Abstract
Introduction:
Despite the known harmful effects of smoking during pregnancy, the highly addicted find it difficult to quit. Decreased smoking may be regarded as a means of harm reduction. There is limited information on the benefits of smoking reduction short of quitting. This study used salivary cotinine to assess the impact of change in smoking exposure on birth weight in full-term infants.
Methods:
In a prenatal smoking cessation study, smoking status was validated by saliva cotinine at baseline and end of pregnancy (EOP). Salivary cotinine ≥15ng/ml defined active smoking. Based on salivary cotinine, women were grouped as nonsmoking/quit, light exposure (<150ng/ml), and heavy exposure (≥150ng/ml) at baseline and EOP. EOP and baseline smoking status were stratified to form smoking exposure change groups. Mean birth weight was compared among those who quit, reduced, maintained, and increased.
Results:
Smoking cessation was associated with a 299g increase in birth weight compared with sustained heavy smoking, p = .021. Reduced exposure from heavy to light was associated with a 199g increase in birth weight compared with sustained heavy exposure, a 103g increase compared with increased exposure, and a 63g increase compared with sustained light exposure. Differences among continuing smokers were not statistically significant.
Conclusions:
Although not statistically significant, the increase in infant birth weight associated with reduction from heavy to light exposure suggests potential for benefit. The only statistically significant comparison was between quitters and sustained heavy smokers, confirming that smoking cessation should remain the goal for pregnant women.
Introduction
The harmful effects of exposure to cigarette smoke during pregnancy and the benefits of quitting have long been established (Butler, Goldstein, & Ross, 1972; Lumley, Oliver, Chamberlin, & Oakley, 2009; Murin, Rafii, & Bilello, 2011; Vardavas et al., 2010; Wickström, 2007). Still, many women who smoke find it difficult to quit upon learning they are pregnant. The spontaneous quit rate among pregnant smokers has been estimated to be between 20% and 40% (Morasco, Dornelas, Fischer, Oncken, & Lando, 2006; Ockene et al., 2002; Quinn, Mullen, & Ershoff, 1991; Solomon & Quinn, 2004), which means that the majority continue to smoke throughout gestation. Those who manage to quit are generally lighter smokers, at lower levels of addiction (Giglia, Binns, & Alfonso, 2006; Stotts et al., 2009; Tong, Jones, Dietz, D’Angelo, & Bombard, 2009).
Complete cessation of cigarette smoking prior to the third trimester of pregnancy is recommended, as the toxic components of tobacco are thought to exert the most impact on the growth and development of the fetus at this critical stage (Cliver et al., 1995; Lieberman, Gremy, Lang, & Cohen, 1994; Ohmi, Hirooka, & Mochizuki, 2002; Rush & Cassano, 1983). Given widespread awareness of the potential adverse effects of smoking on the unborn baby, there is a tendency among pregnant women to at least cut back on the amount smoked—an attempt at harm reduction (Windsor, Li, Boyd, & Hartmann, 1999). However, there is limited information on the benefits of smoking reduction.
Among women who fail to quit, decreased consumption is an appealing compromise. This notion of harm reduction may appeal to health care providers as well (Walsh, Redman, Brinsmead, & Arnold, 1995). When facing resistant smokers, providers may temper their advice about smoking and recommend reduction. Indeed, a gradual reduction to quitting is an effective smoking cessation strategy (Stead & Lancaster, 2007; Wang et al., 2008); however, in the case of pregnant women, complete cessation may not be realized before delivery. In terms of pregnancy outcomes, there is a lack of conclusive evidence of an advantage to changing smoking behavior short of quitting. The level at which a reduction in smoking can be considered beneficial is unknown and advice on the number of cigarettes that can be “safely” smoked is variable (England et al., 2001; Li, Windsor, Perkins, Goldenberg, & Lowe, 1993; Secker-Walker, Vacek, Flynn, & Mead, 1998).
The objective of this study was to explore the impact of reduction in exposure to cigarette smoke during pregnancy on birth weight of full-term infants. Salivary cotinine, used to validate smoking status in the targeted population of women enrolled in a prenatal smoking cessation study, was taken as the measure of smoking exposure for this analysis. For several reasons, biochemical measures are considered more valid compared with self-report. As a primary metabolite of nicotine, cotinine serves as a direct measure of smoking consumption and has been used in studies assessing the impact of smoking on fetal growth restriction (Lambers & Clark, 1996; Pastrakuljic, Derewlany, & Koren, 1999; Petersen, Leite, Chatkin, & Thiesen, 2010; Walsh, 1994). The long half-life of cotinine, measurable in saliva for up to 20hr, makes it a useful indicator of exposure (Etter & Perneger, 2001; Jarvis, Russell, Benowitz, & Feyerabend, 1988). Validity of self-reported smoking is questionable, particularly among pregnant women (Britton, Brinthaupt, Stehle, & James, 2004; England et al., 2007; Russell, Crawford, & Woodby, 2004; Shipton et al., 2009). In addition, reported cigarettes smoked as a measure of nicotine exposure is subject not only to deception but also is impacted by numerous other factors, including, nicotine yield of different cigarette brands; personal smoking patterns, that is, depth of inhalation and how close to the filter the cigarette is smoked; variation in individual metabolism of nicotine; and environmental, or secondhand, exposure. These factors influence nicotine levels in the body and are largely responsible for the less-than-perfect correlation between number of cigarettes smoked and biochemical measures (England et al., 2001; Klebanoff, Levine, Clemens, DerSimonian, & Wilkins, 1998; Secker-Walker et al., 1998). The evidence of a stronger correlation between biochemical measures of smoking and infant birth weight compared with self-reported cigarettes smoked is another compelling reason for use of cotinine as a measure of exposure (Haddow, Knight, Palomaki, Kloza, & Wald, 1987; Secker-Walker et al., 1998; Wang, Tager, Van Vunakis, Speizer, & Hanrahan, 1997).
