Abstract
Posterior reversible encephalopathy syndrome (PRES) is characterized by headache, nausea, vomiting, seizures and visual disturbances. PRES has been usually associated with hypertension, chronic renal disease, malignancy and chemotherapeutic agents. We report the association of PRES with Autoimmune lymphoproliferative syndrome, which to our best knowledge has not been reported before.
Keywords: Autoimmune lymphoproliferative syndrome, children, posterior reversible encephalopathy syndrome
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) is an inherited disorder manifesting with cytopenia, lymphadenopathy, and hepatosplenomegaly, characterized by the accumulation of double negative T (DNT) cells (CD3+, CD4-, and CD8-) in the peripheral blood.[1] Posterior reversible encephalopathy syndrome (PRES) is characterized by headache, nausea, vomiting, seizures, and visual disturbances.[2] We report the association of PRES with ALPS, which to our best knowledge has not been reported before.
Case Report
A 15-year-old adolescent boy presented with swelling on both sides of the neck for 2 months and fever with weight loss for 1 month. He was reported to have swellings on both sides of the neck on and off for the past 8 years. He has undergone four lymph node biopsies elsewhere which were all reported as reactive lymphadenitis. He has not received any other specific therapy so far. On physical examination, he had severe pallor, cervical lymphadenopathy, palpable liver of 4 cm, and spleen of 3 cm below the costal margin. Laboratory investigations revealed hemoglobin of 6.2 g/L, platelets of 6 × 109/L, and Erythrocyte Sedimentation Rate of 122 mm/h. Liver function tests were normal except elevated total protein of 9.4 g/dl. Serum electrolytes, renal function tests, serum uric acid, and lactate dehydrogenase were within normal limits. Peripheral smear showed microcytic, hypochromic anemia. Direct Coombs test (DCT) and antinuclear antibody (ANA) was positive. Urine microscopy did not reveal any proteinuria or hematuria. Serum complements (C3 and C4) were normal. Double-stranded DNA and anti-Smith ELISA, lupus anticoagulant, anticardiolipin antibodies were negative. Chest X-ray was normal and Mantoux was negative. HIV ELISA and other viral serology including Cytomegalovirus, Ebstein Barr Virus (EBV) and toxoplasmosis were negative. Serum immunoglobulin study revealed hypergammaglobulinemia (IgG, 3426 mg/100 ml; IgA, 353 mg/100 ml; IgM, 79 mg/100 ml; IgE, 941.3 mg/100 ml). Peripheral blood lymphocytes were analyzed by flow cytometry for DNT cells (CD3+, CD4-, and CD8-). Healthy control showed the expected < 1% DNT cells, while the patient's sample showed 19% of double negative cells consistent with the diagnosis of ALPS. Since the diagnosis is conclusive with flow cytometry, he was started with prednisolone (1 mg/kg/day) after counseling about nature of disease process and need for long-term treatment. During follow-up after 2 weeks, his lymphadenopathy, hepatosplenomegaly regressed, and cytopenias resolved completely. His blood pressure was monitored and found to be within normal limits. We did not repeat the lymph node and bone marrow biopsy since he responded very well.
Two months after therapy, he presented with generalized tonic–clonic seizures which were preceded by severe headache. His Glasgow coma scale score was 12/15. He was drowsy and disoriented. On central nervous system (CNV) examination, fundus was normal and pupils were bilaterally central, circular, reacting to light. Bilateral deep tendon reflexes were brisk and plantars were extensor. Rest of the systemic examination was normal.
His blood pressure was 180/110 mm of Hg - more than 99th centile for his age. His blood investigations including serum glucose, electrolytes, calcium, and magnesium were normal. Cerebrospinal fluid examination also was within normal limits. Magnetic Resonance Imaging of the brain showed abnormal T2-weighted and fluid attenuated inversion recovery (FLAIR) hyperintense signals seen in bilateral parieto-occipital regions along with effacement of adjacent sulci, consistent with the diagnosis of PRES [Figure 1a and b]. He was treated with anticonvulsants and antihypertensives. He recovered completely without any neurological deficit. Currently, he is on tapering doses of oral steroids and added on mycophenolate mofetil. He is on regular follow-up for 1 year now.
Figure 1.
