Abstract
Purpose
To report the identification of five novel nonsense mutations in the zinc finger E-box binding homeobox 1 (ZEB1) gene and exclusion of promoter region mutations in individuals without ZEB1 coding region mutations in posterior polymorphous corneal dystrophy (PPCD).
Methods
Slit-lamp examination and DNA collection were performed for individuals diagnosed with PPCD and, when available, affected and unaffected family members. Genomic DNA prepared from peripheral blood leukocytes and buccal epithelial cells underwent PCR amplification and automated sequencing of the ZEB1 gene and 1 kb 5′ of ZEB1, presumably containing the ZEB1 promoter region.
Results
Thirteen unrelated individuals with PPCD were identified, and genomic DNA was collected from each individual. ZEB1 mutations were identified in six of the 13 probands, five of which were novel: p.(Gly150Alafs*36; spontaneous), p.(His230Argfs*7), p.(Ser638Cysfs*5), p.(Glu1039Glyfs*6), and p.(Gln884Argfs*37). Screening of the ZEB1 promoter region in 31 probands with PPCD without a ZEB1 coding region mutation identified only two known single nucleotide polymorphisms (SNPs) whose frequency in the affected probands did not differ significantly from that in the general population.
Conclusions
We report five novel frame-shift mutations, one confirmed as spontaneous, in the ZEB1 gene associated with PPCD, bringing the total number of reported pathogenic mutations to 24, and the percentage of PPCD associated with ZEB1 mutations to 32%. The absence of ZEB1 promoter region mutations in probands without a ZEB1 coding region mutation indicates that other genetic loci, such as the PPCD1 locus, are involved in the pathogenesis of PPCD.
Introduction
Posterior polymorphous corneal dystrophy (PPCD; MIM 122000) is an autosomal dominant corneal dystrophy associated with characteristic morphologic endothelial abnormalities and, in severe cases, endothelial decompensation. Although an uncommon inherited disorder, PPCD has been associated with a number of other ocular disorders, including primary open angle and secondary angle-closure glaucoma [1,2], as well as non-keratoconic corneal steepening [3,4] and keratoconus [5,6]. A number of associated extraocular manifestations, including abdominal hernia and hydrocele formation, distinguish PPCD from the majority of the other corneal dystrophies, which are traditionally considered isolated corneal disorders [7,8]. PPCD also differs from the majority of other corneal dystrophies in that locus heterogeneity has been reported, with linkage reported to chromosomes 10 (the PPCD3 locus) [9] and 20 (the PPCD1 locus) [1]. Krafchak and colleagues reported frameshift mutations in the zinc finger E-box binding homeodomain 1 gene (ZEB1 gene; OMIM 189909) in the PPCD3 locus in five of 11 probands and demonstrated altered endothelial expression of the collagen IV, alpha 3 (COL4A3; OMIM 120070) gene in the corneal endothelium of an affected individual, leading to their proposed theory of pathogenesis of PPCD3 [8]. We confirmed the role of ZEB1 in PPCD3 by reporting eight additional frameshift mutations in 32 probands who were screened, and provided additional evidence to support the role of ZEB1 in negative regulation of COL4A3 transcription [7].
To date, 19 coding region mutations, all nonsense, have been identified in the ZEB1 gene in 65 families with PPCD [7,8,10-12]. We report the identification of six ZEB1 coding region mutations in 13 additional probands, five of which are novel and one that was confirmed to be spontaneous. We also report the absence of pathogenic sequence variants in the ZEB1 promoter region in 31 probands without ZEB1 coding region mutations. Our results indicate that truncating ZEB1 mutations are present in approximately one third of probands with PPCD, with a unique mutation identified in every proband except one screened to date.
Methods
The authors followed the tenets of the Declaration of Helsinki in the treatment of the subjects. Study approval was obtained from the Institutional Review Board at the University of California, Los Angeles (UCLA IRB # 94–07–243-(14–33A), 02–10–092-(4,11), 10–001932).