As smoking reduction is more likely than complete cessation during pregnancy (Hebel, Fox, & Sexton, 1988; Pickett, Wakschlag, Dai, & Leventhal, 2003), there has been interest in the impact of reduced smoking on birth outcomes. Typically, studies have compared reduction with no change, assessing the effect of some percent decrease in smoking, frequently 50% (Secker-Walker et al., 1998; Secker-Walker & Vacek, 2002; Windsor et al., 1993, 1999, 2000). However, the impact on birth weight of a 50% reduction in exposure is likely to depend on the level of smoking at baseline. As such, some studies have explicitly controlled for baseline level of exposure (England et al., 2001; Li et al., 1993). Li et al. (1993) specified a 20 and 60ng/ml reduction for light and heavy smokers, respectively, and England et al. (2001) grouped smokers as light and heavy and stratified baseline and follow-up status.
Similar to Li and England, we controlled for baseline exposure, using stratification to examine the impact of change from one level to another. We assessed the impact of smoking exposure change from study entry to the end of pregnancy (EOP) by comparing mean birth weights among women who quit smoking, reduced from heavy to light, maintained light, increased from light to heavy, and maintained heavy smoking. This study adds to the current limited body of knowledge about the effect of smoking reduction during pregnancy.
Methods
Study Population
Among a cohort of women in a prenatal smoking cessation study, 13% quit and 45% were able to reduce smoking by the EOP, based on a saliva cotinine cutpoint of 15ng/ml. “Resistant smokers,” defined as women continuing to smoke into the second trimester of pregnancy, were targeted for a smoking cessation intervention in a study funded by the Robert Wood Johnson Foundation and conducted at the University of Texas Health Science Center Houston. Between 2001 and 2004, 360 women were enrolled in a randomized trial comparing three interventions for smoking cessation: best-practice (BP) counseling alone, BP plus an ultrasound accompanied by information on the potential harmful effects of smoking on the fetus, and motivational interviewing plus the information-guided ultrasound. Eligible women were those who reported cigarette smoking during the past 7 days and were between 16 and 26 weeks gestation. Women were recruited in Houston and Harris County area Women, Infants, and Children (WIC) centers and by advertisement. Details of the study are reported elsewhere (Stotts et al., 2009).
Information on average number of cigarettes per day in the past week was collected by questionnaire and was validated by salivary cotinine on three occasions: (a) baseline (between 16 and 26 weeks gestation), (b) EOP (at approximately 36 weeks), and (c) 6 weeks postpartum. All participants gave informed consent and the study was approved by the University of Texas Health Science Center Houston Institutional Review Board.
Cigarette smoking during pregnancy is thought to contribute to decreased birth weight by two mechanisms, shortened gestation and fetal growth restriction (Kramer, 1987). As the focus of this analysis was fetal growth restriction, only women with full-term (gestational age at delivery ≥37 weeks), singleton pregnancies were included to control for reduced birth weight associated with preterm delivery and multifetal gestation.
Other eligibility criteria for this analysis included availability of saliva cotinine measures at the two time points of interest, baseline and EOP, and information on well-established correlates of infant birth weight, including sex of the infant, maternal age, parity, education, income, and race/ethnicity. Additionally, in a subset of the cohort, information had been collected on maternal prepregnancy weight, height, and predelivery weight for an ancillary study, providing data on prepregnancy body mass index (BMI) and gestational weight gain. Of the 360 women enrolled in the smoking cessation study, 260 met inclusion criteria. Complete cessation was defined as salivary cotinine <15ng/ml, a cutpoint found to have high sensitivity and specificity (Florescu et al., 2009; Wagenknecht, Burke, Perkins, Haley, & Friedman, 1992), and was used in previous research (Peacock et al., 1998). As this study aimed to observe the effect of change in smoking exposure, women who had salivary cotinine values consistent with nonactive smoking at both time points, baseline and EOP, were excluded. Thirty-five met this criterion, bringing the number to 225 for this analysis.
Study Measures
The outcome variable infant birth weight was self-reported at the postpartum visit in pounds and ounces and converted to grams. This information was not confirmed in birth records; however, studies have shown maternal report of birth weight to be highly reliable and accurate within a few grams for much longer periods than 6 weeks after birth (Catov et al., 2006; Elliott et al, 2010; Troude et al., 2008). The primary predictor variable was change in exposure to cigarette smoke, measured by salivary cotinine. Saliva was collected by a dental roll, which participants were instructed to place between the cheek and the gums and hold for approximately 5min or until well saturated. Samples were batched and frozen at −70°F until shipped to J-2 Laboratory in Tuscan, AZ for analysis by method of Gas Chromatographic Thermionic Specific Detector (GC-TSD).
