead to a long-term survival benefit.
The majority of women diagnosed with invasive ovarian cancer in Canada or the United States will succumb to their disease, but approximately 35% of women with ovarian cancer (including 20% of patients with serous cancers) are expected to be long-term survivors and ultimately cured (1). Overall, 13% of unselected case patients of ovarian cancer are attributable to mutations in BRCA1 or BRCA2 (2,3). Several studies have examined survival after ovarian cancer for women with BRCA1 or BRCA2 mutations (4–14), and most report better survival for women with mutations (4,6–14). Observed differences in survival may be the result of differences in the intrinsic aggressiveness of hereditary vs nonhereditary cancers, differences in the ages of diagnosis and/or histologic subtypes, or differences in the response to chemotherapy. Many studies published to date have followed case patients for relatively short periods of time. This approach is valid if the relative hazard associated with a gene mutation is constant over time, but if the assumption of hazards proportionality is violated, then a long period of follow-up is necessary to permit proper comparison of survivorship. In many studies, the hereditary case patients and the group of comparison patients were derived from different populations (5,6,9,11,13,14). Additionally, many studies involved less than 50 hereditary case patients (4,7–9,12), and some studies did not properly adjust for survivorship bias (5,11–13). We sought to estimate 10-year survival for women with ovarian cancer, with and without mutations in BRCA1 or BRCA2, to determine whether or not the observed short-term survival benefit for mutation carriers is associated with a better prospect for cure.
Subjects and Methods
We included two large series of unselected ovarian cancer patients who had been studied previously for germline mutations in BRCA1 and BRCA2 and who were under active follow-up. In total, 3367 unselected patients were diagnosed with invasive ovarian cancer in the period between 1995 and 1999 and the period between 2002 and 2004 in Ontario, Canada. These patients were identified by monitoring acquisitions of the Ontario Cancer Registry. Patients were aged between 20 and 79 years and were residents in Ontario at the time of diagnosis of a new primary epithelial ovarian tumor. Of 3367 potentially eligible case patients, we were able to obtain and test blood samples from 1414 (42%). For eight additional case patients, a genetic test was done outside of the study, and these case patients were also included, bringing the total studied to 1422. For 1081 patients, the patient had died before contact was able to be made, and these individuals were not included. Other reasons for nonparticipation included subject refusal (n = 216 patients), subject too ill (n = 137 patients), physician refusal (n = 150 patients), inability to locate (n = 108 patients), and other (n = 252 patients). One patient had a mutation in both genes and was excluded. In addition, 204 unselected patients from Tampa, Florida were included. These patients were diagnosed among seven gynecologic oncologists affiliated with teaching hospitals in Tampa, Florida (3). All of the women in the study gave written informed consent, and the study was approved by the institutional review boards of the University of Toronto and Moffitt Cancer Centre.
For all participating patients, the BRCA1 and BRCA2 genes were screened in their entirety, using methods previously described (2). Genetic testing for Ontario patients was performed in the Narod laboratory (Toronto, Canada) and testing for the Florida patients was done by Myriad Genetics (Salt Lake City, UT). Germline deletions were sought using the multiplex ligation-dependent probe amplification test on all case patients from Ontario (2) but not on the patients from Florida. All of the observed mutations included in this article are considered to be deleterious.
Of the 1626 women who participated in the study, the average time elapsed from date of diagnosis to date of ascertainment (blood draw) was 20.0 months.
Pathology Review
We included only women with invasive ovarian cancer. For each case patient, the investigators reviewed pathology reports to determine eligibility and tumor histologic type. We classified the cancers by histologic category and by grade. We also obtained medical charts for 1289 patients (79%) and recorded information on size of cancer, extent of involvement of local and regional lymph nodes, and presence of metastatic disease. Based on the chart review and using International Federation of Gynecology and Obstetrics criteria, we assigned clinical stage to the individual patients (15). The Ontario patient database was then linked to records of the Ontario Cancer Registry. The Ontario Registry records contain date of death and cause of death of all cancer patients diagnosed in Ontario. Deaths were recorded that occurred before September 2010. Dates of death for Florida patients were established using the National Death Index of the United States for deaths through February 2012.
