Abstract
Introduction:
There is increasing evidence that response to pharmacological treatment for nicotine dependence may be moderated by genetic polymorphisms. However, the feasibility, acceptability, and impact of genetically tailoring treatment in real-world clinical settings are unknown.
Methods:
We conducted a multiphased, mixed-methods feasibility study with current smokers to develop and evaluate a patient-centered, theoretically grounded personalized medicine treatment protocol. The initial research phase included formative work to develop intervention materials. The second phase included a randomized pilot trial to evaluate the intervention. Trial participants (n = 36) were genotyped for ANKK1 rs1800497 and were randomized to receive genetic feedback (GF) plus standard behavioral counseling (BC) for smoking cessation or BC without GF. All participants received genetically tailored pharmacotherapy (nicotine patch or bupropion).
Results:
The intervention was feasible to implement and was acceptable to participants based on satisfaction ratings and objective measures of participation. There was no evidence that the GF resulted in adverse psychological outcomes (e.g., depression, fatalism, reduced perceived control over quitting, differential motivation for quitting) based on quantitative or qualitative outcomes.
Conclusions:
Study results suggest that it is feasible to offer treatment within a health care setting that includes genetically tailored pharmacotherapy and doing so had no apparent adverse psychological impacts. Further evaluation of pharmacogenetically tailored smoking cessation interventions appears warranted.
Introduction
Best practice for the treatment of nicotine dependence calls for a combination of behavioral counseling (BC) and pharmacotherapy (Fiore et al., 2008). However, even with our best combination therapies, most smokers relapse or do not maintain long-term abstinence (Fiore et al., 2008). The limited efficacy of our current standard, “one-size-fits-all” treatments has prompted researchers to explore whether individual treatment response varies by genotype (David et al., 2008, 2011). Emerging evidence from candidate gene and genome-wide association studies have identified associations between genetic polymorphisms in multiple pharmacological pathways (drug receptor, signaling, or metabolic) and efficacy of or side effects from nicotine replacement therapy (NRT), bupropion, or varenicline (Gold & Lerman, 2012; Kortmann, Dobler, Bizarro, & Bau, 2010; Uhl et al., 2008, 2010). This suggests that individual treatment response may be influenced by one’s genetic profile. In addition, when people believe their health problems have a genetic cause, the perceived effectiveness of pharmacological treatment increases (Marteau & Weinman, 2006). Since perceived effectiveness predicts treatment use, there is good reason to assume that genetically tailoring pharmacotherapy could improve treatment outcomes through a combination of improved adherence (Marteau et al., 2012) and better treatment response.
At present, it remains unclear which genetic polymorphisms will be most informative for treatment tailoring, but personalized medicine is expected to play a larger role in standard clinical care in the future (Altman, 2011; Collier, 2012). This innovation has been met with both enthusiasm and caution (Allison, 2008; Goldsmith, Jackson, O’Connor, & Skirton, 2012; Hamburg & Collins, 2010). Barriers include cost (Dean, 2009), the need for greater education and genetic literacy among patients and providers (Baars, Henneman, & Ten Kate, 2005; Collier, 2012; Quaak, Smerecnik, van Schooten, de Vries, & van Schayck, 2012; Suther & Goodson, 2003), and individuals’ skepticism about genetic testing and its benefits for treatment (Park et al., 2011). Despite these concerns, rapid developments in industry-based direct-to-consumer marketing of pharmacogenetic products could drive demand from consumers. Thus, there is a need to understand how to best design and deliver genetically tailored interventions, in addition to understanding their effectiveness. It is also important to determine that these interventions are safe (Collins, Green, Guttmacher, Guyer, & U.S. National Human Genome Research Institute, 2003; Secretary’s Advisory Committee on Genetics Health and Society, 2008). That is, that they do not result in adverse psychological outcomes such as increased depression and that they do not undermine important cognitive treatment mediators such as motivation, intent to quit, self-efficacy, or perceived control over one’s ability to quit smoking. These are issues that warrant further research.
The goal of the current study was to develop and evaluate a patient-centered personalized medicine protocol for smokers ready to quit. We examined the feasibility of offering the personalized intervention within the context of a health care setting, the acceptability of the intervention to smokers, and the preliminary psychological and behavioral impacts of offering genetic feedback (GF) and tailored pharmacotherapy to smokers. Findings from this pilot work are intended to inform future research.