Based on previous research and review of the data, a cotinine level of 150ng/ml was selected as the cutpoint to define light and heavy smoking. In a study among African American women, the greatest suppression of infant birth weight was found to be at salivary cotinine levels ≥100ng/ml (El-Mohandes, Kiely, Gantz, Blake, & El-Khorazaty, 2009). Li et al. (1993) used a salivary cotinine cutpoint of 100ng/ml to distinguish light and heavy smokers. Examination of the functional relationship between EOP salivary cotinine and infant birth weight in our data revealed the level at which infant birth weight began a notable, consistent decline to be around 150ng/ml. Dichotomous variables, in which women were classified as heavy (cotinine ≥150ng/ml) or light (cotinine <150ng/ml) smokers, were created for both baseline and EOP time points. Additional categories for nonsmokers at baseline and EOP (salivary cotinine <15ng/ml) were included for a total of three categories for each time point.
Smoking status at EOP (nonsmoking, light, and heavy) was stratified by baseline exposure categories (nonsmoking, light, and heavy). The variable created from this stratification included the following nine groups: (1) baseline nonsmoking and EOP light, (2) baseline nonsmoking and EOP heavy, (3) baseline light and EOP light, (4) baseline light and EOP heavy, (5) baseline heavy and EOP light, (6) baseline heavy and EOP heavy, (7) baseline light and EOP nonsmoking, (8) baseline heavy and EOP nonsmoking, and (9) baseline nonsmoking and EOP nonsmoking. Table 1 shows the number of participants in each baseline by EOP stratum.
Table 1.
Number of Participants in Each Smoking Change Stratum
| Baseline | ||||
|---|---|---|---|---|
| EOP | NS | Light | Heavy | Total |
| NS | 35a | 28 | 8 | 71 |
| Light | 6 | 67 | 20 | 93 |
| Heavy | 2 | 20 | 74 | 96 |
| Total | 43 | 115 | 102 | 260 |
Note. EOP = end of pregnancy; NS = nonsmoking.
aThis group, classified as nonsmoking at baseline and EOP, was excluded.
As stated previously, individuals in category 9 were deemed nonsmokers and excluded. Given the number of categories, several small groups were combined with others. Women with cotinine levels consistent with nonsmoking at baseline and consistent with light or heavy smoking at EOP were included in the light/light and light/heavy groups, respectively. Additionally, as only eight women with heavy exposure at baseline quit, the light/quit and heavy/quit categories were collapsed into one “quit” group, forming the five-group “exposure change” variable used in this analysis.
Other factors associated with infant birth weight were collected at the baseline visit (maternal age, number of previous births/parity, race and ethnicity, annual household income, and years of school completed) and at the postpartum visit (sex of the baby, birth weight, height, prepregnancy weight, and predelivery weight). Information on pregnancy complications, such as diabetes, hypertension, preeclampsia, and anemia was available on a subset, self-reported at the postpartum visit. Seven percent of women reported a diagnosis of diabetes, 7% hypertension, 6% preeclampsia, and 15% anemia. However, these factors were not significantly associated with birth weight in this sample and, as such, they were not included in this analysis. The influence of gestational age at delivery (GAD) on infant birth weight was controlled for by the exclusion of preterm deliveries; however, birth weight has been shown to vary significantly between weeks 37–40 (Cogswell & Yip, 1995; Nahum, Stanislaw, & Huffaker, 1995) and, as such, GAD was included as a covariate. Two thirds of the women in the study received an ultrasound, which was used to determine GAD, whereas reported date of last menstrual period was used for the other one third.
Data Analysis
In bivariate analysis, one-way analysis of variance (ANOVA) was used to assess the effect of change in smoking exposure status on infant birth weight. Pairwise comparisons, using the Bonferroni method to control for multiplicity, assessed difference in mean infant birth weight across exposure change groups. Multiple regression analysis was conducted to adjust for other birth weight–associated factors, including maternal age, race/ethnicity, parity, education, income, sex of the baby, GAD, prepregnancy BMI, and gestational weight gain. Given the large number of factors to be included in the model and the sample size, general linear modeling (GLM) was used to generate reliable effect estimates in the likely case of unbalanced, small, and/or empty cells (Neter, Kutner, Nachtsheim, & Wasserman, 1996). All data analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 18.0.
Results
A description of the study population is shown in Table 2. The smoking cessation study cohort was comprised largely of low income women, diverse in race and ethnicity. A majority, 79%, reported annual household incomes <25K. Reflecting national racial/ethnic trends for women in which smoking is most prevalent among non-Hispanic Whites and least prevalent among Hispanics (Tong et al., 2009), the study population was 48% non-Hispanic White, 35% African American/Black, and 15% Hispanic. Educational attainment was at or below high school graduate level for the majority (80%) of the women. Mean age was 25, and ranged from 16 to 45. Number of previous births ranged from 0 to 6, and 38% of the women were nulliparous. There were no significant differences by these sociodemographics between the women included in this analysis and those excluded due to missing cotinine values.
Table 2.