Patient identification/deoxyribonucleic acid collection and preparation
Patients examined on the Cornea Service at the Jules Stein Eye Institute (by Dr. Anthony Aldave) or at the Kansas University Eye Center (by Dr. John Sutphin) were diagnosed with PPCD based on the presence of characteristic corneal endothelial changes in one or both eyes: an endothelial band with parallel borders typically associated with white flaky-appearing material along the edge of the band; single or grouped vesicular endothelial changes, typically associated with a surrounding gray halo; and either discreet or confluent geographic gray endothelial opacities. After individuals were offered enrollment in the study by Dr. Anthony Aldave, and after informed consent was obtained, a peripheral blood sample, a buccal epithelial sample (Cyto-Soft Cytology Brush; Medical Packaging Corporation, Camarillo, CA), or a saliva sample (Oragene saliva collection kits; DNA Genotek, Inc., Kanata, Canada) was collected as a source of genomic DNA. Unrelated, unaffected, healthy volunteers were recruited to serve as controls. Genomic DNA was prepared from the peripheral blood leukocytes and buccal epithelial cells using the FlexiGene DNA and QIAamp DNA Blood Mini Kits (Qiagen, Valencia, CA), respectively.
Polymerase chain reaction amplification
Each of the nine exons of ZEB1 was amplified using primers and conditions previously described by Krafchak et al. with the exception of exon 1, which was amplified using a custom-designed oligonucleotide pair described previously [7,8]. An alternative exon 1 and the 1 Kb upstream of the initiation methionine (ATG), containing the core and putative distal promoter regions were amplified using custom-designed oligonucleotide pairs (Table 1). Each 25 μl reaction contained 50 mM Tris-HCl (pH 9.0, 25 °C), 20 mM NH4Cl, 2.5 mM MgSO4, 200 mM each deoxynucleotide triphosphate (dNTP) plus 20 mM 7-deaza-2´-deoxyguanosine 5´-triphosphate, 0.5 M Betaine, 2.5 μl dimethyl sulfoxide, 150 mM Trehalose, 0.002% Tween-20, 0.12 mM of each primer, 0.5 units of RedTaq Genomic DNA Polymerase (Sigma-Aldrich, St. Louis, MO), and approximately 20 ng of genomic DNA. Thermal cycling was performed in an iCycler Thermal Cycler (Bio-Rad, Hercules, CA).
Table 1. Primers used for screening alternative exon 1 and the 1 Kb upstream of the zinc finger E-box binding homeobox 1 (ZEB1) gene.
| ZEB1 | Forward primer 5’-3’ | Reverse primer 5’-3’ | Annealing Temp |
|---|---|---|---|
| Alternative exon 1 |
GTGGAGAGATGACTTGTTATAGCA |
GTGGTTCAGACTCACAGTC |
54 °C |
| Promoter: Proximal Region |
GCCGATGCTTCTTGCCTTAAG |
GCTGTCGGAGTTGGAAAGGTAAAG |
56 °C |
| Promoter: Distal Region | CCAGACCGCGATCCCTTCCTTG | CTCCGCCACTCACCGTATTG | 56 °C |
Deoxyribonucleic acid sequencing
Purification of the PCR products was achieved by incubating 15–30 ng of amplified DNA with 5 U Exonuclease I and 0.5 U shrimp alkaline phosphatase (USB Corp., Cleveland, OH) for 15 min at 37 °C. After inactivation of the nucleases for 15 min at 80 °C, sequencing reactions were performed by adding 2 µl of Big Dye Terminator Mix v3.1 (Applied Biosystems, Foster City, CA), 2 µl of SeqSaver (Sigma-Aldrich), and 0.2 µl of primer (10 pmoles/µl). Samples were denatured at 96 °C for 2 min and then cycled 25 times at 96 °C for 10 s, 50 °C for 5 s, and 60 °C for 4 min. Unincorporated nucleotides were removed using the CleanSeq reagent and an SPRI plate (Agencourt Bioscience Corporation, Beverly, MA) following the manufacturer’s instructions and then analyzed on an ABI-3100 Genetic Analyzer (Applied Biosystems) after resuspension in 0.1 mM EDTA. Coding region nucleotide sequences (including the donor and acceptor splice sites) were read manually by comparison to the ZEB1 cDNA sequences (GenBank accession number NM_030751 and NM_001128128.2), while promoter region sequences were compared to the ZEB1 RefSeqGene sequence (GenBank accession number NG_017048.1). The description of the identified sequence variants adhered to the Human Genome Variation Society (HGVS) nomenclature guidelines.
