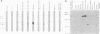Abstract
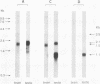
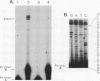
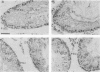
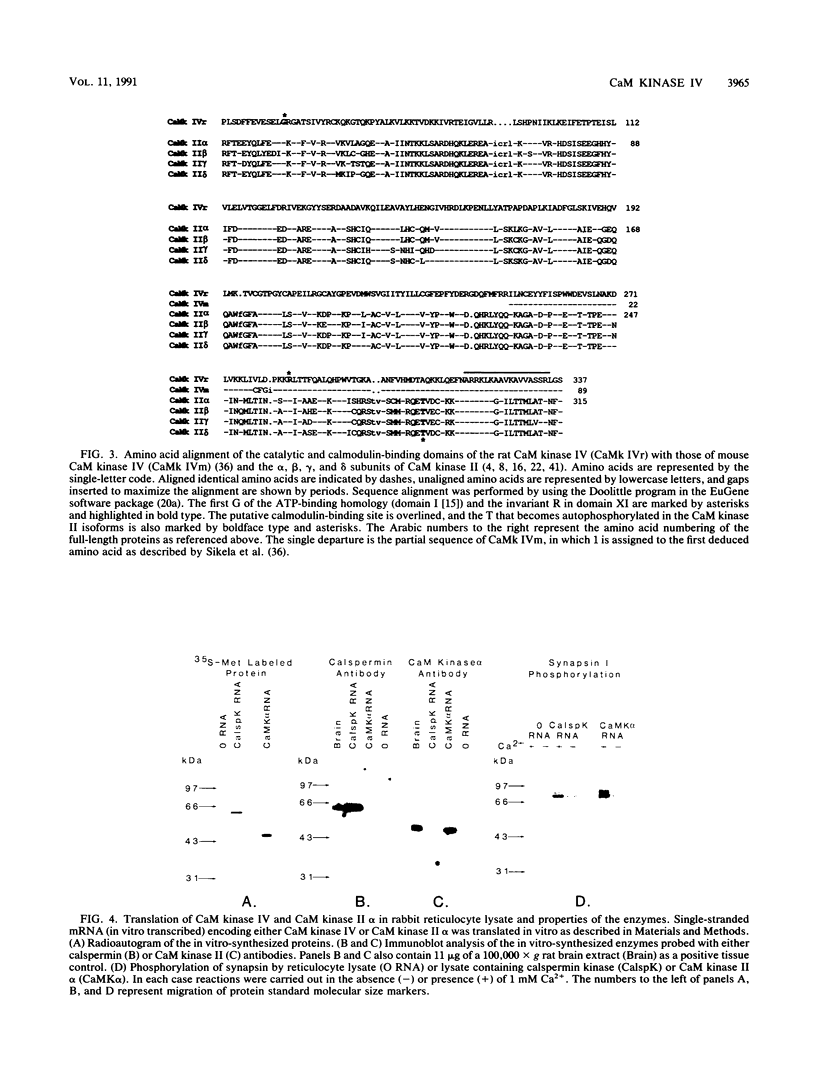
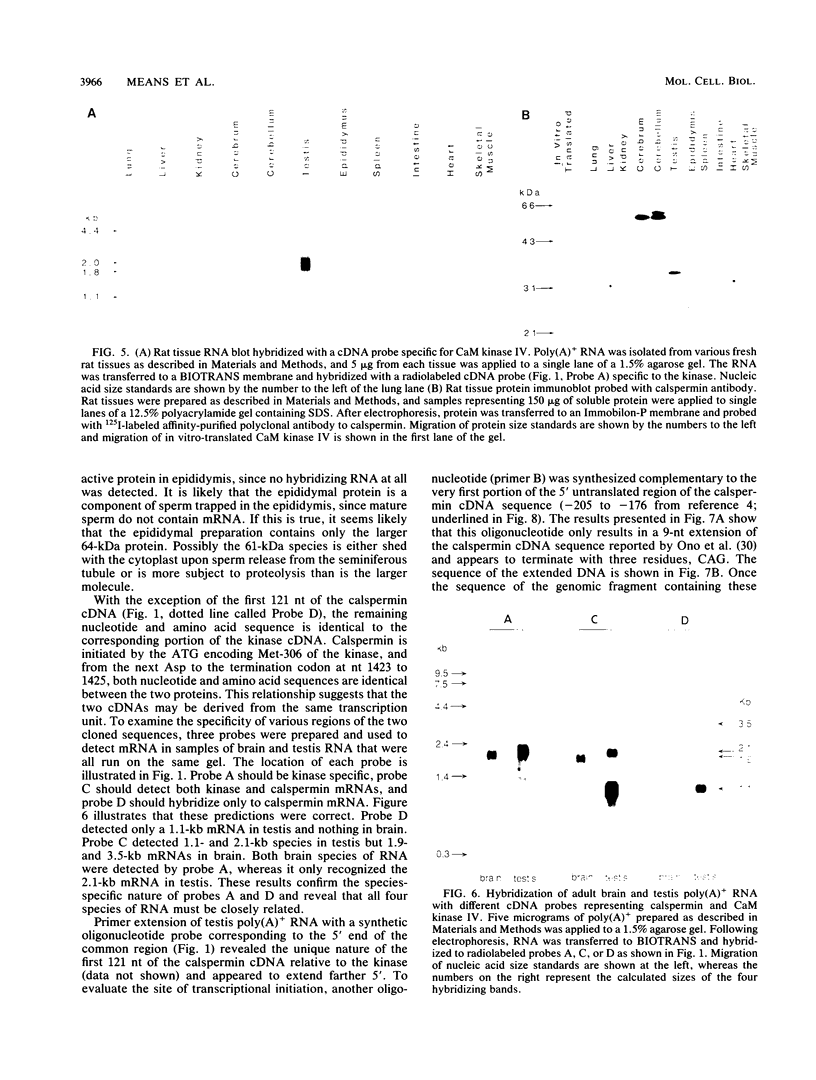
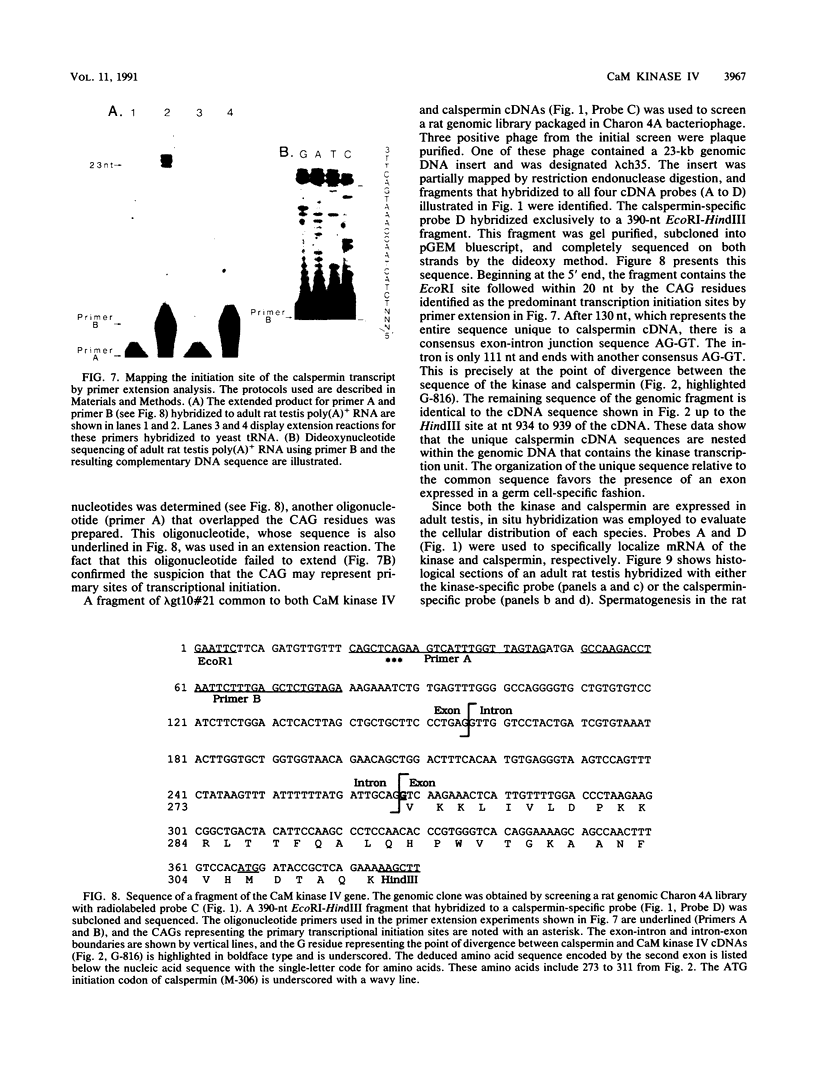
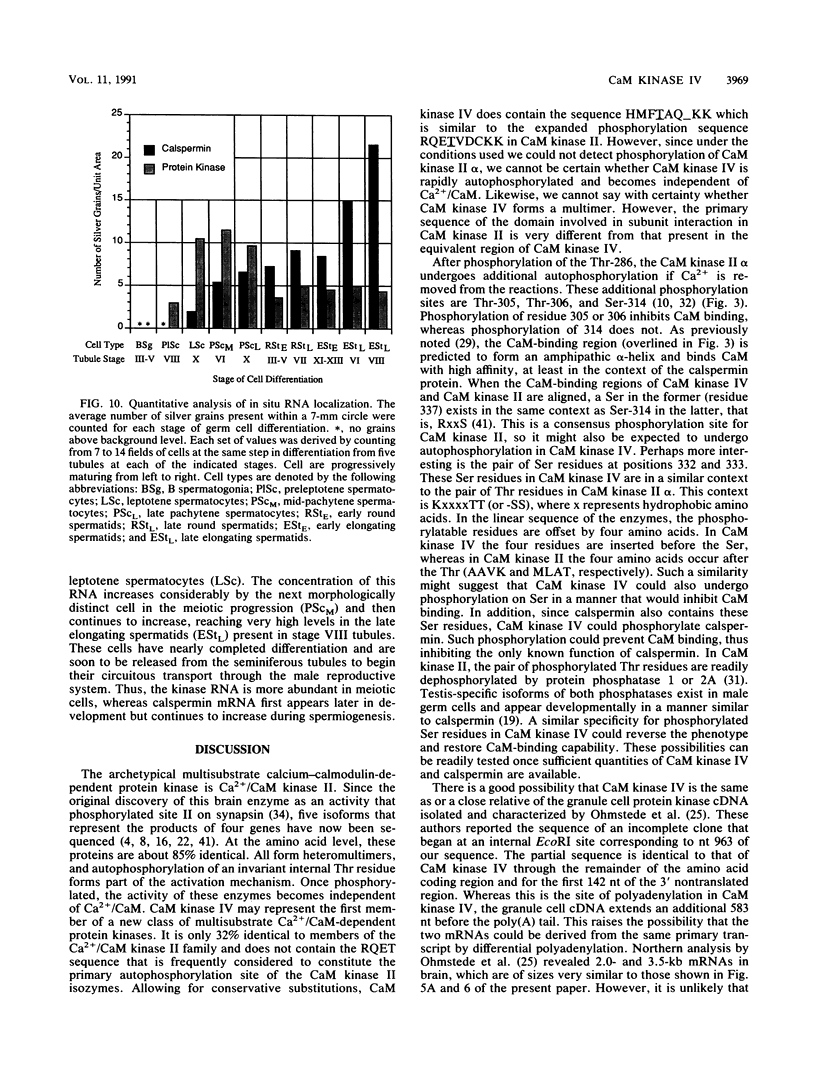
A cDNA representing a unique Ca2+/calmodulin-dependent protein kinase has been cloned and sequenced from a rat brain cDNA library. This enzyme, expressed in brain, testis, and spleen, is only 32% identical to the various isoforms of Ca2+/calmodulin-dependent protein kinase II. The sequence of the COOH-terminal 169 amino acids is identical to that of a previously described male germ cell-specific calmodulin-binding protein called calspermin (T. Ono, G.R. Slaughter, R.G. Cook, and A.R. Means, J. Biol. Chem. 264:2081-2087, 1989). This identity extends to the nucleic acid sequence and includes all but the first 130 nucleotides of the calspermin cDNA. Primer extension and sequence of a genomic fragment containing the unique calspermin sequence reveals that this mRNA is derived from the kinase transcription unit by germ cell-specific use of a unique exon. In situ hybridization was used to demonstrate that both kinase and calspermin mRNAs are expressed during spermatogenesis. The kinase mRNA is first detected in early meiotic cells and declines to a low level in haploid cells. Calspermin mRNA first appears in pachytene primary spermatocytes and continues to increase as cells complete meiosis and undergo terminal differentiation. These results show that differential utilization of a single gene during spermatogenesis is used to generate mRNAs that