Characteristics of the Study Population
| Characteristic | Number (%) |
|---|---|
| Age (M ± SD) | 25±6.25 |
| Age groups | |
| ≤18 | 18 (8) |
| 19–29 | 153 (68) |
| ≥30 | 53 (24) |
| Race/ethnicity | |
| Hispanic | 34 (15) |
| African American/Black | 78 (35) |
| Non-Hispanic White | 109 (48) |
| Other | 4 (2) |
| Education | |
| <High school | 85 (38) |
| High school | 95 (42) |
| >High school | 45 (20) |
| Income | |
| <$15,000 | 107 (47) |
| $15,000–24,999 | 69 (31) |
| ≥$24,000 | 49 (22) |
| Parity | |
| 0 births | 85 (38) |
| 1–2 births | 94 (42) |
| ≥3 births | 46 (20) |
| Prepregnancy BMI | |
| <20 | 30 (13) |
| 20–26 | 125 (56) |
| ≥27 | 70 (31) |
| Gestational weight gain | |
| ≤15 lbs | 20 (9) |
| 16–54 lbs | 181 (80) |
| ≥55 lbs | 24 (11) |
There was significant change in smoking exposure, measured both as reported cigarettes per day and salivary cotinine. Mean cigarettes per day decreased from 12 at baseline to 9 at EOP, p < .001, and mean salivary cotinine decreased from 160 to 140ng/ml, p = .015. Baseline and EOP correlations were significant for both cigarettes per day and salivary cotinine (Pearson correlation: .410, p < .001 and .521, p < .001, respectively). Reported cigarettes per day and cotinine were significantly correlated at both time points (Pearson correlation: .423 at baseline, p < .00; .400 at EOP, p < .001). Despite these correlations among measures, only EOP cotinine was significantly correlated with infant birth weight, r = −.166, p = .012.
Seventy-four (33%) had sustained heavy exposure, with saliva cotinine levels ≥150ng/ml at both baseline and EOP. Twenty-two (10%) had increased exposure, saliva cotinine <150ng/ml at baseline and ≥150ng/ml at EOP. Seventy-three (32%) had consistent light exposure, saliva cotinine <150ng/ml at both baseline and EOP. Twenty (9%) reduced exposure from heavy, saliva cotinine ≥150ng/ml, to light, <150ng/ml. Finally, 36 (16%) were quit, having saliva cotinine levels ≥15ng/ml at baseline and <15ng/ml at EOP. Table 3 shows mean cotinine levels at baseline and EOP.
Table 3.
Mean, Standard Deviation, and Difference Between Salivary Cotinine Levels at Baseline and End of Pregnancy by Smoking Change Category
| Smoking change category | n | Baseline | EOP | Difference |
|---|---|---|---|---|
| Heavy/heavy | 74 | 269±111 | 248±81 | −21* |
| Light/heavy | 22 | 80±47 | 248±91 | +168* |
| Light/light | 73 | 80±42 | 76±39 | −4* |
| Heavy/light | 20 | 244±78 | 94±30 | −150 |
| Quita | 36 | 94±77 | 9 ± .8 | −85 |
Note. aReferent group.
*Statistically significant at p < .005.
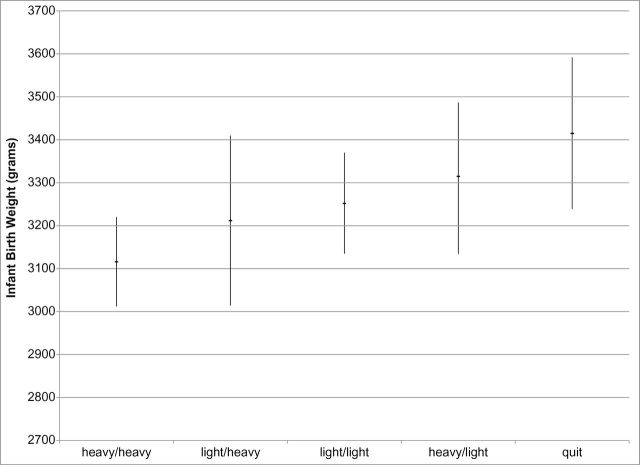
Overall, mean birth weight was 3,235±479g. Mean birth weight was highest among quitters, 3,415±521g, 95% CI (3239, 3592); followed by those who reduced from heavy to light exposure, 3,315±368g, 95% CI (3143, 3487); sustained light exposure 3,252±504g, 95% CI (3135, 3370); increased exposure from light to heavy, 3,212±447g, 95% CI (3014, 3410); and sustained heavy exposure, 3,116±447g, 95% CI(3012, 3220), F = 2.66, p = .034. The Levene statistic for homogeneity of variances was 1.15, p = 0335, indicating that group variances were not significantly different. Pairwise comparisons among the exposure change categories, using the Bonferroni method to control for multiplicity, revealed the only statistically significant difference to be between sustained heavy smokers and quitters, p = .021. Figure 1 depicts mean birth weight and 95% CI of each smoking exposure change group.
Figure 1.
Mean birth weight and 95% CI by smoking exposure change group.
Mean birth weight for the excluded group of nonsmokers was 3,160±497g, lower than the smoking groups except for heavy/heavy. This finding is not unique to this study. In the study reported by Li et al. (1993), the birth weight of never-smokers was lower than that of quitters and was comparable to reducers. As the lower mean birth weight among the women excluded because they had saliva cotinine values consistent with nonsmoking was counter to what would be expected, we examined the data for potential contributing factors. The nonsmoking group was majority African American (52%), had a greater proportion of participants <18 years of age, and a greater proportion without medical coverage compared with the other groups. These factors likely contributed to the lower mean birth weight.
Using GLM, a “full model” assessed the contribution of each predictor variable to the variation in infant birth weight. To achieve the most efficient model, variables were eliminated if they were nonsignificant at p ≥ .20 and, based on the F test for model significance (Jaccard & Turrisi, 2003), removal did not significantly change the model R-square value. Education and parity fit these criteria and were removed. The overall effect of the smoking exposure change variable was nonsignificant, p = .122, in the adjusted model. Pairwise comparison of parameter estimates, however, showed the difference between complete cessation and sustained heavy smoking remained significant, p = .05.