Statistical Analysis
We conducted a Kaplan–Meier survival analysis on the members of the cohort from the date of diagnosis until the date of death from ovarian cancer. To account for the time elapsed between the date of diagnosis and the date of ascertainment (genetic testing), we performed a left-truncated survival analysis, implemented in SAS. This adjustment is done to eliminate the survivorship bias that may occur because patients who died shortly after diagnosis were often missed in our ascertainment scheme (16). Patients were censored at the date of death from another cause or the end of September 2010 (based on the Ontario record linkage described above) or February 29, 2012 (for the Florida patients). Kaplan–Meier survival curves were constructed for subgroups defined by histopathologic type (serous, endometrioid, clear cell, mucinous, other) and by BRCA mutation status. To compare statistical significance or differences associated with the Kaplan–Meier analysis, the score test was used (this test is equivalent to the log-rank test if there are no ties in the survival times). To estimate hazard ratios (HRs) associated with mutation status, we conducted univariable and multivariable survival analyses. The latter were adjusted for age at diagnosis, histologic subtype, tumor grade, and clinical stage. Hazard ratios were examined for 3-year, 5-year and 10-year periods after diagnosis. We then estimated annual mortality for members of the cohort based on a life-table approach for each 1-year period after date of diagnosis until 10 years after diagnosis.
Results
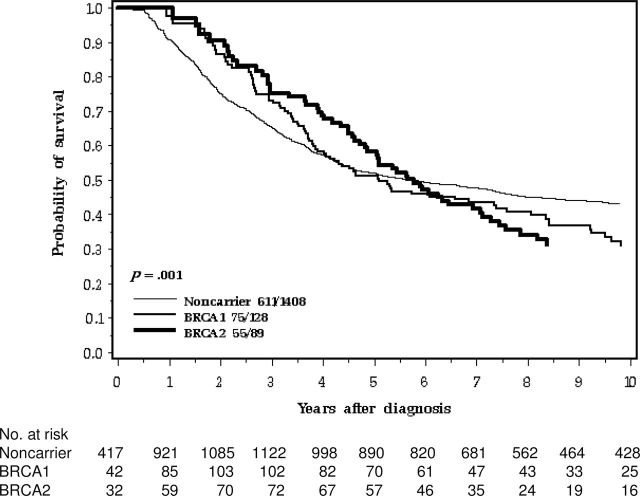
Among the 1626 women in our study with invasive cancer, 129 were identified as carriers of BRCA1 mutations, 89 were identified as carriers of BRCA2 mutations, and 1408 were classified as noncarriers. The overall proportion of mutation carriers was 13.4%. The characteristics of the 1626 women are presented in Table 1. The mortality experience of the women by genetic mutation status is presented in Figure 1 and Table 2. The crude 3-year survival for the hereditary case patients was better than that of the nonhereditary case patients, but after 10 years of follow-up, no survival advantage was apparent.
Table 1.
Characteristics of women with ovarian cancer in the study
| Characteristic | BRCA1 (n = 129) | BRCA2 (n = 89) | No mutation (n = 1408) |
|---|---|---|---|
| Age at diagnosis, y, mean (range) | 51.1 (31–78) | 57.6 (34–73) | 57.7 (19–81) |
| Histology, No. (%) | |||
| Serous | 95 (73.6%) | 65 (73.0%) | 748 (53.1%) |
| Mucinous | 0 | 0 | 127 (9.0%) |
| Endometriod | 19 (14.7%) | 8 (8.9%) | 301 (21.4%) |
| Clear cell | 1 (0.8%) | 2 (2.3%) | 104 (7.4%) |
| Other | 14 (10.9%) | 14 (15.7%) | 128 (9.1%) |
| Stage, No. (%) | |||
| I | 7 (5.4%) | 6 (6.7%) | 267 (19.0%) |
| II | 23 (17.8%) | 4 (4.5%) | 241 (17.1%) |
| III | 79 (61.2%) | 60 (67.4%) | 692 (49.9%) |
| IV | 17 (13.2%) | 15 (16.9%) | 187 (13.3%) |
| Missing | 3 | 4 | 21 |
| Grade | |||
| I | 2 (1.6%) | 0 | 205 (14.6%) |
| II | 20 (15.5%) | 19 (21.4%) | 269 (19.1%) |
| III | 73 (56.6%) | 47 (52.8%) | 450 (32.0%) |
| Unknown | 34 (26.4%) | 23 (25.8%) | 484 (34.4%) |
| Year of diagnosis, mean (range) | 1999.6 (1995–2004) | 1999.3 (1995–2004) | 1999.5 (1995–2004) |
| Residence, No. (%) | |||
| Ontario | 109 (85.4%) | 68 (76.4%) | 1245 (88.4%) |
| Florida | 20 (15.5%) | 21 (23.6%) | 163 (11.6%) |
| Years of follow-up, mean (range) | 6.2 (6.9–12.0) | 6.4 (1.1–12.0) | 7.0 (0.3–12.0) |
Figure 1.