Statistical analyses
The Fisher exact test was used to identify an association between ZEB1 promoter region sequence variants and PPCD in probands without a ZEB1 coding region mutation. The binomial proportion test was used to compare the percentage of all probands with PPCD demonstrating a ZEB1 promoter region sequence variant with the prevalence in the general population as reported in HapMap.
Results
Patient Identification and screening of the zinc finger E-box binding homeobox 1 gene coding region
Thirteen probands diagnosed with PPCD based on the presence of characteristic clinical features were enrolled into the study and provided DNA for genetic analysis. Ten of the 13 probands were female, and the average age at the time of enrollment was 36 years of age (range, 5-77 years). Screening of the ZEB1 coding region in the 13 PPCD probands identified six nonsense mutations in the heterozygous state, of which five are novel: c.449delG (p.(Gly150Alafs*36)), c.689_690delAT (p.(His230Argfs*7)), c.1913_1914delCA (p.(Ser638Cysfs*5)), c.2650delC (p.(Gln884Argfs*37)), and c.3116_3117delAG (p.(Glu1039Glyfs*6)). The sixth mutation identified, c.1576dupG (p.(Val526Glyfs*3)), has been previously reported [8]. Located in exons 4 and 5, the p.(Gly150Alafs*36) and p.(His230Argfs*7) mutations, respectively, are predicted to result in the loss or disruption of multiple important functional domains of the ZEB1 protein, including the C-terminal binding protein and SMAD binding domains, the homeodomain, and the N- and C-terminal zinc-finger clusters (Figure 1). The p.(Ser638Cysfs*5) mutation located in exon 7 is predicted to result in the loss of the CtBP binding domains, the homeodomain, and the C-terminal zinc-finger cluster. Located in exon 8, the p.(Gln884Argfs*37) mutation is predicted to result in the loss of the C-terminal zinc-finger cluster. The p.(Glu1039Glyfs*6) mutation located in exon 9 is not predicted to result in the loss of any of these important functional domains.
Figure 1.

Depiction of the zinc finger E-box binding homeobox 1 (ZEB1) protein, demonstrating the location of the five novel mutations and 19 previously reported mutations. The mutation nomenclature is presented according the Human Genome Variation Society (HGSV) guidelines, and thus may be different from the nomenclature used in original publications. Important functional domains are also depicted.