There are hundreds of candidate genes that could ultimately inform cessation treatment outcome, but at the time this study was designed in 2006, the most widely replicated gene associations with abstinence outcomes were with dopamine pathway genotypes with bupropion and NRT. Data on varenicline were not yet available. Candidate gene selection for this trial was largely informed by two placebo-controlled trials of bupropion and one of transdermal NRT (David, Brown et al., 2007; David, Strong et al., 2007; E. Johnstone et al., 2004; Lerman et al., 2003). Observed abstinence effect sizes for the most widely studied of these polymorphisms (rs1800497) suggested that NRT was more effective among persons with the A1/A1 or A1/A2 alleles and bupropion was more effective among persons with the A2/A2 allele. These results are consistent with more recent trials that have also shown that persons with A1/A1 or A1/A2 genotypes have more favorable response to NRT patch (Breitling et al., 2011; Stapleton, Sutherland, O’Gara, Spirling, & Ball, 2011). Others have suggested a gene (ANKK1) × sex interaction (E. C. Johnstone et al., 2004; Yudkin et al., 2003) or gene (ANKK1) × gene (CYP2B6 or SLC6A3) interactions (David, Brown et al., 2007; Lerman et al., 2003; Swan et al., 2007), but the limited state of the science in 2006 precluded these details from informing the design of the current pilot trial, which is aimed at understanding the psychological and behavioral impact of the GF as opposed to the efficacy of the genetically tailored treatment.
Methods
Overview of Mixed-Methods Study Design
We conducted a two-phased, mixed-methods study. Phase 1 involved formative research to develop and refine a patient-centered, theoretically grounded behavioral intervention for delivering genetically tailored smoking cessation treatment. Phase 2 assessed the feasibility and acceptability of delivering the comprehensive, genetically tailored smoking cessation intervention and the impact of this intervention on key psychological and behavioral outcomes. The study was not designed to examine gene × drug interactions. Rather, our intent was to evaluate the effect of the GF on our outcomes of interest. All activities were reviewed and approved by the Institutional Review Boards at Group Health Cooperative (GHC; protocol ID HS-09-040), Stanford University (protocol ID 16513), and SRI International (DHHS Registration/ID No. IRB00000110 and Assurance No. FWA00007933).
Study Population and Recruitment: Phases 1 and 2
All Phase 1 and Phase 2 participants were recruited from GHC, a large, regional not-for-profit health plan in the Pacific Northwest. Potential participants were identified based on electronic health records (Phases 1 and 2) and a subset of participants in a previous smoking cessation study (Phase 2; Swan et al., 2010), who had been previously genotyped and provided consent to be recontacted. Potential participants were mailed an invitation letter, contacted by phone, and screened for eligibility. Additional screening and enrollment details are described below.
Phase 1 Methods
Phase 1A: Expert Opinion Panel
We convened a panel of doctorate-level experts (n = 10) in pharmacogenetics; smoking cessation treatment; ethical, legal, and social implications of genetics research; genetic literacy; patient–clinician communications; and mixed-methods research to guide development of the pharmacogenetic treatment, GF, and evaluation. The panel recommended that participants receive supplemental written materials addressing the following topics: the role of genes in medication outcomes and side effects, the role of genes in nicotine dependence, the implications of one’s ANKK1 genotype (A1 vs. A2) on pharmacotherapy selection (NRT vs. bupropion), and the rationale for genetically matching pharmacotherapy selection based on enhanced treatment outcomes. It was recommended that materials be kept brief and written in plain language. The panel further advised that phone-based counseling was a reasonable treatment approach and was consistent with the standard care quitline counseling offered to health plan members through their insurance coverage. Despite the limitations of our current knowledge base, it was recommended that the role of genetics in treatment outcome not be downplayed too much, as this could diminish GF participants’ confidence in quitting. This advice informed the design of the pilot study educational materials and treatment protocol. Material design was also influenced by an earlier, transdisciplinary conference of physicians, clinical geneticists, genetic counselors, genetic epidemiologists, and others convened by the National Cancer Institute (NCI) and the National Human Genome Research Institute to educate primary care physicians how to translate genetic technology to medical education and practice (David & Gramling, 2003).