Ten-year survival of ovarian cancer patients by BRCA mutation.
Table 2.
Annual mortality for years 1 to 10 after diagnosis of ovarian cancer*
| BRCA1 | BRCA2 | No mutation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year from diagnosis | Person-years | Deaths | Annual mortality† | Person-years | Deaths | Annual mortality | Person-years | Deaths | Annual mortality | Odds ratio (95% CI)‡ |
| <1 | 20.7 | 1 | 0.048 | 17.1 | 0 | 0 | 213 | 29 | 0.136 | 0.19 (0.03–1.47) |
| (1,2] | 61.8 | 9 | 0.145 | 41.9 | 4 | 0.095 | 563 | 110 | 0.195 | 0.64 (0.35–1.18) |
| (2,3] | 82.9 | 14 | 0.169 | 58.5 | 11 | 0.188 | 902 | 123 | 0.136 | 1.30 (0.81–2.06) |
| (3,4] | 88.7 | 20 | 0.225 | 64.3 | 6 | 0.093 | 992 | 133 | 0.134 | 1.27 (0.81–2.00) |
| (4,5] | 74.1 | 10 | 0.135 | 61.4 | 10 | 0.163 | 928 | 90 | 0.097 | 1.52 (0.90–2.55) |
| (5,6] | 64.7 | 7 | 0.108 | 51.0 | 11 | 0.216 | 854 | 42 | 0.049 | 3.16 (1.76–5.68) |
| (6,7] | 53.9 | 3 | 0.056 | 39.8 | 5 | 0.126 | 756 | 27 | 0.036 | 2.39 (1.06–5.41) |
| (7,8] | 45.1 | 3 | 0.066 | 29.0 | 6 | 0.207 | 621 | 35 | 0.056 | 2.16 (1.00–4.66) |
| (8,9] | 36.8 | 4 | 0.109 | 21.2 | 2 | 0.094 | 504 | 3 | 0.026 | 4.01 (1.47–10.9) |
| (9,10] | 28.2 | 5 | 0.177 | 17.3 | 0 | 0 | 444 | 9 | 0.020 | 5.41 (1.74–16.8) |
| All years | 596.4 | 77 | 0.129 | 426.6 | 56 | 0.131 | 7511.8 | 616 | 0.082 | |
* CI = confidence interval.† Annual mortality is the proportion of women alive at beginning of interval who died of ovarian cancer during the interval.
‡ Odds for dying of ovarian cancer in 1–year period for women alive at beginning of interval, carriers vs noncarriers; Breslow–Day heterogeneity of odds ratios P = .0001.
