Abstract
Mesenchymal stromal cells (MSCs) are multipotent progenitor cells capable of differentiating into adipocytes, osteoblasts, and chondroblasts as well as secreting a vast array of soluble mediators. This potentially makes MSCs important mediators of a variety of therapeutic applications. They are actively under evaluation for immunomodulatory purposes such as graft-versus host disease (GvHD) and Crohn’s disease, as well as regenerative applications such as stroke and congestive heart failure. Here we report our method of generating clinical-grade MSCs together with suggestions gathered from manufacturing experience in our Good Manufacturing Practices (GMP) facility.
Keywords: Clinical Trials, Good manufacturing practices (GMP), Mesenchymal stromal cell (MSC), Phase I
Introduction
First identified by Friedenstein et al over three decades ago, mesenchymal stromal cells (MSCs) are multipotent cells of the non-hematopoietic lineage that reside in bone marrow and the stroma (1). They are capable of differentiating into adipocytes, chondroblasts, and osteoblasts in vitro (2;3). In bone marrow, MSCs are very rare, composing 0.01–0.001% of all cells. The highest frequency is in newborns with a decrease in frequency with age (4;5). MSCs are routinely generated from bone marrow (BM), but they can also be expanded from adipose tissue, cord blood, amniotic fluid, placenta, as well as fetal liver, blood, lung, and spleen (6–9).
The manufacture of MSCs is a routine and relatively simple procedure that involves culturing whole adherent BM cells or isolated bone marrow mononuclear cells (BM MNC). This heterogeneous cell population is initially plated in tissue culture flasks and the adherent cells—containing the MSC progenitors—are passaged to produce a homogenous population of MSCs that have a similar morphology to fibroblasts (10;11).
Given the physical properties of MSCs (large size, adherence), expanding clinically- applicable numbers of MSCs can be difficult using conventional methods, as this may require many hundreds of culture flasks and a large number of open procedures. Alternatively, MSCs can be plated in cell factories and bioreactors for large-scale expansion, however, the optimal culture conditions are still under investigation (10). One concern when expanding large numbers of MSCs is the number of passages (P) required to meet dose requirements (4). Many groups limit their passage number to retain differentiation potential but ignore the number of cell doublings occurring during each passage. In light of reports of telomere shortening and reduced proliferation in vivo correlating with reduced telomere length, the number of cell doublings should be minimized (12).
Another variable to consider during the expansion is the protein source (fetal bovine serum vs. human platelet lysate vs. serum-free media). All of these conditions are able to support MSC growth, however, they also contribute to variability between MSC lots (13). Moreover, the use of fetal bovine serum may lead to immunologic reactions in the patients (14).
The absence of co-stimulatory molecules and human leukocyte antigen (HLA) Class II molecules, as well as low HLA Class I expression on MSCs make them ideal cells for allogeneic, or “off-the-shelf” use in both regenerative medicine and immunomodulatory applications (15;16). Work is still in progress to characterize the optimal profile of MSC lots used in specific applications and cultured using different techniques, media, and passage numbers. Although the Food and Drug Administration (FDA) has not issued standard release criteria for investigational new drug applications that involve MSCs, the International Society of Cellular Therapy (ISCT) has established the minimum criteria that should be used to define MSCs and these and other regulatory issues are discussed elsewhere (10;11;17;18).
Applications
The unique immunosuppressive properties of MSCs–as well as their ability to function in the allogeneic setting—has sparked interest in the use of these cells clinically (19). The release of immunosuppressive factors like prostaglandins (20) and Factor H (21) inhibit the innate immune system, while the release of Transforming Growth Factors (TGF), prostaglandins, indoleamine 2,3-dioxygenase (IDO) and IL-10, as well as the expression of PDL-1 and HLA-G, modulate the adaptive immune system (20). This makes MSCs an attractive therapy for autoimmune or inflammatory diseases such as GvHD, diabetes, and inflammatory bowel disease (9). There are currently over 40 clinical trials listed under “Mesenchymal Stromal Cells” on www.clinicaltrials.gov, ranging from treatments for acute GvHD to therapies for Crohn’s disease, emphysema, congestive heart failure, and stroke. Allogeneic MSCs are also being produced by biotechnology companies and pursued in phase III clinical trials for acute GvHD and Crohn’s disease (22). In light of their relative ease of manufacture, their potential to be used allogeneically, and their long standing record of safety, we anticipate that the number of applications will increase rapidly in the next five years.