Phase 1B: Formative Patient Interviews
Participants were eligible for Phase 1 if they were aged 18–65, were a current smoker, smoked at least 5 cigarettes/day, could read and speak English, did not have a diagnosis or history of treatment for schizophrenia or a psychotic disorder in the past 5 years, and self-identified as non-Hispanic, White. All participants provided consent to participate. Interviews were conducted by research staff trained in cognitive interviewing and using a structured guide. Smokers were asked about their familiarity with genetic concepts (e.g., DNA, genes), understanding of the roles genes play in smoking behavior and treatment response, reaction to the concept of genetically tailoring pharmacotherapy, familiarity with the Genetic Information Nondiscrimination Act, concerns about privacy of genetic information, and interest in genetically tailored treatment. Each interview was audiotaped and transcribed. Participants received $25 and reimbursement for parking, as applicable. Interviews were continued until response saturation (i.e., when responses became redundant and no new themes emerged) was achieved (n = 10; six females, mean age = 46.1). Transcripts were reviewed by the study investigators (SPD, JM) for key themes and specific feedback that then informed the content, tone, and best format for delivering GF to pilot participants.
Phase 1C: Protocol Development
Based on the feedback from smokers and our expert panel, we drafted a one-page information sheet describing how genes influence medications; two one-page GF forms, called Personal Treatment Profiles (one for persons with the A1 genotype and one for persons with the A2 genotype), which informed participants of their genotype, the relevance of this to smoking cessation pharmacotherapy outcomes, and the medication (NRT or bupropion) that had been assigned to them based on their genotype and a cover letter for these materials. Next, we created a cognitive behavioral counseling program based on best practice standards (Fiore et al., 2008).
Phase 2: Randomized Pilot Trial and Summative Interviews
Phase 2A: Recruitment and Enrollment
Pilot participants were recruited from April 2010 through February 2011. Potential participants were mailed an invitation letter, contacted by phone, and if willing, screened for eligibility. Participants were eligible if they were aged 18–65; currently smoked at least 10 cigarettes/day and wanted to stop smoking in the next 3–6 weeks; self-identified as White, non-Hispanic; were comfortable reading and writing in English; had a telephone; were currently enrolled in GHC and eligible to receive medications from the mail order pharmacy; and were willing to receive information about their genotype. Individuals were ineligible if they reported medical contraindications for NRT or bupropion, or evidenced current depression as assessed by a Patient Health Questionnaire (PHQ; Kroenke et al., 2009) score >10. Persons with documented evidence of psychotic, mood, or other Axis I disorders were also excluded based on their medical records and were prescreened out of the invitation pool. Ethnic and racial minorities were excluded because the current evidence base does not yet allow informed decisions regarding how to genetically tailor NRT or bupropion in these populations. All participants who did not have a genotype on file were genotyped for rs1800497 prior to randomization. Participants were mailed a written consent form and saliva collection kits, which they returned directly to the study lab for analysis. Upon return of the consent form and prior to genotyping, baseline assessments were scheduled. Randomization occurred within approximately 1 week from the baseline assessment.
Phase 2B: Intervention
Following the baseline assessment, participants were randomized to treatment (GF or BC) using an automated algorithm that stratified by gender and genotype. Treatment groups differed in whether they were informed that their treatment had been selected based on their genotype but each received the same motivationally enhanced cognitive behavioral counseling (up to three brief calls delivered by a certified tobacco treatment specialist), a self-help guide for smoking cessation (Clearing the Air, NCI), and a standard 8-week course of pharmacotherapy. Smokers with the A1 allele (TT/CT) were assigned to receive NRT and participants with the A2 allele (CC) were assigned to receive bupropion. Each counseling call was designed to last ~20min. The first call preceded the target quit date (TQD), the second call was scheduled at about 2 weeks post-TQD, and the third call scheduled approximately 2 weeks after the second call.