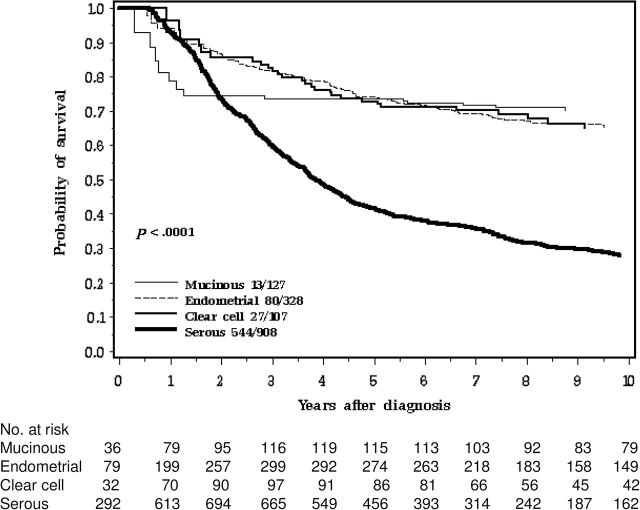
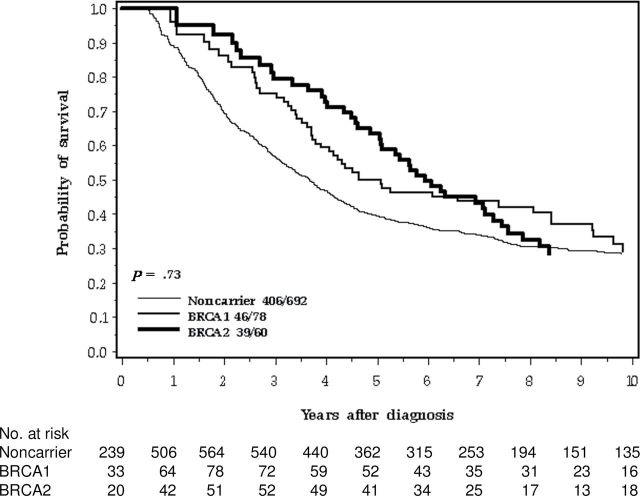
Women with serous cancers experienced worse survival than women with other histologic subtypes (Figure 2). Seventy-three percent of the hereditary case patients and 53% of the nonhereditary case patients were of serous subtype (P < .01). Among patients with serous cancers, the 10-year survival of the three groups of patients (BRCA1, BRCA2, no mutation) were similar (Table 3; Figure 3 ).
Figure 2.
Ten-year survival of ovarian cancer patients by histology.
Table 3.
Hazard ratios for survival at 5 and 10 years after diagnosis of serous ovarian cancers associated with various factors*
| 5-year survival | 10-year survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Unadjusted HR (95% CI) | P | Adjusted† HR (95% CI) | P | Unadjusted HR (95% CI) | P | Adjusted† HR (95% CI) | P |
| BRCA status | ||||||||
| No Mutation | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | ||||
| BRCA1 | 0.74 (0.53 to 1.04) | .08 | 0.83 (0.59 to 1.18) | .31 | 0.89 (0.67 to 1.17) | .40 | 0.96 (0.72 to 1.28) | .78 |
| BRCA2 | 0.68 (0.45 to 1.02) | .06 | 0.65 (0.43 to 0.99) | 0.04 | 0.94 (0.68 to 1.29) | .69 | 0.88 (0.64 to 1.22) | .45 |
| Either | 0.71 (0.54 to 0.93) | .01 | 0.75 (0.57 to 0.99) | 0.04 | 0.90 (0.72 to 1.12) | .34 | 0.92 (0.74 to 1.16) | .49 |
| Age at diagnosis, y | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | ||||
| <50 | 1.37 (1.05 to 1.79) | .02 | 1.09 (0.83 to 1.44) | .53 | 1.38 (1.09 to 1.74) | .007 | 1.10 (0.86 to 1.39) | .45 |
| 50–59 | 1.63 (1.25 to 2.13) | .0004 | 1.38 (1.04 to 1.83) | .02 | 1.52 (1.20 to 1.92) | .0005 | 1.33 (1.04 to 1.70) | .02 |
| 60–69 | 1.50 (1.10 to 2.03) | .01 | 1.22 (0.89 to 1.67) | .21 | 1.46 (1.11 to 1.91) | .006 | 1.23 (0.93 to 1.62) | .14 |
| ≥70 | ||||||||
| Grade | ||||||||
| I | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | ||||
| II | 2.40 (1.46 to 3.96) | .0006 | 1.97 (1.18 to 3.27) | .009 | 2.55 (1.65 to 3.94) | <.001 | 2.06 (1.32 to 3.21) | .001 |
| III | 2.24 (1.38 to 3.63) | .001 | 1.66(1.01 to 2.71) | .05 | 2.43 (1.59 to 3.69) | >.0001 | 1.72 (1.12 to 2.64) | .01 |
| Stage | ||||||||
| I | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | ||||
| II | 3.88 (0.90 to 16.7) | .07 | 3.43 (0.80 to 14.8) | .10 | 6.63 (1.58 to 27.7) | .01 | 6.17 (1.47 to 25.9) | .01 |
| III | 12.4 (3.08 to 49.7) | .0004 | 11.4 (2.82 to 48.2) | .0006 | 20.6 (5.15 to 82.8) | <.0001 | 20.1 (4.98 to 80.8) | <.0001 |
| IV | 18.6 (4.60 to 75.5) | <.001 | 18.9 (4.62 to 77.6) | <.001 | 32.5 (8.05 to 131) | <.0001 | 35.6 (8.73 to 145) | <.0001 |
* CI = confidence interval; HR = hazard ratio.