encode proteins with distinct functions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcivar A. A., Hake L. E., Hardy M. P., Hecht N. B. Increased levels of junB and c-jun mRNAs in male germ cells following testicular cell dissociation. Maximal stimulation in prepuberal animals. J Biol Chem. 1990 Nov 25;265(33):20160–20165. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer-LeLievre C., Olson L., Ebendal T., Hallbök F., Persson H. Nerve growth factor mRNA and protein in the testis and epididymis of mouse and rat. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2628–2632. doi: 10.1073/pnas.85.8.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Kennedy M. B. Deduced primary structure of the beta subunit of brain type II Ca2+/calmodulin-dependent protein kinase determined by molecular cloning. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1794–1798. doi: 10.1073/pnas.84.7.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower P. A., Yelick P. C., Hecht N. B. Both P1 and P2 protamine genes are expressed in mouse, hamster, and rat. Biol Reprod. 1987 Sep;37(2):479–488. doi: 10.1095/biolreprod37.2.479. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Behringer R. R., Peschon J. J., Brinster R. L., Palmiter R. D. Genetically haploid spermatids are phenotypically diploid. Nature. 1989 Jan 26;337(6205):373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Bulleit R. F., Bennett M. K., Molloy S. S., Hurley J. B., Kennedy M. B. Conserved and variable regions in the subunits of brain type II Ca2+/calmodulin-dependent protein kinase. Neuron. 1988 Mar;1(1):63–72. doi: 10.1016/0896-6273(88)90210-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colbran R. J., Soderling T. R. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990 Jul 5;265(19):11213–11219. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goto M., Koji T., Mizuno K., Tamaru M., Koikeda S., Nakane P. K., Mori N., Masamune Y., Nakanishi Y. Transcription switch of two phosphoglycerate kinase genes during spermatogenesis as determined with mouse testis sections in situ. Exp Cell Res. 1990 Feb;186(2):273–278. doi: 10.1016/0014-4827(90)90306-u. [DOI] [PubMed] [Google Scholar]
- Guerriero V., Jr, Russo M. A., Olson N. J., Putkey J. A., Means A. R. Domain organization of chicken gizzard myosin light chain kinase deduced from a cloned cDNA. Biochemistry. 1986 Dec 30;25(26):8372–8381. doi: 10.1021/bi00374a007. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanley R. M., Means A. R., Ono T., Kemp B. E., Burgin K. E., Waxham N., Kelly P. T. Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science. 1987 Jul 17;237(4812):293–297. doi: 10.1126/science.3037704. [DOI] [PubMed] [Google Scholar]
- Hecht N. B., Distel R. J., Yelick P. C., Tanhauser S. M., Driscoll C. E., Goldberg E., Tung K. S. Localization of a highly divergent mammalian testicular alpha tubulin that is not detectable in brain. Mol Cell Biol. 1988 Feb;8(2):996–1000. doi: 10.1128/mcb.8.2.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T. E., Shai S. Y., Langford K. G., Martin B. M., Bernstein K. E. Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol Cell Biol. 1990 Aug;10(8):4294–4302. doi: 10.1128/mcb.10.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y., Sasaki K., Shima H., Shibuya M., Sugimura T., Nagao M. Protein phosphatases possibly involved in rat spermatogenesis. Biochem Biophys Res Commun. 