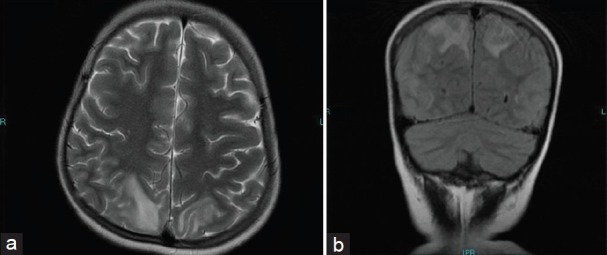
(a and b) Abnormal T2-weighted and fluid attenuated inversion recovery (FLAIR) hyperintense signals with no restricted diffusion seen in bilateral parieto-occipital regions with effacement of adjacent sulci
Discussion
ALPS is an inherited lymphoid disorder whose etiology has been attributed to a primary defect in apoptosis or programmed cell death. It results from mutations in molecules involved in the Fas–Fas ligand pathway and is characterized by non-malignant lymphoproliferation (lymphadenopathy, hepatosplenomegaly), autoimmunity, and cytopenias. There is accumulation of DNT cells (CD3+, CD4-, and CD8-) in the peripheral blood.[1]
PRES is a clinico-radiological syndrome characterized by headache, confusion, nausea, vomiting, seizures, and visual disturbances and radiological findings of bilateral grey and white matter abnormalities suggestive of edema in the posterior regions of cerebral hemispheres.[2] This was first described in 1996.[3] In the pediatric population, PRES has been associated with hypertension, chronic renal disease, autoimmune disorders, intra-abdominal neurogenic tumors like ganglioneuroma, Henoch-Schφnlein purpura, Hemolytic uremic syndrome, acute lymphoblastic leukemia, steroids therapy, porphyria, Addison's disease, administration of chemotherapeutic agents like L-asparaginase, immunosuppressive agents like cyclosporine, Intravenous Immuno Globulin (IIG) therapy, and bone marrow transplantation. PRES has been rarely reported with post-streptococcal glomerulonephritis, neuromyelitis optica spectrum disorders. Bronchial asthma, Non Steroidal Anti Inflammatory Drugs (NSAID)-induced acute tubular interstitial nephritis, atopic dermatitis with group A beta-hemolytic streptococcus skin infection, sickle cell disease, and also tumor lysis.[4–10]
The pathophysiology of PRES is complex which involves vasogenic rather than cytotoxic edema. It is probably a brain capillary leak syndrome related to hypertension, fluid retention, and possibly the cytotoxic effects of immunosuppressive agents on the vascular endothelium. The relative paucity of sympathetic innervation in the posterior brain leads to susceptibility to hyperperfusion and vasogenic edema during acute blood pressure elevations which may explain the presence of majority of the lesions in the vascular territory of posterior circulation. Cerebral autoregulation maintains a relatively constant cerebral blood flow over a range of mean arterial blood pressure, thus protecting the brain from acute changes in blood pressure. However, at high mean arterial pressures, autoregulation fails, leading to arteriolar vasodilation and endothelial dysfunction, resulting in disruption of the blood–brain barrier and capillary leakage. The white matter is predominantly affected as it is less tightly packed than the cortex. Children with acute onset of hypertension are probably more at risk than those with chronic hypertension because they have not yet developed adaptive vascular changes. The pathophysiology of PRES caused by immunosuppressive therapy is less clear, but may involve damage to the vascular endothelium resulting in vasospasm, reduced tissue perfusion, activation of the coagulation cascade, and extravasation of fluid.[1,11]
In patients of PRES, seizures (frequently new onset, secondary generalized occipital lobe seizure) almost always occur during the course of illness.
The most common abnormalities on computed tomography (CT) and/or magnetic resonance imaging (MRI) scans are focal regions of vasogenic edema involving the white matter in the posterior cerebral hemispheres, often asymmetrically and most commonly involving the parieto-occipital lobes bilaterally, often in a watershed-type distribution. While neuroimaging abnormalities in PRES are seen maximally in the white matter of the parietal and occipital regions, lesions can be more extensive, spreading into the cerebellum, frontal regions, basal ganglia, thalami, brain stem, and corpus callosum, suggesting a diagnosis of demyelinating disease. Primarily, posterior fossa involvement is uncommon in PRES syndrome. The calcarine and paramedian parts of the occipital lobe are classically spared, helping to distinguish PRES from bilateral posterior cerebral artery infarction.[1] Although abnormalities may be seen on CT scan, FLAIR MRI sequences are much better at showing the extent of the hyperintense lesions and the degree of cortical involvement. Diffusion-weighted MR imaging (DWI) sequences are helpful to discriminate mitochondrial encephalopathy and venous infarction, in which the lesions typically show restricted diffusion. The majority of lesions in PRES show iso or slightly hyperintense signal on DWI but increased apparent diffusion coefficient (ADC) consistent with vasogenic edema, as opposed to decreased ADC values usually seen with infarction. Rarely, very high tissue perfusion pressure may lead to decreased cerebral blood flow, with resulting ischemia and cytotoxic edema, manifested by an area of increased signal on DWI and decreased signal on the ADC map. The lesions of PRES usually do not enhance on post-contrast images. The MR spectroscopy results have varied, but evidence of subtle metabolic changes including high lactate and increased choline and creatine and mildly decreased N-acetyl aspartate has been reported.[12–15]
Although reversible by definition, secondary complications, such as status epilepticus, intracranial hemorrhage, and massive ischemic infarction, can cause substantial morbidity with neurodevelopmental sequelae and mortality. At least one case of death due to PRES-related brainstem swelling has been reported. Hence as the name indicates, this is not always posterior and reversible. Hence, early recognition of PRES is important for timely institution of therapy, which typically consists of gradual blood pressure control and withdrawal of potentially offending agents.[16,17]
Recurrence is infrequent, even though trigger factors for PRES were repeatedly experienced by the patients but it can be very rarely recurrent also.[18] Mesial temporal sclerosis can occur after PRES as a sequelae.[19] Differential diagnosis of PRES includes PCA territory infarcts, venous thrombosis, demyelinating disorders, vasculitis, and encephalitis.