During the first counseling call, GF participants were informed of their genotype, assigned pharmacotherapy, and provided the rationale for this assignment based on their genotype. BC participants were simply informed the medication they would receive, based on the study physician’s recommendation, with no GF. Following this call, GF participants were mailed a self-help guide for smoking cessation, a reminder when their next counseling call was scheduled, the one-page Personal Treatment Profile, and a one-page description of how genes influence the ways drugs work. The Personal Treatment Profile echoed each participant’s ANNK1 genotype, the implications of this for smoking cessation treatment outcome, and the medication that was chosen for them based on their genotype. For example, people with the A1 genotype were informed, “people with the A1 gene are more likely to quit smoking using the nicotine patch than people who have the A2 gene. People with the A1 gene are no more likely to quit smoking using bupropion SR than when they use placebo. In short, if you have the A1 gene, you are more likely to benefit from the nicotine patch than bupropion. Based on your genotype, we recommend you use the nicotine patch and counseling to quit smoking.” This level of probabilistic language was considered to be more straightforward for participants to understand than presenting numerically based probabilities and less likely to conflict with new findings that could emerge during the study period.
Phase 2C: Assessment
Participants were assessed by phone at baseline, approximately 1 week prior to the TQD, and 12 weeks following the TQD. Participants who reported abstinence at the 12-week assessment were mailed a saliva collection kit for cotinine analyses to biochemically confirm abstinence (Hughes et al., 2003; Hukkanen, Jacob, & Benowitz, 2005). Psychological outcomes, baseline descriptors, and mediational process measures included the following: the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991); the Center for Epidemiological Studies Depression Scale (CES-D; Ratloff, 1977); four items from the Smoking Risk Perceptions Scale (SRPS; Park et al., 2007); the eight-item Rapid Assessment of Adult Literacy for Genetics (REAL-G) (Erby, Roter, Larson, & Cho, 2008); the Schwartz Numeracy Scale (Schwartz, Woloshin, Black, & Welch, 1997); and the Morisky Scale of medication adherence (Morisky, Green, & Levine, 1986). To assess smokers’ fatalistic beliefs about the genetic determinism of smoking, we modified the Powe Fatalism Inventory (Powe, 1995) by rephrasing items to reflect fatalistic beliefs about the determined nature of smoking, as opposed to fatalistic beliefs about cancer. Five items from the original scale were dropped because they were not relevant for the revised scale, for example, “I believe if someone gets cancer their time to die is near.” The modified 10-item scale had high internal validity (Cronbach’s α = 0.93 at follow-up). Additional measures assessed smokers’ perceived control over quitting smoking, their belief that their behavior can mitigate the risks of smoking (threat minimization; Wright, French, Weinman, & Marteau, 2006), self-efficacy for quitting smoking, motivation for quitting, intent to quit, treatment interest, and a series of items assessing general satisfaction with their interventionist in the following domains: general communication, trust in the clinician, and overall satisfaction (Schneider, Kaplan, Greenfield, Li, & Wilson, 2004). All preceding constructs were measured using Likert scale items (see Tables 1 and 2 for scale score ranges).
Table 1.
Baseline Characteristics of Pilot Sample
| Genetic feedback (n = 19) | Behavioral counseling (n = 17) | p value | |
|---|---|---|---|
| Demographics | |||
| Age (mean, SD) | 52.8 (8.6) | 49.4 (10.6) | .39 |
| Female (n, %) | 11 (63%) | 10 (58%) | .83 |
| Married (n, %) | 15 (88%) | 12 (75%) | .52 |
| High school education or greater (n, %) | 17 (100%) | 16 (100%) | 1.00 |
| Employed (n, %) | 17 (88%) | 15 (88%) | .95 |
| Genotype (n, %) | |||
| A1/A1 or A1/A2 | 4 (24%) | 6 (32%) | — |
| A2/A2 | 13 (76%) | 13 (68%) | .74 |
| Tobacco dependence and history | |||
| Nicotine dependence (FTND; mean, SD) (range: 0-8) | 4.3 (1.7) | 4.6 (1.5) | .63 |
| Cigarettes/day (mean, SD) | 16.5 (4.6) | 20.9 (10.1) | .13 |
| Previous quit attempts, past year (mean, SD) | 1.9 (1.8) | 0.9 (1.2) | .07 |
| Longest abstinent period (years; mean, SD) | 0.9 (1.2) | 0.9 (1.2) | .87 |
| Psychosocial measures (mean, SD) | |||
| Genetic literacy (Real-G; range: 0–8) | 7.1 (1.5) | 7.6 (0.6) | .18 |
| Numeracy (range: 0–3) | 1.8 (0.9) | 2.1 (0.8) | .44 |
| Risk perception (range: 4–20) | 16.7 (2.1) | 16.1 (2.6) | .47 |
| Threat minimization (range: 2–14) | 6.5 (3.5) | 8.8 (2.9) | .04 |
| Depression (CES-D; range: 0–60) | 5.3 (7.6) | 4.2 (3.9) | .62 |
| Motivation to quit (range: 1–7) | 5.7 (1.2) | 5.5 (1.5) | .64 |
| Intent to quit (range: 3–21) | 15.5 (5.1) | 15.3 (5.1) | .92 |
| Self-efficacy for quitting (range: 3–21) | 14.2 (4.0) | 13.4 (3.9) | .52 |
| Perceived control over quitting (range: 3–21) | 11.4 (4.0) | 11.8 (3.2) | .75 |
Note. CES-D = Center for Epidemiological Studies Depression Scale; FTND = Fagerström Test for Nicotine Dependence; SD = standard deviation. Ranges refer to range of possible scale scores.