† Adjusted for age at diagnosis, histologic subtype, tumor grade, and clinical stage.
Figure 3.
Ten-year survival of ovarian cancer patients by BRCA mutation, serous cancer only.
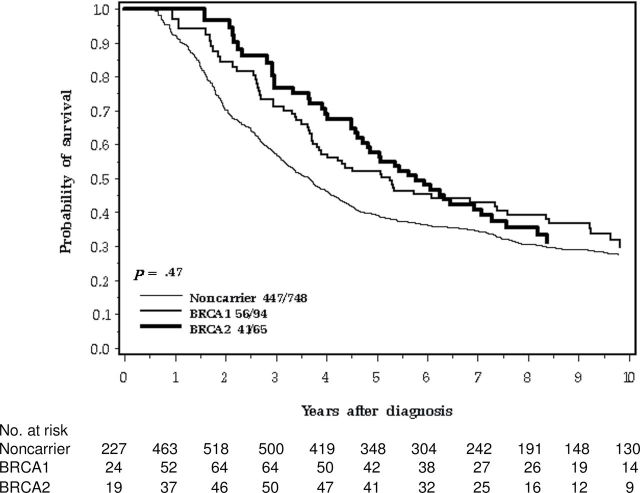
Eighty-one percent of the women with BRCA1 or BRCA2 mutations presented with stage III or IV tumors, compared with 63% of the noncarriers (P < .0001). Despite this, carriers had a favorable short-term prognosis (Figure 1). The survival advantage was more pronounced when the comparison was limited to women with stage III cancers (Figure 4).
Figure 4.
Ten-year survival of ovarian cancer patients by BRCA mutation, stage III cancer only.
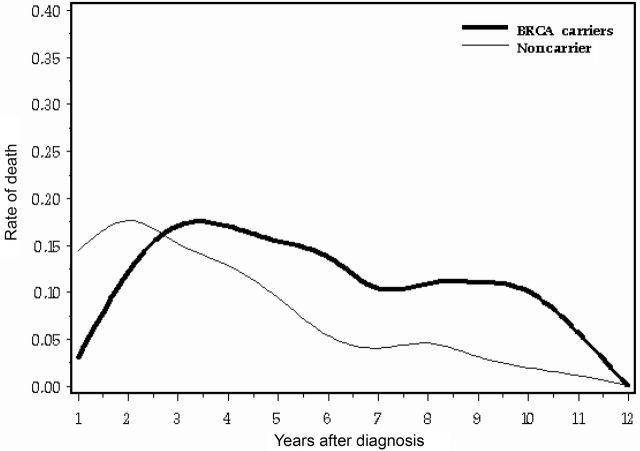
Annual mortality for the three subgroups by 1-year intervals from time of diagnosis are presented in Table 2 and in Figure 5. The annual mortality of the carriers was better than that of noncarriers for years 1 and 2, but thereafter was similar or worse. The odds of dying of ovarian cancer for carriers vs noncarriers increased with time from diagnosis, and the null hypothesis that the odds ratio was consistent throughout the follow-up period was rejected (P = .0001; Breslow–Day test for heterogeneity). At 12 years, ovarian cancer mortality reached baseline (Figure 5), and therefore 12-year survival appears to be a reasonable surrogate for cure. Among 309 women who survived for 12 years, only one death from ovarian cancer occurred thereafter in 588 person-years of observation. At 12 years after diagnosis, 29.5% of BRCA1 mutation carriers, 29.1% of BRCA2 carriers, and 41.2% of noncarriers were alive (Kaplan–Meier estimates). Among women with serous ovarian cancers, 27.4% of BRCA1 mutation carriers, 27.7% of BRCA2 carriers, and 27.1% of noncarriers were alive at 12 years after diagnosis. Among women with stage III ovarian cancers, 39.2% of BRCA1 mutation carriers, 35.0% of BRCA2 carriers, and 40.8% of noncarriers were alive at 12 years after diagnosis.
