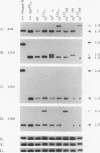
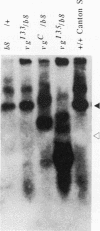
Abstract
Mammalian multidrug-resistant cell lines, selected for resistance to a single cytotoxic agent, display cross-resistance to a broad spectrum of structurally and functionally unrelated compounds. These cell lines overproduce a membrane protein, the P-glycoprotein, which is encoded by a member(s) of a multigene family, termed mdr or pgp. The amino acid sequence of the P-glycoprotein predicts an energy-dependent transport protein with homology to a large superfamily of proteins which transport a wide variety of substances. This report describes the isolation and characterization of two Drosophila homologs of the mammalian mdr gene. These homologs, located in chromosomal sections 49EF and 65A, encode proteins that share over 40% amino acid identity to the human and murine mdr P-glycoproteins. Fly strains bearing disruptions in the homolog in section 49EF have been constructed and implicate this gene in conferring colchicine resistance to the organism. This work sets the foundation for the molecular and genetic analysis of mdr homologs in Drosophila melanogaster.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler P. N., Charlton J., Brunk B. Genetic interactions of the suppressor 2 of zeste region genes. Dev Genet. 1989;10(3):249–260. doi: 10.1002/dvg.1020100314. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Arceci R. J., Croop J. M., Horwitz S. B., Housman D. The gene encoding multidrug resistance is induced and expressed at high levels during pregnancy in the secretory epithelium of the uterus. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4350–4354. doi: 10.1073/pnas.85.12.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk B. P., Adler P. N. Aristapedioid: a gain of function, homeotic mutation in Drosophila melanogaster. Genetics. 1990 Jan;124(1):145–156. doi: 10.1093/genetics/124.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Chin J. E., Soffir R., Noonan K. E., Choi K., Roninson I. B. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol. 1989 Sep;9(9):3808–3820. doi: 10.1128/mcb.9.9.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Croop J. M., Gros P., Housman D. E. Genetics of multidrug resistance. J Clin Invest. 1988 May;81(5):1303–1309. doi: 10.1172/JCI113455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J. M., Guild B. C., Gros P., Housman D. E. Genetics of multidrug resistance: relationship of a cloned gene to the complete multidrug resistant phenotype. Cancer Res. 1987 Nov 15;47(22):5982–5988. [PubMed] [Google Scholar]
- Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., Housman D. E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989 Mar;9(3):1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Gros P. Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol. 1990 Apr;10(4):1652–1663. doi: 10.1128/mcb.10.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen T. D., Johnson D. H., Henikoff S. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol Cell Biol. 1988 Dec;8(12):5206–5215. doi: 10.1128/mcb.8.12.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dura J. M., Randsholt N. B., Deatrick J., Erk I., Santamaria P., Freeman J. D., Freeman S. J., Weddell D., Brock H. W. A complex genetic locus, polyhomeotic, is required for segmental specification and epidermal development in D. melanogaster. Cell. 1987 Dec 4;51(5):829–839. doi: 10.1016/0092-8674(87)90106-1. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S. J., Kyle D. E., Martin R. K., Oduola A. M., Forsyth K., Kemp D. J., Cowman A. F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990 May 17;345(6272):255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Georges E., Bradley G., Gariepy J., Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. I., Lothstein L., Horwitz S. B. Differential overexpression of three mdr gene family members in multidrug-resistant J774.2 mouse cells. Evidence that distinct P-glycoprotein precursors are encoded by unique mdr genes. J Biol Chem. 1989 Jul 15;264(20):12053–12062. [PubMed] [Google Scholar]
- Itoh N., Slemmon J. R., Hawke D. H., Williamson R., Morita E., Itakura K., Roberts E., Shively J. E., Crawford G. D., Salvaterra P. M. Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4081–4085. doi: 10.1073/pnas.83.11.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko P. F., Pardue M. L. Studies of the genetic organization of the vestigial microregion of Drosophila melanogaster. Genetics. 1988 Oct;120(2):495–502. doi: 10.1093/genetics/120.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni G., Wise J., Young J. E., Otto E. Metallothionein gene duplications and metal tolerance in natural populations of Drosophila melanogaster. Genetics. 1987 Dec;117(4):739–744. doi: 10.1093/genetics/117.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Mokdad R., Debec A., Wegnez M. Metallothionein genes in Drosophila melanogaster constitute a dual system. Proc Natl Acad Sci U S A. 1987 May;84(9):2658–2662. doi: 10.1073/pnas.84.9.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Murphy C., Levis R., Rubin G. M. DNA sequence of the white locus of Drosophila melanogaster. J Mol Biol. 1984 Dec 15;180(3):437–455. doi: 10.1016/0022-2836(84)90021-4. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M., Rose E., Housman D. E., Gros P. Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Mol Cell Biol. 1990 Apr;10(4):1642–1651. doi: 10.1128/mcb.10.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Samuelson J., Ayala P., Orozco E., Wirth D. Emetine-resistant mutants of Entamoeba histolytica overexpress mRNAs for multidrug resistance. Mol Biochem Parasitol. 1990 Jan 15;38(2):281–290. doi: 10.1016/0166-6851(90)90031-g. [DOI] [PubMed] [Google Scholar]
- St Johnston R. D., Hoffmann F. M., Blackman R. K., Segal D., Grimaila R., Padgett R. W., Irick H. A., Gelbart W. M. Molecular organization of the decapentaplegic gene in Drosophila melanogaster. Genes Dev. 1990 Jul;4(7):1114–1127. doi: 10.1101/gad.4.7.1114. [DOI] [PubMed] [Google Scholar]
- Tearle R. G., Belote J. M., McKeown M., Baker B. S., Howells A. J. Cloning and characterization of the scarlet gene of Drosophila melanogaster. Genetics. 1989 Jul;122(3):595–606. doi: 10.1093/genetics/122.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Serrano A. E., Wasley A., Bogenschutz M. P., Shankar A. H., Wirth D. F. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989 Jun 9;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Jones R. S., Lasko P. F., Gelbart W. M. Homeosis and the interaction of zeste and white in Drosophila. Mol Gen Genet. 1989 Sep;218(3):559–564. doi: 10.1007/BF00332424. [DOI] [PubMed] [Google Scholar]
- Ziemer A., Tietze K., Knust E., Campos-Ortega J. A. Genetic analysis of enhancer of split, a locus involved in neurogenesis in Drosophila melanogaster. Genetics. 1988 May;119(1):63–74. doi: 10.1093/genetics/119.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A. M., Kooiman P. M., Schneider C., Borst P. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene. 1988 Nov 30;71(2):401–411. doi: 10.1016/0378-1119(88)90057-1. [DOI] [PubMed] [Google Scholar]