Methods
In this report we describe our current method of generating MSCs from human bone marrow (for the manufacturing SOP see Supplemental data Figure S1). To address some of the concerns mentioned above, we limit our expansions to four passages and attempt to reach required cell numbers with less than 30 cell doublings. In these cultures we uniformly used platelet lysate as the protein source. We are also investigating other cell culture devices to reduce manufacturing time to obtain clinical doses and to limit the passage number in a cost effective manner.
Manufacturing Bone Marrow-derived MSCs
All cell culture manipulations, quality and release testing, and flow cytometry are performed by the Center for Cell and Gene Therapy good manufacturing practice (GMP) facilities in Houston, TX, USA.
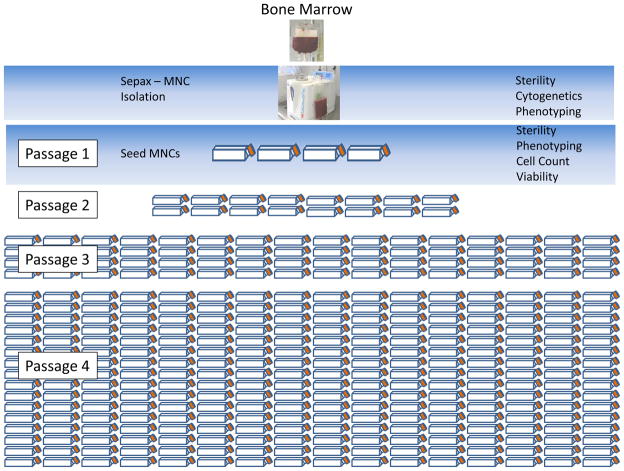
Bone Marrow Mononuclear Cell (BM MNC) Isolation
Upon receiving bone marrow, aliquots are sent for phenotyping, cytogenetics, and sterility testing. The marrow is then passed through a 200 um filter (Pall, Port Washington, NY, USA) to remove residual bone fragments. To enrich the MSC-containing BM population, MNCs are isolated using a Sepax device (Biosafe, Geneva, Switzerland). Briefly, cells are loaded into the Sepax device which layers them onto a Ficoll density cushion. The cells are then centrifuged and the BM MNC are harvested and washed by the device. Other systems, such as the Bone Marrow MSC Separation Device (Kaneka Corp., Osaka, Japan), also offer closed systems that may more efficiently enrich MSCs directly from fresh marrow leading to improved final cell yields. Manual MNC separation can be substituted, but is an open system and increases the likelihood of contamination.
Plating MNCs
After isolating MNCs, cells are again sent for phenotyping and sterility and a viable count performed by trypan blue exclusion and 7AAD staining. MNCs are then plated in T-175 cm2 flasks at approximately 5×105 cells per cm2 (Table 1). The medium used is D-5 containing 5% human platelet lysate (see D-5 media SOP in supplemental data Figure S2). Many groups seed cells at a much lower density and there are reports showing that such cells reach target cell numbers faster, on average, than those seeded at a higher density, probably due to the delay in contact inhibition. We chose to seed at 5×105 cells/cm2 to reduce the number of flasks.
Table 1.