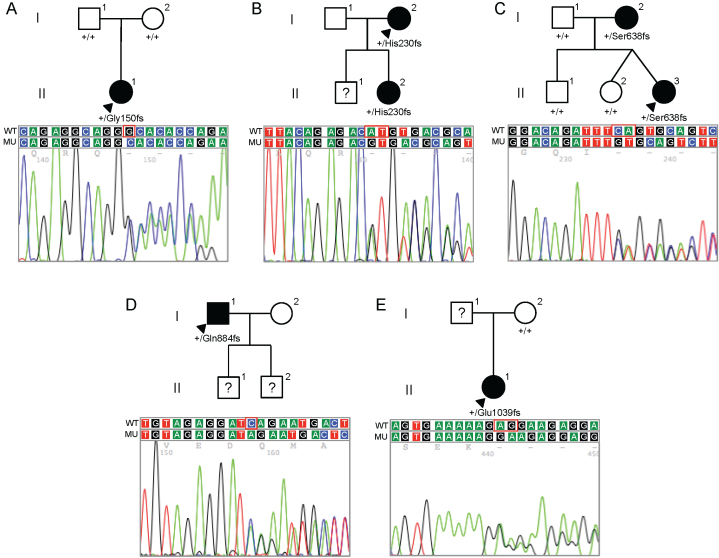
Screening of available affected and unaffected family members of each affected proband was performed (Figure 2). Both parents of the proband in whom the p.(Gly150Alafs*36) mutation was identified were clinically unaffected, and neither demonstrated the mutation identified in the proband. Paternity testing confirmed that the father was the biologic father, indicating that the mutation present in the proband most likely arose spontaneously. The only available parent of the proband demonstrating the p.(Glu1039Glyfs*6) mutation was unaffected and did not demonstrate the mutation identified in the heterozygous state in the proband. In contrast, the p.(His230Argfs*7) and p.(Ser638Cysfs*5) mutations were identified in an affected parent and child in the heterozygous state. The p.(Gln884Argfs*37) mutation was identified in the heterozygous state in an affected individual, although no other family members were available for analysis (Figure 3).
Figure 2.
Pedigrees and zinc finger E-box binding homeobox 1 (ZEB1) sequences for the five families in which novel zinc finger E-box binding homeobox 1 mutations were identified. In each pedigree, the presence of the wild-type allele (designated by the + symbol) or the mutant allele is indicated below the symbol of each individual in whom DNA collection and zinc finger E-box binding homeobox 1 (ZEB1) gene screening were performed. Filled symbols represent affected individuals, open symbols represent unaffected individuals, and question marks indicate individuals of undetermined affected status. Arrowheads indicate probands. Beneath each pedigree, chromatograms demonstrating the identified mutation (MU) and the wild-type DNA sequence (WT) are shown.
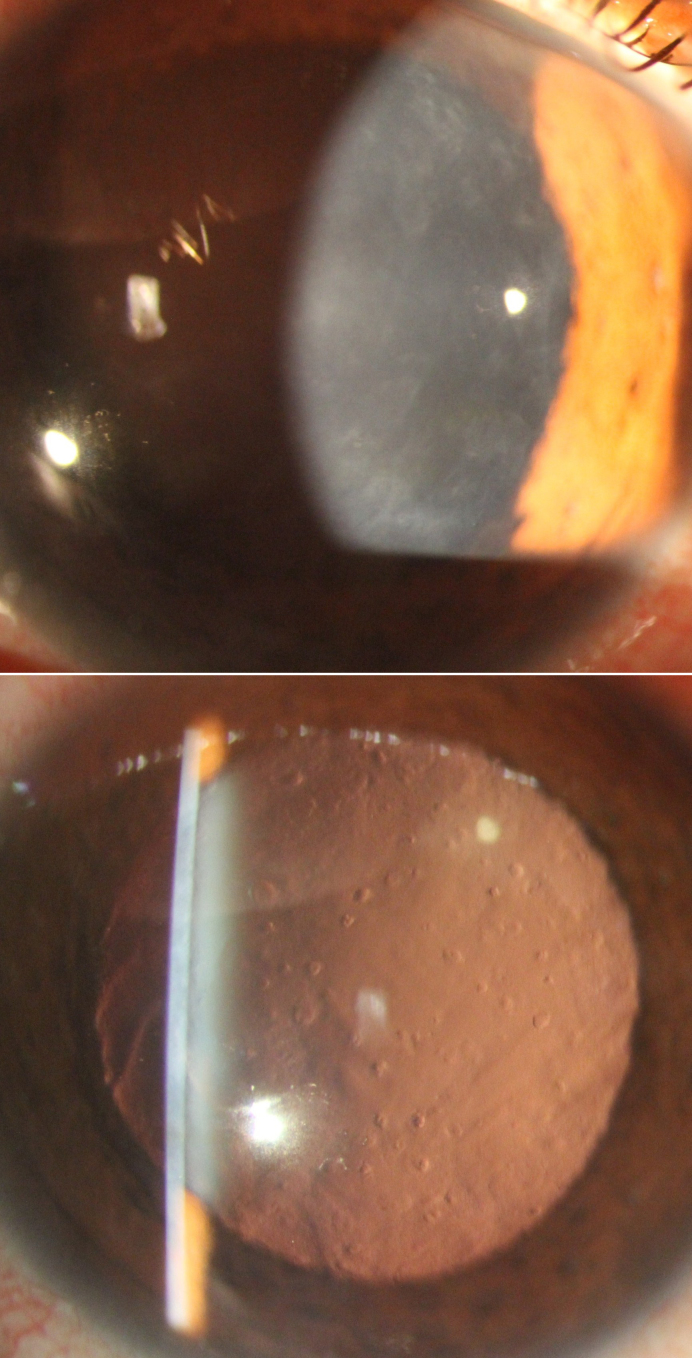
Figure 3.