1990 Aug 31;171(1):230–235. doi: 10.1016/0006-291x(90)91381-2. [DOI] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Kapiloff M. S., Durgerian S., Tatemoto K., Russo A. F., Hanson P., Schulman H., Rosenfeld M. G. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmstede C. A., Jensen K. F., Sahyoun N. E. Ca2+/calmodulin-dependent protein kinase enriched in cerebellar granule cells. Identification of a novel neuronal calmodulin-dependent protein kinase. J Biol Chem. 1989 Apr 5;264(10):5866–5875. [PubMed] [Google Scholar]
- Oliva R., Mezquita J., Mezquita C., Dixon G. H. Haploid expression of the rooster protamine mRNA in the postmeiotic stages of spermatogenesis. Dev Biol. 1988 Feb;125(2):332–340. doi: 10.1016/0012-1606(88)90216-3. [DOI] [PubMed] [Google Scholar]
- Ono T., Koide Y., Arai Y., Yamashita K. Heat-stable calmodulin-binding protein in rat testis. Inhibition of calmodulin-stimulated cyclic nucleotide phosphodiesterase activity. J Biol Chem. 1984 Jul 25;259(14):9011–9016. [PubMed] [Google Scholar]
- Ono T., Koide Y., Arai Y., Yamashita K. Tissue specificity of calspermin: a heat-stable Mr 32,000 calmodulin-binding protein. Arch Biochem Biophys. 1987 May 15;255(1):102–108. doi: 10.1016/0003-9861(87)90299-2. [DOI] [PubMed] [Google Scholar]
- Ono T., Slaughter G. R., Cook R. G., Means A. R. Molecular cloning sequence and distribution of rat calspermin, a high affinity calmodulin-binding protein. J Biol Chem. 1989 Feb 5;264(4):2081–2087. [PubMed] [Google Scholar]