Appropriate seeding density of BM MNC and suggested media volume.
| Flask Size (cm2 per flask) | Media Volume (mL) | # of cells/cm2 | Total # of cells |
|---|---|---|---|
| INITIAL PLATING OF CULTURE | |||
| 75 | 15 | 1–10 × 105 cells/cm2 range; 4–6 × 105 recommended | ~3.75 × 107 |
| 175 | 35 | 1–10 × 105 cells/cm2 range; 4–6 × 105 recommended | ~8.75 × 107 |
| 10-stack cell factory (6,360cm2) | 1200 | 1–10 × 105 cells/cm2 range; 4–6 × 105 recommended | ~3.18 × 109 |
Passaging MSCs
MSCs should be fed with fresh medium every 3–4 days. When the cells are 70–80% confluent, they can be split at a 1:4 flask ratio (Figure 1). We typically pool the cells into one common vessel and split the cells after counting, but do not seed based on cell counts. At each split cell samples are also sent for phenotyping. In our experience, the cells are typically split 10–14 days after culture initiation. After P1, the split times vary from 4–7 days depending on the passage number, donor, and growth factors in the lot of pooled platelet lysate.
Figure 1.
Harvesting MSCs
Depending on the targeted cell dose, the cells can be harvested any time after initiation, but optimally before passage 5 or 30 cell doublings. To harvest, the medium is aspirated from the flasks and the cells are washed with Phosphate Buffered Saline (PBS) (Sigma, St. Louis, MO, USA). TrypLE Select (Invitrogen, Carlsbad, CA, USA) is used to remove the adherent cells from the plastic (Table 2). Comparable dissociation reagents such as TrypZean and Trypsin may be used, however, we prefer TrypLE Select because it is gentle on cells, does not require neutralization, does not contain xenogeneic proteins, and is readily available, whereas some recombinant enzymes are often in short supply. After 5–10 minutes, the cells should be in suspension. Medium is then added to the cells, which are harvested, and samples sent for phenotypic analysis, cytogenetics, sterility, tri-lineage potential testing, and Colony Forming Unit (CFU) assays.
Table 2.
Supplies and reagents used in the production of MSCs
| Supply/Reagent | Manufacturer | Catalog # |
|---|---|---|
| Human Bone Marrow | Lonza | 1M-125 |
| DMEM | Lonza | 12-614F |
| GlutaMAX | Invitrogen | 35050 |
| Heparin | APP Pharmaceutical | 504001 |
| Platelet Lysate | Gulf Coast Blood Center | |
| Pipet-Aid | Drummond | 4000101 |
| Serological Pipettes | Falcon | 357543, 357551, 357550 |
| Lymphoprep | Nycomed | 1114545 |
| Trypan Blue | Gibco | T8154 |
| TrypLE Select | Invitrogen | 12563-029 |
| PBS (without Mg and Ca) | Sigma | D8537 |
| Luer-lock syringes | BD Biosciences | 309646, 309653 |
| Sterile conical tubes | Corning | 430776 |
| Sterile centrifuge tubes | BD Biosciences | 352092 |
| Dimethylsulfoxide | McKesson | 630491 |
| Cryovials | Nalgene | 66008-710 |
| Sepax Device | Biosafe | |
| Sepax Disposable Set | Biosafe | 9CS-600.1 |
| Plasmalyte | Baxter | 2B2543Q |
| 25% Human Serum Albumin | Baxter | 2G0201 |
| T-175 cm2 Tissue Culture Flasks | Corning | 431079 |
| Heparin (1000 U/mL) | AAP | 504001 |
Cryopreservation of MSCs
Before cryopreservation, the MSCs are centrifuged at 500×g for 10 minutes and washed with a “wash medium” containing Plasmalyte (Baxter, Deerfield, IL, USA) and 5% human serum albumin (HSA) (Baxter) (Table 2). The cells are then centrifuged again, the supernatant aspirated, and the cells resuspended in wash medium at ½ the final freeze volume. After placing the cells on ice for approximately ten minutes, 2× freeze media containing 20% DMSO, 5% HSA and 75% Plasmalyte (for a final concentration of 10% DMSO) is added. Cells are cryopreserved using a controlled-rate freezer and freezing ramps that are in use for cryopreservation of bone marrow for transplantation.