Figure 5.
Annualized probability of death among ovarian cancer patients according to carriage of a BRCA1 or BRCA2 mutation vs noncarriage.
The mutation carriers had an unfavorable distribution of stage, grade, and histology, compared with noncarriers (Table 1), and therefore an adjusted analysis was conducted. In multivariable survival analysis, adjusted for histologic subtype, age at diagnosis, disease stage, and grade, the presence of a BRCA1 or BRCA2 mutation was associated with a better prognosis at 3 years after diagnosis (HR = 0.68, 95% confidence interval [CI] = 0.48 to 0.98; P = .03). The advantage waned over time; at 5 years after diagnosis, the hazard ratio was 0.79 (95% CI = 0.63 to 1.01; P = .06), and at 10 years after diagnosis, the hazard ratio was 1.00 (95% CI = 0.83 to 1.22; P = .90). Among women with stage III ovarian cancer, the adjusted hazard ratio was 0.60 (95% CI = 0.35 to 1.03; P = .07) at 3 years after diagnosis, 0.73 (95% CI = 0.52 to 1.03; P = .08) at 5 years after diagnosis, and 0.95 (95% CI = 0.72 to 1.25; P = .70) at 10 years after diagnosis. A subgroup analysis restricted to women with serous ovarian cancers showed similar results (Table 3).
Discussion
In this study of 1626 unselected ovarian cancer case patients from Ontario, Canada, and Tampa, Florida, we confirmed that the short-term survival of ovarian cancer patients with BRCA1 or BRCA2 mutations was better than that of noncarriers, but the survival advantage was relatively short lived and did not impact long-term survival. In the first 2 years after diagnosis, annual mortality rates were lower for the carriers than for noncarriers, but in years 3 to 10, mortality rates were higher for carriers than for noncarriers (Figure 5; Table 2). These results suggest that hereditary and nonhereditary case patients have distinct survival patterns. These differences are unlikely to be because of differences in the intrinsic aggressiveness of the cancers but may reflect a better acute response to chemotherapy in the hereditary case patients. This would be the case if the initial response to chemotherapy were superior for carriers than for noncarriers, but the ultimate probability of relapse were similar. Tan et al. (9) reported that BRCA1 carriers had better responses to platinum-based chemotherapy than noncarriers, both in terms of complete response (82% vs 43%) and for time from relapse to death (5.0 vs 1.6 years). Cass et al. (8) reported that women with ovarian cancer and BRCA1 or BRCA2 mutations were more likely to respond to chemotherapy than women without mutations and also had improved survival. The number of carriers in that study was small (n = 29). In a larger study from the Netherlands, Vencken et al. (13) reported that 88% of 112 patients with BRCA1 or BRCA2 mutations had a complete response or no evidence of disease after first-line chemotherapy, compared with 71% of noncarrier patients.
In our study, peak mortality occurred 2 years after diagnosis for noncarriers but approximately 3.5 years after diagnosis for carriers. A hazard ratio that is estimated based solely on survival for the first 5 years will be misleading. The odds ratio for dying in any given 1-year interval initially favored carriers in years 1 and 2, but the size of the odds ratio increased throughout the follow-up period. The (null) hypothesis that the relative odds of dying for carriers and noncarriers were the same throughout the follow-up period was rejected.