- Patton B. L., Miller S. G., Kennedy M. B. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990 Jul 5;265(19):11204–11212. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M., Kuhn R., Bosse F., Schäfer U. A conserved element in the leader mediates post-meiotic translation as well as cytoplasmic polyadenylation of a Drosophila spermatocyte mRNA. EMBO J. 1990 Dec;9(13):4519–4525. doi: 10.1002/j.1460-2075.1990.tb07903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikela J. M., Hahn W. E. Screening an expression library with a ligand probe: isolation and sequence of a cDNA corresponding to a brain calmodulin-binding protein. Proc Natl Acad Sci U S A. 1987 May;84(9):3038–3042. doi: 10.1073/pnas.84.9.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikela J. M., Law M. L., Kao F. T., Hartz J. A., Wei Q., Hahn W. E. Chromosomal localization of the human gene for brain Ca2+/calmodulin-dependent protein kinase type IV. Genomics. 1989 Jan;4(1):21–27. doi: 10.1016/0888-7543(89)90309-1. [DOI] [PubMed] [Google Scholar]
- Slaughter G. R., Means A. R. Analysis of expression of multiple genes encoding calmodulin during spermatogenesis. Mol Endocrinol. 1989 Oct;3(10):1569–1578. doi: 10.1210/mend-3-10-1569. [DOI] [PubMed] [Google Scholar]
- Slaughter G. R., Needleman D. S., Means A. R. Developmental regulation of calmodulin, actin, and tubulin RNAs during rat testis differentiation. Biol Reprod. 1987 Dec;37(5):1259–1270. doi: 10.1095/biolreprod37.5.1259. [DOI] [PubMed] [Google Scholar]
- Stallard B. J., Collard M. W., Griswold M. D. A transferrinlike (hemiferrin) mRNA is expressed in the germ cells of rat testis. Mol Cell Biol. 1991 Mar;11(3):1448–1453. doi: 10.1128/mcb.11.3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu T., Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989 Oct 25;264(30):17907–17912. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]