Table 2.
Quantitative Measures of Treatment Acceptability
| Genetic feedback mean (sd) | Behavioral counseling mean (sd) | Genetic feedback effect (se) | T value | p value | |
|---|---|---|---|---|---|
| Morisky adherence (range: 0–8) | 1.8 (1.6) | 2.7 (1.9) | −0.90 (0.64) | −1.41 | .170 |
| Trust (range: 5–30) | 18.8 (2.3) | 19.3 (2.5) | −0.46 (0.88) | −0.53 | .604 |
| Communication (range: 4–20) | 19.4 (1.5) | 19.4 (1.4) | −0.18 (0.51) | −0.03 | .972 |
| Satisfaction (range: 4–20) | 19.5 (0.8) | 19.4 (1.8) | 0.16 (0.51) | 0.31 | .757 |
| Treatment interest (range: 1–10) | 9.6 (0.74) | 9.0 (1.6) | 0.60 (0.44) | 1.36 | .184 |
Note. SD = standard deviation; SE = standard error. Ranges refer to range of possible scale scores.
Summative Interviews
Nineteen participants, representing both genders and genotypes, were randomly chosen to participate in a summative interview following the first assessment. Interviews were conducted using an in-depth interview guide and explored participants’ overall satisfaction with the interventionist, the patient-centeredness of the GF and counseling, the overall acceptability of the treatment, understanding and recall of the GF results, self-efficacy, any negative emotional or cognitive reactions to the GF, and areas of concern about the intervention.
Pilot Trial Data Analyses
Descriptive statistics were used to characterize the baseline sample and t tests used to compare mean differences at baseline. Linear regression was used to estimate the effect of group on follow-up psychological and process measure scores, controlling for baseline scores.
Results
Phase 1: Formative Interview Results
All Phase 1 participants had some level of familiarity with basic genetic concepts, with moderate genetic literacy overall. One third thought that genetics probably was important in their personal smoking behavior, but only one participant thought it clearly played no role. However, most (n = 6) accepted the idea that genetics were important to others’ smoking behavior and most thought genetics could be important for cessation (n = 7). These findings are in contrast to other published reports, which indicate both higher and lower proportions of people who attribute smoking, at least in part, to genetics (Park et al., 2011; Wright et al., 2007).
Confidentiality was raised as a concern but not an apparent barrier to genetic testing for most (7/10) participants. All respondents were interested in genetic testing for smoking cessation. These findings are consistent with those reported by others (Park et al., 2011). Finally, all participants were receptive to phone-based counseling with supplemental written materials.