There are several limitations to our study. We did not ascertain all case patients of ovarian cancer in Ontario or in Tampa, and participation rate may have differed by age or ethnic group. We excluded patients who were deceased at the time of ascertainment, essentially selecting our study sample in favor of survivors; however, our estimated survival accounted for this potential bias through left-truncated survival analysis (16,17). We did not use multiplex ligation-dependent probe amplification to screen for large rearrangements in the Florida samples, but the expected number of missed mutations would be small. We used the Cox proportional hazards model in the analysis of the survival data; we acknowledge that the assumption of proportional hazards is inappropriate here, but these results were included in order to provide comparisons with previous studies and to illustrate the necessity for long term follow-up data. We did not include data on treatment in our model, but treatment regimens are relatively standard across North America and the treating physicians were not aware of the genetic status of the patient.
Advantages of our study include the large size of the studied cohort (N = 1626) and of the mutation-positive subset (n = 218). Most important, our mutation carriers comprised a subset of the total tested cohort (ie, the noncarrier control group was derived from the same patient population as the carrier case patients, and all patients were diagnosed in the same years and treated in the same hospitals. In many previous studies, hereditary case patients and nonhereditary comparison groups were derived from different sources (5,6,9,11,13,14). None of the patients were aware of their genetic status at the time of diagnosis, and the results were not returned to the patients until a minimum of 1 year had elapsed from diagnosis. Thus, treatment decisions were not influenced by the genetic status. In previous studies, the patients with mutations might have been aware of their genetic status before diagnosis and, as a consequence, underwent close surveillance (or were treated differently). We followed our patients for a mean of 6.9 years. We were able to distinguish between deaths from ovarian cancer and deaths from other causes.
Our results and conclusions differ to some extent from those of earlier studies, many of which have reported a survival advantage for women with BRCA1 or BRCA2 mutations (6–14). However, our results for short-term survival are similar to those of other studies, and the differences can be explained mostly by differences in sample size and length of follow-up.
In one early, large study of familial and hereditary ovarian cancer patients in the United Kingdom, no survival differences were noted for patients with BRCA1, BRCA2, or no mutations (5). Patients were diagnosed from 1970 to 1998, and it is not clear how many received platinum-based chemotherapy. Boyd et al. (6) reported a survival advantage for BRCA1/2 carriers with ovarian cancer, in particular for advanced stage cancers. In the Boyd et al. study, the control group of noncarriers was external and was selected from the Gynecology Oncology Group clinical trials. In our study, a survival advantage at 5 years was seen for women with stage III cancers (55% for BRCA1 and BRCA2 mutation carriers combined vs 39% for noncarriers; P = .02). In a well-conducted study from Israel, 779 women with invasive ovarian cancer were tested for mutations and were followed for 9 years (10). The most remarkable result was that for stage IV cancers, the 5-year survival was 36% for mutation carriers (BRCA1 and BRCA2 combined) and 8% for noncarriers. In our study, for women with stage IV cancers, 5-year survival was 27% for BRCA1 and BRCA2 carriers combined vs 25% for noncarriers (P = .90); we observed a survival advantage only among the subgroup with stage III ovarian cancers.
Da Yang et al. (11) studied 37 BRCA1 mutation carriers and 29 BRCA2 carriers and compared them with 250 noncarriers. All of these patients had high-grade serous cancers and 96% had stage III or IV cancer. Among the 29 case patients with BRCA2 mutations, the 5-year survival was 61% and was superior to that of the patients in the other two categories. Only one BRCA2 carrier died within 2 years of diagnosis. The authors attribute the low risk of death in the first few years to a high sensitivity to chemotherapy. All 25 patients with BRCA2 mutations had good initial responses to cisplatinum. These data are consistent with our data, but their sample size was small and the follow-up time was relatively short. The five-year survival for 75 patients in our study with BRCA2-positive, stage III or IV ovarian cancer was 54%; similar to the result reported by da Yang et al (11).