Discussion
An effect of reduced smoking exposure was not supported by the data in this study, as the only statistically significant difference among the exposure change groups was between quitters and sustained heavy smokers. Sustained heavy exposure was associated with a 299 g decrement in infant birth weight compared with quitting. This finding is consistent with previous evidence of the superior effect of smoking cessation on infant birth weight compared with sustained smoking (El-Mohandes et al., 2009; McCowan et al., 2009; Murin et al., 2011).
Although nonsignificant, a trend toward a positive effect of smoking reduction was observed. Mean infant birth weight was highest among women who reduced from heavy to light smoking compared with other persistent smokers. Reduction from heavy to light exposure was associated with a 199g increase in birth weight compared with heavy/heavy exposure, a 103g increase compared with light/heavy, and a 63g increase compared with light/light. This finding of improved birth weight among those who reduced from heavy to light exposure is contrary to that of England et al. (2001), who found that infant birth weight was not affected by decreased smoking exposure among heavy smokers, only light smokers. The finding of this study is, however, congruent with that of Li et al. (1993), who found an increase in birth weight associated with reduction among White women with salivary cotinine levels >100ng/ml. Secker-Walker, Vacek, Flynn, & Mead (1998) also reported increased birth weight associated with reduction among heavier smokers.
This study used salivary cotinine as a measure of smoking exposure to assess the impact of reduction on growth restriction in full-term infants. Other studies have used both self-report and biochemical measures (England et al., 2001; Li et al., 1993; Secker-Walker & Vacek, 1998). However, report of cigarettes per day in this study was poorly correlated with infant birth weight and did not yield any useful findings. Recall bias is a likely explanation. A review of the data showed that in some cases there was gross incongruence between report of cigarettes smoked and salivary cotinine at both baseline and EOP. Also, as biochemical measures of cotinine are influenced by other factors, particularly environmental exposure, secondhand smoke may account for some of the discrepancy observed between self-report and salivary cotinine values. A majority of the women had partners who continued to smoke or lived in households with other smokers (81%).
Given the potential for confounding in previous studies that assessed the impact of change without regard for baseline levels, we stratified EOP cotinine by baseline cotinine for a straightforward assessment of change from heavy to light exposure. Similar strategies of assessing the impact of change relative to baseline levels of exposure have been reported by others. Li et al. (1993) specified the amount of reduction in salivary cotinine for heavy (≥100ng/ml) and light (<100ng/ml) smokers as 60 and 20ng/ml, respectively. England et al. (2001) stratified EOP and baseline smoking exposure and compared the impact of change from heavy smoking (≥11 cigarettes/day and urine cotinine ≥1,500ng/ml) to light smoking (<11 cigarettes/day and urine cotinine <1,500ng/ml) on infant birth weight.
There were limitations that may have impacted the findings of this study. As this is a substudy, we were constrained to the data collected in the parent study, limiting our ability to control for some potential confounders. The estimate of the effect of smoking exposure change may have been obscured by lack of control for timing of change and/or duration of reduced exposure. The time at which smoking cessation or smoking reduction occurs during pregnancy impacts infant birth weight (Lieberman et al., 1994; Ohmi et al., 2002). Most benefits are associated with change before the third trimester. Greater gains in birth weight would be expected if reduction occurred earlier, say in the second trimester, than late in the third trimester (Secker-Walker et al., 1998). As information on time and duration of change was not available, it was not possible to control for this effect. Additionally, information on other substances, that is, illicit drugs and alcohol, were not available, precluding control for these birth weight correlates.
The broad categories used to define light and heavy exposure and the combining of strata due to small numbers likely resulted in heterogeneity within exposure change categories, increasing difficulty of detecting differences in mean birth weight across groups (Bentley, Weinstein, & Kuntz, 2009). A larger sample size would have allowed a greater number of strata for increased within-group homogeneity, the result of which would have been sharper differences between groups and increased power.
Another limitation was the use of a self-selected, nonprobability sample. Those electing to participate may differ from the population of women who smoke during pregnancy, limiting generalizability. Further, as a substudy, using data collected for evaluation of other outcomes, interpretation of statistical significance requires caution (Moye, 2000).
The strengths of this study include the prospective design; use of a biochemical measure of smoking exposure, which is free of information bias and represents active and passive exposure; and the use of stratification as a straightforward, easy to interpret means of assessing the impact of change relative to baseline.
Despite the aforementioned shortcomings, the findings of this study, taken together with previous reports, suggests a potential benefit of smoking reduction. Though not statistically significant, the near 200g increase in birth weight associated with reduction from heavy to light exposure compared with sustained heavy exposure is clinically significant. Smoking-related birth weight deficits of 150–250g have been reported and, on average, maternal smoking reduces birth weight by 200g (Butler, Goldstein, & Ross, 1972; Murin et al., 2011). Although small, the 200g decrement in birth weight due to smoking contributes to low birth weight and small for gestational age (Lambers & Clark, 1996). This study and others suggest that reduction in exposure to cigarette smoke has the potential to substantially decrease the 200g smoking-related birth weight decrement, even among heavy smokers.