Slit-lamp photomicrographs of a patient with posterior polymorphous corneal dystrophy 3 (PPCD3) secondary to p.(Gln884Argfs*37) mutation in the zinc finger E-box binding homeobox 1 gene (ZEB1). Diffusely distributed geographic gray-white Descemet membrane opacities are seen on direct illumination (A) that appear as round and oval-shaped “vesicles” on retroillumination, consistent with posterior polymorphous corneal dystrophy (PPCD).
Screening of the zinc finger E-box binding homeobox 1 gene promoter region
Thirty-one probands with PPCD in whom ZEB1 coding region mutations were not identified were selected for screening of the putative ZEB1 promoter located within a 1 kb region upstream of the ATG start codon. Two known single nucleotide polymorphisms, −933T>G (rs3737179) and −803G>C (rs3737180), were identified together in the heterozygous state in six of the 31 (19.3%) probands (the minor allele haplotype). To test for an association between the minor allele haplotype and the absence of ZEB1 coding sequence variants, we compared the prevalence of this haplotype in the general population to that in 14 probands with PPCD3 (in whom a ZEB1coding region mutation had been identified). None of the probands with PPCD3 demonstrated the minor allele of either single nucleotide polymorphism, although the 0% prevalence was not statistically significantly less than the 12.4% prevalence of the haplotype in the general population (data from HapMap; binomial proportion test p=0.19) or the 19.3% prevalence in probands with PPCD in whom ZEB1 coding region mutations were not identified (Fisher exact test p=0.16). Additionally, the 13.3% (6/45) prevalence of this haplotype in all probands with PPCD (ZEB1 and non-ZEB1) was not significantly different from that in the general population (binomial proportion test p=0.85).
Discussion
With the current report of five novel ZEB1 mutations in 13 probands with PPCD, the total number of reported mutations identified in ZEB1 associated with PPCD increases to 24, and the percentage of probands with PPCD in whom a ZEB1 coding region mutation has been identified increases to 32% (25/78) [7,8,10-12]. As no pathogenic sequence variants were identified in the putative promoter region in the 31 probands with PPCD without a ZEB1 coding region mutation, we can conclude that other genetic loci, such as the PPCD1 locus on chromosome 20, are involved in the pathogenesis of PPCD.
We report the first ZEB1 mutation that is not unique to the family in which it was identified, p.(Val526Glyfs*3), which was originally reported by Krafchak and colleagues [8]. The Caucasian pedigree in whom we identified this mutation could be related to the Caucasian family reported by Krafchak and colleagues. However, as the mutation was not identified in either parent of the proband that we report, it may also represent a spontaneous mutation (although this could not be confirmed as we did not perform paternity testing). Two different spontaneous mutations in ZEB1 have been reported by Krafchak and colleagues, and thus, the novel p.(Gly150Alafs*36) mutation that we report represents the third reported spontaneous mutation [8]. Although spontaneous mutations are therefore implicated in 12% (3/25) of probands with PPCD with ZEB1 mutations, these mutations have been identified in only one other gene associated with a corneal dystrophy, the transforming growth factor, beta-induced (TGFBI) gene [13,14]. Given this, as well as the fact that the majority of the corneal dystrophies are dominantly inherited and associated with complete penetrance, clinicians commonly rely on the presence of a positive family history in diagnosing corneal dystrophies. Therefore, identifying and reporting spontaneous pathogenic mutations associated with corneal dystrophies is important to ensure that clinicians consider the possibility of a dominantly inherited corneal dystrophy even in the absence of a family history.
