Abstract
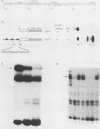
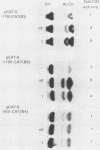
The Ly-6E/A antigen is expressed on activated murine T cells. Using probes made from the previously characterized cDNA, we have isolated a genomic DNA clone encoding the Ly-6A antigen. We determined the DNA sequence of the genomic clone and conducted a functional analysis of the promoter region. Mouse fibroblast BALB/3T3 cells transfected with this genomic clone constitutively expressed Ly-6A antigen on their cell surface. This expression was inducible by alpha/beta and gamma interferons. The Ly-6E 5'-flanking region was analyzed by chloramphenicol acetyltransferase assays in fibroblast cells for cis-acting elements. At least two positive elements were found to be needed for maximum constitutive promoter activity in L cells. One of the positive elements was specifically bound by a CCAAT box-binding protein from crude nuclear extract, as shown by electrophoretic mobility shift assays and footprinting. The other element, which contains a GGAAA motif and has homology to various known enhancers, also showed a specific binding activity. This second positive element when multimerized became a very powerful enhancing element. Interferon treatment could enhance expression of the chloramphenicol acetyltransferase gene fused to the Ly-6E 5'-flanking region in stably transfected BALB/3T3 cells. The elements responsible for this enhancement lie, at least in part, between positions -1760 and -900 of the gene. Surprisingly, there is no sequence homology between this region of Ly-6E and the established consensus for the interferon-stimulated response element, which has been shown functionally important to all previously characterized alpha/beta interferon-inducible promoters. The Ly-6E gene may prove to be a novel system for the study of interferon induction.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C. Transient gene expression control: effects of transfected DNA stability and trans-activation by viral early proteins. Mol Cell Biol. 1985 May;5(5):1034–1042. doi: 10.1128/mcb.5.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Binding of a nuclear factor to a regulatory sequence in the promoter of the mouse H-2Kb class I major histocompatibility gene. Mol Cell Biol. 1987 Jan;7(1):305–313. doi: 10.1128/mcb.7.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D. W., Philbrick W. M., Bothwell A. L. Identification of a novel 9-kDa polypeptide from nuclear extracts. DNA binding properties, primary structure, and in vitro expression. J Biol Chem. 1988 Jun 15;263(17):8450–8457. [PubMed] [Google Scholar]
- Borrelli E., Heyman R., Hsi M., Evans R. M. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A., Pace P. E., LeClair K. P. Isolation and expression of an IFN-responsive Ly-6C chromosomal gene. J Immunol. 1988 Apr 15;140(8):2815–2820. [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Cohen B., Vaiman D., Chebath J. Enhancer functions and in vitro protein binding of native and mutated interferon-responsive sequences. Nucleic Acids Res. 1989 Feb 25;17(4):1679–1695. doi: 10.1093/nar/17.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClair K. P., Bridgett M. M., Dumont F. J., Palfree R. G., Hämmerling U., Bothwell A. L. Kinetic analysis of Ly-6 gene induction in a T lymphoma by interferons and interleukin 1, and demonstration of Ly-6 inducibility in diverse cell types. Eur J Immunol. 1989 Jul;19(7):1233–1239. doi: 10.1002/eji.1830190713. [DOI] [PubMed] [Google Scholar]
- LeClair K. P., Palfree R. G., Flood P. M., Hammerling U., Bothwell A. Isolation of a murine Ly-6 cDNA reveals a new multigene family. EMBO J. 1986 Dec 1;5(12):3227–3234. doi: 10.1002/j.1460-2075.1986.tb04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity S. N., Golumbek P. T., Karsenty G., de Crombrugghe B. Selective activation of transcription by a novel CCAAT binding factor. Science. 1988 Jul 29;241(4865):582–585. doi: 10.1126/science.3399893. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Chan C., Kroczek R. A., Shevach E. M. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986 Sep 1;164(3):709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfree R. G., LeClair K. P., Bothwell A., Hämmerling U. cDNA characterization of an Ly-6.2 gene expressed in BW5147 tumor cells. Immunogenetics. 1987;26(6):389–391. doi: 10.1007/BF00343712. [DOI] [PubMed] [Google Scholar]
- Porter A. C., Chernajovsky Y., Dale T. C., Gilbert C. S., Stark G. R., Kerr I. M. Interferon response element of the human gene 6-16. EMBO J. 1988 Jan;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Coligan J., Palmer E., Benacerraf B., Rock K. L. Cloning and expression of a cDNA for the T-cell-activating protein TAP. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2255–2259. doi: 10.1073/pnas.85.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Reiser H., Bamezai A., McGrew J., Benacerraf B. The LY-6 locus: a multigene family encoding phosphatidylinositol-anchored membrane proteins concerned with T-cell activation. Immunol Rev. 1989 Oct;111:195–224. doi: 10.1111/j.1600-065x.1989.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Su B., Bothwell A. L. Biosynthesis of a phosphatidylinositol-glycan-linked membrane protein: signals for posttranslational processing of the Ly-6E antigen. Mol Cell Biol. 1989 Aug;9(8):3369–3376. doi: 10.1128/mcb.9.8.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Janeway C. A., Jr Antigen-dependent selection of B lymphoma cells varying in Ia density by cloned antigen-specific L3T4a+ T cells: a possible in vitro model for B cell adaptive differentiation. J Mol Cell Immunol. 1984;1(4):253–265. [PubMed] [Google Scholar]
- Tsang S. Y., Nakanishi M., Peterlin B. M. B-cell-specific and interferon-gamma-inducible regulation of the HLA-DR alpha gene. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8598–8602. doi: 10.1073/pnas.85.22.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Bamezai A., Rock K. L. TAP transcription and phosphatidylinositol linkage mutants are defective in activation through the T cell receptor. Cell. 1988 Mar 11;52(5):665–674. doi: 10.1016/0092-8674(88)90404-7. [DOI] [PubMed] [Google Scholar]
- van de Rijn M., Heimfeld S., Spangrude G. J., Weissman I. L. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]