We conclude, as others have, that smoking cessation should be the goal for pregnant women. However, the trend of increased gains in birth weight with reduced smoking exposure among women smoking into the second trimester of pregnancy observed in this study, taken together with previous findings, warrants continued exploration. Research in this area is important to inform the care of highly addicted pregnant women for whom smoking cessation is difficult.
Funding
This work was supported by a grant from the Robert Wood Johnson Foundation.
Declaration of Interests
Neither of the authors of this manuscript has any competing interests.
Acknowledgments
We would like to acknowledge the Clinical Research Unit (UL1RR024148), formerly the General Clinical Research Center (MO1RR02558) of the University of Texas Health Science Center at Houston, where the research was conducted.
References
- Bentley T. G., Weinstein M. C., Kuntz K. M. (2009). Effects of categorizing continuous variables in decision-analytic models. Medical Decision Making, 29 549–556 doi:10.1177/0272989X09340238 [DOI] [PubMed] [Google Scholar]
- Britton G. R., Brinthaupt J., Stehle J. M., James G. D. (2004). Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 33 306–311 doi:10.1177/0884217504264866 [DOI] [PubMed] [Google Scholar]
- Butler N. R., Goldstein H., Ross E. M. (1972). Cigarette smoking in pregnancy: Its influence on birth weight and perinatal mortality. British Medical Journal, 2 127–130 doi:10.1136/bmj.2.5806.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov J. M., Newman A. B., Kelsey S. F., Roberts J. M., Sutton-Tyrrell K. C., Garcia M., et al. (2006). Accuracy and reliability of maternal recall of infant birth weight among older women. Annals of Epidemiology, 16 429–431 doi:10.1016/annepidem.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Cliver S. P., Goldenberg R. L., Cutter G. R., Hoffman H. J., Davis R. O., Nelson K. G. (1995). The effect of cigarette smoking on neonatal anthropometric measurements. Obstetrics and Gynecology, 85 625–630 doi:10.1016/0029-7844(94)00437-I [DOI] [PubMed] [Google Scholar]
- Cogswell M. E., Yip R. (1995). The influence of fetal and maternal factors on the distribution of birthweight. Seminars in Perinatology, 19 222–240 doi:10.1016/S0146-0005(05)80028-X [DOI] [PubMed] [Google Scholar]
- Elliott J. P., Desch C., Istwan N. B., Rhea D., Collins A. M., Stanziano G. J. (2010). The reliability of patient-reported pregnancy outcome data. Population Health Management, 13 27–32 doi:10.1089/pop.2009.0008 [DOI] [PubMed] [Google Scholar]
- England L. J., Kendrick J. S., Wilson H. G., Merritt R. K., Gargiullo P. M., Zahniser S. C. (2001). Effects of smoking reduction during pregnancy on the birth weight of term infants. American Journal of Epidemiology, 154 694–701 doi:10.1093/aje/154.8.694 [DOI] [PubMed] [Google Scholar]
- England L. J., Grauman A., Qian C., Wilkins D. G., Schisterman E. F., Yu K. F., Levine R. J. (2007). Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine & Tobacco Research, 9 1005–1013 doi:10.1080/14622200701491255 [DOI] [PubMed] [Google Scholar]
- El-Mohandes A. A., Kiely M., Gantz M. G., Blake S. M., El-Khorazaty M. N. (2009). Prediction of birth weight by cotinine levels during pregnancy in a population of African American smokers. Pediatrics, 124 e671–e680 doi:10.1542/peds.2008–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J. F., Perneger T. V. (2001). Measurement of self reported active exposure to cigarette smoke. Journal of Epidemiology and Community Health, 55 674–680 doi:10.1136/jech.55.9.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florescu A., Ferrence R., Einarson T., Selby P., Soldin O., Koren G. (2009). Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Therapeutic Drug Monitoring, 31 14–30 doi:10.1097/FTD.0b013e3181957a3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglia R. C., Binns C. W., Alfonso H. S. (2006). Which women stop smoking during pregnancy and the effect on breastfeeding duration. BioMed Central Public Health, 6 195 doi:10.1186/1471-2458-6-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow J. E., Knight G. J., Palomaki G. E., Kloza E. M., Wald N. J. (1987). Cigarette consumption and serum cotinine in relation to birthweight. British Journal of Obstetrics and Gynaecology, 94 678–681 doi:10.1111/j.1471-0528.1987.tb03174.x [DOI] [PubMed] [Google Scholar]
- Hebel J. R., Fox N. L., Sexton M. (1988). Dose-response of birth weight to various measures of maternal smoking during pregnancy. Journal of Clinical Epidemiology, 41 483–489 doi:10.1016/0895-4356(88)90050–9 [DOI] [PubMed] [Google Scholar]
- Jaccard J., Turrisi R. (2003). Interaction effects in multiple regression, In Lewis-Beck M. S. (Ed.), Quantitative applications in social sciences (2nd ed., pp. 11–12). Thousand Oaks: CA: Sage; [Google Scholar]