Four of the five novel mutations that we report are predicted to result in the loss of one or more domains critical to the function of the ZEB1 protein. The ZEB1 protein is generated from nine coding exons that contain two zinc finger clusters (N-terminal and C-terminal), a homeodomain, and SMAD and CtBP binding domains (Figure 1). The zinc finger domains are DNA-binding motifs, while the homeodomain has been reported to interact with the N-terminal zinc finger cluster, in an intraprotein interaction [15]. SMAD proteins are the transducers of TGF-β signaling, relaying signals from cell-surface receptors to the nucleus, where the proteins activate transcription of specific target genes [16]. Thus, the loss of any one of these domains could significantly alter the function of the truncated protein product. As the fifth novel mutation that we describe is located downstream of the C-terminal zinc finger cluster in exon 9, haploinsufficiency likely occurs primarily as a consequence of nonsense-mediated messenger ribonucleic acid decay (NMD) [17,18]. Alternatively, putative functional properties of the deleted portion of the p.(Glu1039Glyfs*6) mutant protein may be crucial to protein function (e.g., tertiary folding, intracellular localization, or signal transduction), leading to a non-functional protein and subsequently to PPCD. To determine the effects of the novel ZEB1 mutations that we report, we are currently performing a series of experiments to detect the subcellular localization of the corresponding mutant ZEB1 protein in transfected primary human corneal endothelial cells.
Acknowledegments
The authors thank Dr. John Sutphin for contributing patients to this study. Support provided by National Eye Institute grants 1R01 EY022082 (A.J.A.), P30 EY000331 (core grant), an unrestricted grant from Research to Prevent Blindness and a grant from the Gerald Oppenheimer Family Foundation Center for the Prevention of Eye Disease (A.J.A.).
References
- 1.Héon E, Mathers WD, Alward WL, Weisenthal RW, Sunden SL, Fishbaugh JA, Taylor CM, Krachmer JH, Sheffield VC, Stone EM. Linkage of posterior polymorphous corneal dystrophy to 20q11. Hum Mol Genet. 1995;4:485–8. doi: 10.1093/hmg/4.3.485. [DOI] [PubMed] [Google Scholar]
- 2.Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985;83:413–75. [PMC free article] [PubMed] [Google Scholar]
- 3.Raber IM, Fintelmann R, Chhabra S, Ribeiro MP, Eagle RC, Jr, Orlin SE. Posterior polymorphous dystrophy associated with nonkeratoconic steep corneal curvatures. Cornea. 2011;30:1120–4. doi: 10.1097/ICO.0b013e3182114452. [DOI] [PubMed] [Google Scholar]
- 4.Liskova P, Filipec M, Merjava S, Jirsova K, Tuft SJ. Variable ocular phenotypes of posterior polymorphous corneal dystrophy caused by mutations in the ZEB1 gene. Ophthalmic Genet. 2010;31:230–4. doi: 10.3109/13816810.2010.518577. [DOI] [PubMed] [Google Scholar]
- 5.Cremona FA, Ghosheh FR, Rapuano CJ, Eagle RC, Jr, Hammersmith KM, Laibson PR, Ayres BD, Cohen EJ. Keratoconus associated with other corneal dystrophies. Cornea. 2009;28:127–35. doi: 10.1097/ICO.0b013e3181859935. [DOI] [PubMed] [Google Scholar]
- 6.Driver PJ, Reed JW, Davis RM. Familial cases of keratoconus associated with posterior polymorphous dystrophy. Am J Ophthalmol. 1994;118:256–7. doi: 10.1016/s0002-9394(14)72911-3. [DOI] [PubMed] [Google Scholar]