The diagnosis of PRES should be considered in any child presenting with new onset seizures, encephalopathy, and visual symptoms. This condition responds promptly to adequate control of blood pressure, and the long-term neurological prognosis is extremely favorable. We report the occurrence of PRES in ALPS, which to our best knowledge has not been reported before.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Worth A, Thrasher AJ, Gaspar HB. Autoimmune lymphoproliferative syndrome: Molecular basis of disease and clinical phenotype. Br J Haematol. 2006;133:124–40. doi: 10.1111/j.1365-2141.2006.05993.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–10. doi: 10.1001/archneurol.2007.46. [DOI] [PubMed] [Google Scholar]
- 3.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 4.Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–32. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Goyal VK, Talukdar B. Reversible posterior leucoencephalopathy syndrome in post streptococcal glomerulonephritis. Indian Pediatr. 2010;47:274–6. doi: 10.1007/s13312-010-0037-y. [DOI] [PubMed] [Google Scholar]
- 6.Kurahashi H, Okumura A, Koide T, Ando Y, Hirata H, Magota M, et al. Posterior reversible encephalopathy syndrome in a child with bronchial asthma. Brain Dev. 2006;28:544–6. doi: 10.1016/j.braindev.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Yokobori S, Yokota H, Yamamoto Y. Pediatric posterior reversible leukoencephalopathy syndrome and NSAID-induced acute tubular interstitial nephritis. Pediatr Neurol. 2006;34:245–7. doi: 10.1016/j.pediatrneurol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Kaito E, Terae S, Kobayashi R, Kudo K, Tha KK, Miyasaka K. The role of tumor lysis in reversible posterior leukoencephalopathy syndrome. Pediatr Radiol. 2005;35:722–7. doi: 10.1007/s00247-005-1434-6. [DOI] [PubMed] [Google Scholar]
- 9.Koichihara R, Hamano S, Yamashita S, Tanaka M. Posterior reversible encephalopathy syndrome associated with IVIG in a patient with Guillain-Barré syndrome. Pediatr Neurol. 2008;39:123–5. doi: 10.1016/j.pediatrneurol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Park JM, Oh SH, Kim J, Lee JH. Atopic dermatitis with group A beta-hemolytic Streptococcus skin infection complicated by posterior reversible encephalopathy syndrome. Arch Dermatol. 2009;145:846–7. doi: 10.1001/archdermatol.2009.129. [DOI] [PubMed] [Google Scholar]
- 11.Brubaker LM, Smith JK, Lee YZ, Lin W, Castillo M. Hemodynamic and permeability changes in posterior reversible encephalopathy syndrome measured by dynamic susceptibility perfusion-weighted MR imaging. AJNR Am J Neuroradiol. 2005;26:825–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: Utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000;21:1199–206. [PMC free article] [PubMed] [Google Scholar]
- 13.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: Prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23:1038–48. [PMC free article] [PubMed] [Google Scholar]
- 14.Eichler FS, Wang P, Wityk RJ, Beauchamp NJ, Jr, Barker PB. Diffuse metabolic abnormalities in reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 2002;23:833–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon S, Koo J, Lee S. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol. 2001;24:361–4. doi: 10.1016/s0887-8994(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 16.Lucchini G, Grioni D, Colombini A, Contri M, De Grandi C, Rovelli A, et al. Encephalopathy syndrome in children with hemato-oncological disorders is not always posterior and reversible. Pediatr Blood Cancer. 2008;51:629–33. doi: 10.1002/pbc.21688. [DOI] [PubMed] [Google Scholar]
- 17.Kheir JN, Lawlor MW, Ahn ES, Lehmann L, Riviello JJ, Silvera VM, et al. Neuropathology of a fatal case of posterior reversible encephalopathy syndrome. Pediatr Dev Pathol. 2010;13:397–403. doi: 10.2350/09-04-0634-CR.1. [DOI] [PubMed] [Google Scholar]
- 18.Girişgen I, Tosun A, Sönmez F, Ozsunar Y. Recurrent and atypical posterior reversible encephalopathy syndrome in a child with peritoneal dialysis. Turk J Pediatr. 2010;52:416–9. [PubMed] [Google Scholar]
- 19.Aboian MS, Junna MR, Krecke KN, Wirrell EC. Mesial temporal sclerosis after posterior reversible encephalopathy syndrome. Pediatr Neurol. 2009;41:226–8. doi: 10.1016/j.pediatrneurol.2009.03.007. [DOI] [PubMed] [Google Scholar]