- Jarvis M. J., Russell M. A., Benowitz N. L., Feyerabend C. (1988). Elimination of cotinine from body fluids: Implications for noninvasive measurement of tobacco smoke exposure. American Journal of Public Health, 78 696–698 doi:10.2105/AJPH.78.6.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff M. A., Levine R. J., Clemens J. D., DerSimonian R., Wilkins D. G. (1998). Serum cotinine concentration and self-reported smoking during pregnancy. American Journal of Epidemiology, 148 259–262 Retrieved from aje.oxfordjournals.org/cgi/content/abstract/148/3/259 [DOI] [PubMed] [Google Scholar]
- Kramer M. S. (1987). Determinants of low birth weight: Methodological assessment and meta-analysis. Bulletin of the World Health Organization, 65 663–737 Retrieved from www.ncbi.nlm.nih.gov/pmc/articles/PMC2491072/ [PMC free article] [PubMed] [Google Scholar]
- Lambers D. S., Clark K. E. (1996). The maternal and fetal physiologic effects of nicotine. Seminars in Perinatology, 20 115–126 doi:10.1016/S0146-0005(96)80079-6 [DOI] [PubMed] [Google Scholar]
- Li C. Q., Windsor R. A., Perkins L., Goldenberg R. L., Lowe J. B. (1993). The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. Journal of the American Medical Association, 269 1519–1524 doi:10.1001/jama.1993.03500120057026 [PubMed] [Google Scholar]
- Lieberman E., Gremy I., Lang J. M., Cohen A. P. (1994). Low birthweight at term and the timing of fetal exposure to maternal smoking. American Journal of Public Health, 84 1127–1131 doi:10.2105/AJPH.84.7.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley J., Oliver S., Chamberlin C., Oakley L. (2009). Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews, 4doi:10.1002/ 14651858.CD001055.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan L. M., Dekker G. A., Chan E., Stewart A., Chappell L. C., Hunter M., et al. (2009). Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: Prospective cohort study. British Medical Journal, 338 b1081doi:10.1136/bmj.b1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasco B. J., Dornelas E. A., Fischer E. H., Oncken C., Lando H. A. (2006). Spontaneous smoking cessation during pregnancy among ethnic minority women: A preliminary investigation. Addictive Behaviors, 31 203–210 doi:10.1016/j.addbeh.2005.04.022 [DOI] [PubMed] [Google Scholar]
- Moye L. A. (2000). Statistical reasoning in medicine. The intuitive p-value primer (pp. 261–265). New York, NY: Springer: [Google Scholar]
- Murin S., Rafii R., Bilello K. (2011). Smoking and smoking cessation in pregnancy. Clinics in Chest Medicine, 32 75–91, viii doi:10.1016/j.ccm.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Nahum G. G., Stanislaw H., Huffaker B. J. (1995). Fetal weight gain at term: Linear with minimal dependence on maternal obesity. American Journal of Obstetrics and Gynecology, 172 1387–1394 doi:10.1016/0002-9378(95)90467-0 [DOI] [PubMed] [Google Scholar]
- Neter J., Kutner M. H., Nachtsheim C. J., Wasserman W. (1996). Applied linear statistical models (pp. 1054–1057). Chicago, IL: Irwin; [Google Scholar]
- Ockene J., Ma Y., Zapka J., Pbert L., Valentine Goins K., Stoddard A. (2002). Spontaneous cessation of smoking and alcohol use among low-income pregnant women. American Journal of Preventive Medicine, 23 150–159 doi:10.1016/S0749-3797(02)00492-0 [DOI] [PubMed] [Google Scholar]
- Ohmi H., Hirooka K., Mochizuki Y. (2002). Fetal growth and the timing of exposure to maternal smoking. Pediatrics International, 44 55–59 doi:10.1046/j.1442-200X.2002.01495.x [DOI] [PubMed] [Google Scholar]
- Pastrakuljic A., Derewlany L. O., Koren G. (1999). Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta, 20 499–512 doi:10.1053/plac.1999.0418 [DOI] [PubMed] [Google Scholar]
- Peacock J. L., Cook D. G., Carey I. M., Jarvis M. J., Bryant A. E., Anderson H. R., Bland J. M. (1998). Maternal cotinine level during pregnancy and birthweight for gestational age. International Journal of Epidemiology, 27 647–656 doi:10.1093/ije/27.4.647 [DOI] [PubMed] [Google Scholar]
- Petersen G. O., Leite C. E., Chatkin J. M., Thiesen F. V. (2010). Cotinine as a biomarker of tobacco exposure: Development of a HPLC method and comparison of matrices. Journal of Separation Science, 33 516–521 doi:10.1002/jssc.200900575 [DOI] [PubMed] [Google Scholar]
- Pickett K. E., Wakschlag L. S., Dai L., Leventhal B. L. (2003). Fluctuations of maternal smoking during pregnancy. Obstetrics and Gynecology, 101 140–147 doi:10.1016/S0029-7844(02)02370-0 [DOI] [PubMed] [Google Scholar]
- Quinn V. P., Mullen P. D., Ershoff D. H. (1991). Women who stop smoking spontaneously prior to prenatal care and predictors of relapse before delivery. Addictive Behaviors, 16 29–40 doi:10.1016/0306-4603(91)90037-I [DOI] [PubMed] [Google Scholar]
- Rush D., Cassano P. (1983). Relationship of cigarette smoking and social class to birth weight and perinatal mortality among all births in Britain, 5-11 April 1970. Journal of Epidemiology and Community Health, 37 249–255 doi:10.1136/jech.37.4.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell T. V., Crawford M. A., Woodby L. L. (2004). Measurements for active cigarette smoke exposure in prevalence and cessation studies: Why simply asking pregnant women isn’t enough. Nicotine & Tobacco Research, 6(Suppl. 2)S141–S151 doi:10.1080/14622200410001669141 [DOI] [PubMed] [Google Scholar]