- 7.Aldave AJ, Yellore VS, Yu F, Bourla N, Sonmez B, Salem AK, Rayner SA, Sampat KM, Krafchak CM, Richards JE. Posterior polymorphous corneal dystrophy is associated with TCF8 gene mutations and abdominal hernia. Am J Med Genet A. 2007;143A:2549–56. doi: 10.1002/ajmg.a.31978. [DOI] [PubMed] [Google Scholar]
- 8.Krafchak CM, Pawar H, Moroi SE, Sugar A, Lichter PR, Mackey DA, Mian S, Nairus T, Elner V, Schteingart MT, Downs CA, Kijek TG, Johnson JM, Trager EH, Rozsa FW, Mandal MN, Epstein MP, Vollrath D, Ayyagari R, Boehnke M, Richards JE. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am J Hum Genet. 2005;77:694–708. doi: 10.1086/497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu S, Krafchak C, Fuse N, Epstein MP, Schteingart MT, Sugar A, Eibschitz-Tsimhoni M, Downs CA, Rozsa F, Trager EH, Reed DM, Boehnke M, Moroi SE, Richards JE. A locus for posterior polymorphous corneal dystrophy (PPCD3) maps to chromosome 10. Am J Med Genet A. 2004;130A:372–7. doi: 10.1002/ajmg.a.30267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent AL, Niederer RL, Richards A, Karolyi B, Patel DV, McGhee CN. Phenotypic characterisation and ZEB1 mutational analysis in posterior polymorphous corneal dystrophy in a New Zealand population. Mol Vis. 2009;15:2544–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Liskova P, Tuft SJ, Gwilliam R, Ebenezer ND, Jirsova K, Prescott Q, Martincova R, Pretorius M, Sinclair N, Boase DL, Jeffrey MJ, Deloukas P, Hardcastle AJ, Filipec M, Bhattacharya SS. Novel mutations in the ZEB1 gene identified in Czech and British patients with posterior polymorphous corneal dystrophy. Hum Mutat. 2007;28:638. doi: 10.1002/humu.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen DQ, Hosseini M, Billingsley G, Heon E, Churchill AJ. Clinical phenotype of posterior polymorphous corneal dystrophy in a family with a novel ZEB1 mutation. Acta Ophthalmol (Copenh) 2010;88:695–9. doi: 10.1111/j.1755-3768.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhao XC, Nakamura H, Subramanyam S, Stock LE, Gillette TE, Yoshikawa S, Ma X, Yee RW. Spontaneous and inheritable R555Q mutation in the TGFBI/BIGH3 gene in two unrelated families exhibiting Bowman's layer corneal dystrophy. Ophthalmology. 2007;114:e39–46. doi: 10.1016/j.ophtha.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Tanhehco TY, Eifrig DE, Jr, Schwab IR, Rapuano CJ, Klintworth GK. Two cases of Reis-Bucklers corneal dystrophy (granular corneal dystrophy type III) caused by spontaneous mutations in the TGFBI gene. Arch Ophthalmol. 2006;124:589–93. doi: 10.1001/archopht.124.4.589. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda K, Kawakami K. DNA binding through distinct domains of zinc-finger-homeodomain protein AREB6 has different effects on gene transcription. Eur J Biochem. 1995;233:73–82. doi: 10.1111/j.1432-1033.1995.073_1.x. [DOI] [PubMed] [Google Scholar]
- 16.Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–62. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choe J, Cho H, Lee HC, Kim YK. microRNA/Argonaute 2 regulates nonsense-mediated messenger RNA decay. EMBO Rep. 2010;11:380–6. doi: 10.1038/embor.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner LB. Nonsense-mediated RNA decay regulation by cellular stress: implications for tumorigenesis. Mol Cancer Res. 2010;8:295–308. doi: 10.1158/1541-7786.MCR-09-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]