QA/QC
Quality assurance (QA) and quality control (QC) are provided as part of the Center for Cell and Gene Therapy’s GMP facility. Samples are routinely submitted for sterility testing as well as Mycoplasma and Endotoxin. Cytogenetic testing is a matter of controversy as it is difficult to evaluate the potential clinical impact of minor changes in karyotype (19;23). We currently use karyotyping on the incoming samples to ensure that the donor does not have any chromosomal abnormalities. We also karyotype the final product but the results are not used as a release criterion. If they were grossly abnormal we would not use the cells therapeutically. Chromosomal studies are performed by the Cytogenetic Laboratory at Texas Children’s Hospital. Release testing and the certificate of analysis are performed and issued by the QC and QA sections of the GMP respectively.
Flow Cytometry
MSCs are stained and analyzed as per ISCT standards (17) with the markers CD105, CD73, and CD90 ≥95% positive and ≤2% positive for CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR-II. Our phenotyping consists of a direct staining method using a multicolor panel containing the following monoclonal antibodies (mAb): CD90 (clone5E10), CD105 (clone266), CD271 (clone C40-1457), CD73 (clone AD2), CD45 (clone 2D1), CD34 (clone 8G12), CD14 (clone MϕP9), CD19 (clone SJ25C1), and anti-HLA-DR-II (clone G46-6). All mAb are from BD Biosciences (San Jose, CA, USA) and used at the manufacturer’s suggested concentration. In addition, the marker MSCA-1 is included in the panel as it has been shown to be specific for MSCs with proliferative potential (24).3 Fifty thousand total events were acquired on a FACSCanto-II 3 laser configuration using FACSDiva Software v6.3.1 (BD Bioscience). Day of use instrument quality control was performed using BD™ Cytometer Setup & Tracking Beads (BD Bioscience) and mAb compensation using BD™ CompBead Plus (BD Bioscience). The list-mode data files are analyzed using the same software as for acquisition. MSC data are presented as a series of single parameter histograms to define the positive and negative populations but we also employ logical sequential gating to define the negative populations of CD45, CD34, CD14, CD19 & HLA-DR with a two-axis dot-plot to observe the co-expression of the various positive markers CD105, CD73, CD90, CD271 & MSCA-1 within the negative population; we have found this gating method to be more informative.
Scaling up
Our current method of generating MSCs utilizes approximately 256 × T-175 cm2 cell culture flasks, which increases the likelihood of contamination and considerable time is required to harvest this number of flasks. An alternative is to use multi-layered cell factories, but in our hands, while excellent for viral vector production, these are more difficult to manipulate for efficient harvesting of adherent cells and the yield of MSC is suboptimal. It is also difficult to achive a uniform distribution of cells in the flasks, with the result that some may reach sub-confluence earlier than others. We have found that functional and proliferative assays showed MSCs cultured in cell factories and even 5-layer cell stacks did not grow as well as well as those cultured in T-175 cm2 flasks. The increasing use of MSCs as clinical therapies has led to commercial interest in the large-scale production and bioreactors and large-scale culture devices are becoming available. The CLINIcell by Mabio (25), the Rotary Cell Culture System by Synthecon (26), The Xpansion system by ATMI, and the Quantum by Terumo (27) are all examples of bioreactors with varying degrees of automation, and a recent review by Fossett et al has highlighted the need for such devices (4).
Expected Results
Using our flask-based method, we recover approximately 5×108 MSCs by the end of P4. One of the limitations of the flask-based expansion is the feasibility of handling and harvesting hundreds of flasks. Further, if >5×108 cells are desired it might be better to expand in cell factories or bioreactors, or freeze the MSCs at an earlier passage to create a master cell bank, that is then used to manufacture different lots. The final MSC product should meet the criteria listed in Figure 2 and be free from major chromosomal abnormalities.
Potential Problems
Due to the relative ease of manufacture and multiple clinical applications, MSCs can be an attractive starting product – especially for smaller institutions looking to move into the field of cellular therapy. However, their multifunctional potential has made it difficult to develop potency assays that accurately reflect their desired function in vivo. These will be required as trials progress towards Phase III.