- Secker-Walker R. H., Vacek P. M., Flynn B. S., Mead P. B. (1998). Estimated gains in birth weight associated with reductions in smoking during pregnancy. Journal of Reproductive Medicine, 43 967–974 Retrieved from www.reproductivemedicine.com/toc/auto_abstract.php?id=11361 [PubMed] [Google Scholar]
- Secker-Walker R. H., Vacek P. M. (2002). Infant birth weight as a measure of harm reduction during smoking cessation trials in pregnancy. Health Education & Behavior, 29 557–569 doi:10.1177/109019802237024 [DOI] [PubMed] [Google Scholar]
- Shipton D., Tappin D. M., Vadiveloo T., Crossley J. A., Aitken D. A., Chalmers J. (2009). Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. British Medical Journal, 339 b4347doi:10.1136/bmj.b4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L. J., Quinn V. P. (2004). Spontaneous quitting: Self-initiated smoking cessation in early pregnancy. Nicotine & Tobacco Research, 6(Suppl. 2) S203–S216 doi:10.1080/14622200410001669132 [DOI] [PubMed] [Google Scholar]
- Stead L. F., Lancaster T. (2007). Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews (Online), 3 CD005231doi:10.1002/14651858.CD005231.pub2 [DOI] [PubMed] [Google Scholar]
- Stotts A. L., Groff J. Y., Velasquez M. M., Benjamin-Garner R., Green C., Carbonari J. P., DiClemente C. C. (2009). Ultrasound feedback and motivational interviewing targeting smoking cessation in the second and third trimesters of pregnancy. Nicotine & Tobacco Research, 11 961–968 doi:10.1093/ntr/ntp095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong V. T., Jones J. R., Dietz P. M., D’Angelo D., Bombard J. M. (2009). Trends in smoking before, during, and after pregnancy—pregnancy risk assessment monitoring system (PRAMS), United States, 31 sites, 2000–2005. MMWR Morbidity Mortality Weekly Report, 58 1–29 Retrieved from www.cdc.gov/mmwr/preview/mmwrhtml/ss5804al.htm [PubMed] [Google Scholar]
- Troude P., L’Hélias L. F., Raison-Boulley A. M., Castel C., Pichon C., Bouyer J., de La Rochebrochard E. (2008). Perinatal factors reported by mothers: Do they agree with medical records? European Journal of Epidemiology, 23 557–564 doi:10.1007/s10654-008-9268-9 [DOI] [PubMed] [Google Scholar]
- Vardavas C. I., Chatzi L., Patelarou E., Plana E., Sarri K., Kafatos A., et al. (2010). Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. European Journal of Pediatrics, 169 741–748 doi:10.1007/s00431-009-1107-9 [DOI] [PubMed] [Google Scholar]
- Wagenknecht L. E., Burke G. L., Perkins L. L., Haley N. J., Friedman G. D. (1992). Misclassification of smoking status in the CARDIA study: A comparison of self-report with serum cotinine levels. American Journal of Public Health, 82 33–36 doi:10.2105/AJPH.82.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh R. A., Redman S., Brinsmead M. W., Arnold B. (1995). Smoking cessation in pregnancy: A survey of the medical and nursing directors of public antenatal clinics in Australia. The Australian & New Zealand Journal of Obstetrics & Gynaecology, 35 144–150 doi:10.1111/j.1479-828X [DOI] [PubMed] [Google Scholar]
- Walsh R. A. (1994). Effects of maternal smoking on adverse pregnancy outcomes: Examination of the criteria of causation. Human Biology, 66 1059–1092 Retrieved from www.ncbi.nlm.nih.gov/pubmed/7835872 [PubMed] [Google Scholar]
- Wang D., Connock M., Barton P., Fry-Smith A., Aveyard P., Moore D. (2008). “Cut down to quit” with nicotine replacement therapies in smoking cessation: A systematic review of effectiveness and economic analysis. Health Technology Assessment, 12 1–135 doi:10.3310/hta12020 [DOI] [PubMed] [Google Scholar]
- Wang X., Tager I. B., Van Vunakis H., Speizer F. E., Hanrahan J. P. (1997). Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. International Journal of Epidemiology, 26 978–988 doi:10.1093/ije/26.5.978 [DOI] [PubMed] [Google Scholar]
- Wickström R. (2007). Effects of nicotine during pregnancy: Human and experimental evidence. Current Neuropharma cology, 5 213–222 doi:10.2174/157015907781695955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R. A., Li C. Q., Boyd N. R., Jr., Hartmann K. E. (1999). The use of significant reduction rates to evaluate health education methods for pregnant smokers: A new harm reduction behavioral indicator? Health Education & Behavior, 26 648–662 doi:10.1177/109019819902600506 [DOI] [PubMed] [Google Scholar]
- Windsor R. A., Lowe J. B., Perkins L. L., Smith-Yoder D., Artz L., Crawford M., et al. (1993). Health education for pregnant smokers: Its behavioral impact and cost benefit. American Journal of Public Health, 83 201–206 doi:10.2105/ AJPH.83.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R. A., Woodby L. L., Miller T. M., Hardin J. M., Crawford M. A., DiClemente C. C. (2000). Effectiveness of Agency for Health Care Policy and Research clinical practice guideline and patient education methods for pregnant smokers in Medicaid maternity care. American Journal of Obstetrics and Gynecology, 182(1 Pt. 1)68–75 doi:10.1067/mob.2000.100135 [DOI] [PubMed] [Google Scholar]