In the large, multicenter, pooled analysis of 26 studies by Bolton et al. (14), survival rates were compared for 1213 mutation carriers and 2666 noncarriers. The prevalence of mutations in that study was very high (31%), but some sites provided data on carriers only. Median follow-up time was 38 months (compared with 90 months in our study), and the conclusions of Bolton et al. were based entirely on 5-year survival. In that study, the 5-year survival was 36% for noncarriers, 44% for carriers of BRCA1 mutations, and 52% for carriers of BRCA2 mutations.
We saw only one death from ovarian cancer among the 309 women who survived more than 12 years from diagnosis, and 12-year survival, therefore, seems a reasonable surrogate for cure. Our data indicate that the short-term survival benefit of carrying a BRCA1 or BRCA2 mutation is not reflected in long-term differences in the proportions of women who ultimately survive their ovarian cancer. We believe that there is insufficient evidence to conclude that survival from ovarian cancer differs between carriers and noncarriers, and we disagree with the recommendation (14) that health-care providers should counsel women with ovarian cancer and carrying BRCA mutations that they should expect their survival to be better than that of noncarriers or that treatment could be tailored to reflect the differences in survival.
Funding
This work was supported by the Canadian Institutes of Health Research; by grants from the National Cancer Institute (R01 CA 63682 to HAR; R01 CA 63678 to SAN; and R01CA111914 to TP); and by the Florida Biomedical Grant (IBG09-34198 to TP).
We thank Shiyu Zhang and Song Li for conducting the mutation analysis.
References
- 1. Engel J, Eckel R, Schubert-Fritschle G, et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer. 2002; 38: 2435–2445 [DOI] [PubMed] [Google Scholar]
- 2. Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011; 121:(2):353–357 [DOI] [PubMed] [Google Scholar]
- 3. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005; 104:(12):2807–2816 [DOI] [PubMed] [Google Scholar]
- 4. Pal T, Permuth-Wey J, Kapoor R, Cantor A, Sutphen R. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer. 2007; 6(12): 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pharoah PD, Easton DF, Stockton DL, Gayther S, Ponder BA. Survival in familial, BRCA1-associated, and BRCA2-associated epithelial ovarian cancer. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. Cancer Res. 1999; 59:(4):868–871 [PubMed] [Google Scholar]
- 6. Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000; 283:(17):2260–2265 [DOI] [PubMed] [Google Scholar]
- 7. Ramus SJ, Fishman A, Pharoah PD, Yarkoni S, Altaras M, Ponder BA. Ovarian cancer survival in Ashkenazi Jewish patients with BRCA1 and BRCA2 mutations. Eur J Surg Oncol. 2001; 27:(3):278–281 [DOI] [PubMed] [Google Scholar]
- 8. Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003; 97(9): 2187–2195 [DOI] [PubMed] [Google Scholar]
- 9. Tan DS, Rothermundt C, Thomas K, et al. BRCAness syndrome in ovarian cancer: a case–control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008; 26:(34):5530–5536 [DOI] [PubMed] [Google Scholar]
- 10. Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol. 2008; 26:(1):20–25 [DOI] [PubMed] [Google Scholar]
- 11. Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011; 306:(14):1557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hyman DM, Zhou Q, Iasonos A, et al. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2012; 118:(15): 3703–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vencken PM, Kriege M, Hoogwerf D, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011; 22:(6):1346–1352 [DOI] [PubMed] [Google Scholar]
- 14. Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012; 307:(4):382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. FIGO Committee on Gynecologic Oncology Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int J Gynaecol Obstet. 2009; 105:(1):3–4 [DOI] [PubMed] [Google Scholar]
- 16. Keilding N. Delayed entry.. In: Armitage P, Colton T, eds. Encyclopedia of Biostatistics. Hoboken, NJ: John Wiley and Sons; 2005; [Google Scholar]
- 17. Narod SA, Moody JRK, Rosen B, et al. Survival after ovarian cancer among women tested for BRCA1 and BRCA2 mutations. [published online ahead of print June 8, 2012]. Clin Genet. 2012; doi:10.1111/j 1399-0004 [DOI] [PubMed] [Google Scholar]