Conclusion
MSCs provide a unique and exciting therapeutic option for a multitude of diseases and disorders. Although there are obstacles to overcome, testing MSCs in clinical trials will prove informative and should provide novel insights into the ultimate utility of these cells.
Supplementary Material
Acknowledgments
This project is supported by the NHLBI-NIH Production Assistance for Cellular Therapy (PACT) program, contract # HHSN268201000007C. The authors would also like to thank Keli Sharpe for her technical expertise in flow cytometry, Sara Richman for her meticulous reading of the manuscript, as well as Drs. Malcolm Brenner and Helen Heslop for their support and guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 4.Fossett E, Khan WS. Optimising human mesenchymal stem cell numbers for clinical application: a literature review. Stem Cells Int. 2012;2012:465259. doi: 10.1155/2012/465259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999 Jul;14(7):1115–22. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 6.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3(4):248–69. [PMC free article] [PubMed] [Google Scholar]
- 7.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001 Oct 15;98(8):2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 8.Gotherstrom C, Ringden O, Westgren M, Tammik C, Le BK. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003 Aug;32(3):265–72. doi: 10.1038/sj.bmt.1704111. [DOI] [PubMed] [Google Scholar]
- 9.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007 Nov 15;110(10):3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 10.Sensebe L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther. 2011 Jan;22(1):19–26. doi: 10.1089/hum.2010.197. [DOI] [PubMed] [Google Scholar]
- 11.Bieback K, Kinzebach S, Karagianni M. Translating research into clinical scale manufacturing of mesenchymal stromal cells. Stem Cells Int. 2011;2010:193519. doi: 10.4061/2010/193519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012 Jan 20;21(2):273–83. doi: 10.1089/scd.2010.0589. [DOI] [PubMed] [Google Scholar]
- 13.Muller I, Kordowich S, Holzwarth C, Spano C, Isensee G, Staiber A, et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy. 2006;8(5):437–44. doi: 10.1080/14653240600920782. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999 Mar;5(3):309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 16.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003 May 1;101(9):3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 17.Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 18.Pytlik R, Stehlik D, Soukup T, Kalbacova M, Rypacek F, Trc T, et al. The cultivation of human multipotent mesenchymal stromal cells in clinical grade medium for bone tissue engineering. Biomaterials. 2009 Jul;30(20):3415–27. doi: 10.1016/j.biomaterials.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Philippe B, Luc S, Valerie PB, Jerome R, Alessandra BR, Louis C. Culture and Use of Mesenchymal Stromal Cells in Phase I and II Clinical Trials. Stem Cells Int. 2010;2010:503593. doi: 10.4061/2010/503593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abumaree M, Al JM, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012 Jun;8(2):375–92. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 21.Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010 Nov;19(11):1803–9. doi: 10.1089/scd.2009.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009 Jun;8(2):110–23. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 23.Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010 Feb 25;115(8):1549–53. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 24.Battula VL, Treml S, Bareiss PM, Gieseke F, Roelofs H, de ZP, et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009 Feb;94(2):173–84. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner SJ, Myrup AC. Toward closed-system culture of blood origin endothelial cells. Transfusion. 2005 Jul;45(7):1201–7. doi: 10.1111/j.1537-2995.2005.00180.x. [DOI] [PubMed] [Google Scholar]
- 26.Kedong S, Xiubo F, Tianqing L, Macedo HM, LiLi J, Meiyun F, et al. Simultaneous expansion and harvest of hematopoietic stem cells and mesenchymal stem cells derived from umbilical cord blood. J Mater Sci Mater Med. 2010 Dec;21(12):3183–93. doi: 10.1007/s10856-010-4167-5. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen Kim, McNiece Ian, Antwiler Delbert. Reproducible growth and expansion of bone marrow derived mesenchymal stem cells (MSC), using a novel, automated and closed cell expansion system (CES) Cytotherapy. 2008;10(Suppl 1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.