Phase 2: Pilot Trial Results
Study Population
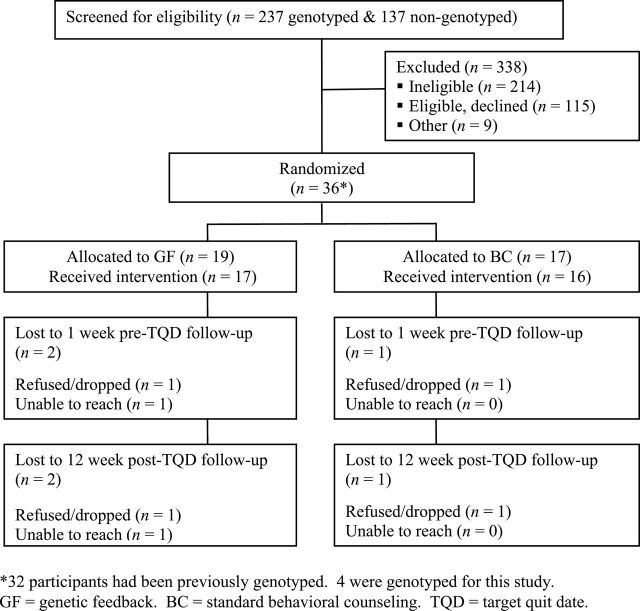
The recruitment flow is presented in Figure 1. Potential participants (n = 374) were screened for eligibility. Primary reasons for ineligibility were not smoking/not smoking enough (n = 139), currently using pharmacotherapy for smoking cessation (n = 125), and having an exclusionary psychiatric diagnosis (n = 48). Recruitment was heavily targeted to previously genotyped participants, as reflected in the higher enrollment of these participants (n = 32) compared with persons who were genotyped as part of the current study (n = 4). The final sample consisted of 36 participants, of whom 10 were A1/A1 or A1/A2 genotype (28%) and 26 were A2/A2 genotype (72%). Of the 10 smokers assigned to NRT patch, 7 had used it previously. Of the 26 assigned bupropion, 10 had used it previously.
Figure 1.
Recruitment flow for pilot trial.
Baseline characteristics are presented in Table 1. The two groups were similar in terms of their education, genetic literacy, numeracy, and other demographic and psychosocial characteristics, with the exception of a significant difference in baseline level of threat minimization. GF participants were less likely to minimize the threat of their smoking to their health.
Treatment Acceptability and Satisfaction
Acceptability and satisfaction were characterized using a variety of quantitative and qualitative measures. Dropout rates were similar across groups (Figure 1) and participants in each group appeared to be equally engaged in the intervention based on their participation in all three counseling calls (GF = 89.5%, BC = 94.1%) Both groups were moderately adherent to treatment as assessed by the Morisky scale (Morisky et al., 1986), although BC scores suggest slightly better adherence than among GF participants (see Table 2). Satisfaction with the interventionist also appeared similar based on ratings of trust, communication, treatment interest, and overall satisfaction (Table 3).
Table 3.
Psychological Outcomes Assessed at 1-Week Post-TQD Follow-Up
| Measure | Genetic feedback mean (sd) | Behavioral counseling mean (sd) | Genetic feedback effect (se) | T value | p value |
|---|---|---|---|---|---|
| Mood | |||||
| Depression (CES-D) | 4.1 (8.2) | 6.1 (7.8) | −2.9 (1.8) | −1.57 | .598 |
| Cognitive measures | |||||
| Fatalism | 0.4 (1.0) | 1.1 (1.8) | −0.23 (0.3) | 0.69 | .496 |
| Intent to quit | 19.5 (1.9) | 18.3 (2.9) | 1.1 (0.7) | 1.42 | .167 |
| Motivation | 6.4 (0.73) | 6.2 (1.0) | 0.14 (0.2) | 0.60 | .555 |
| Perceived control over quitting | 13.4 (3.4) | 14.0 (2.5) | −0.53 (0.97) | −0.55 | .589 |
| Risk perception | 16.1 (2.5) | 15.3 (2.8) | 0.15 (0.65) | 0.24 | .812 |
| Self-efficacy | 15.6 (2.6) | 16.2 (2.5) | −1.05 (0.76) | −1.38 | .177 |
| Threat minimization | 7.3 (3.6) | 8.9 (2.9) | −0.44 (1.23) | −0.36 | .724 |
Note. Regression analyses of genetic feedback vs. standard behavioral counseling. CES-D = Center for Epidemiological Studies Depression Scale; SD = standard deviation; SE = standard error; TQD = target quit date. Analyses were adjusted for age and the interaction of age and group.
Similar themes emerged in the qualitative interviews. All participants in both groups reported that the calls, the counselor, and materials were helpful. When asked what, if anything, did not go well in the participants’ opinion, two respondents in the BC group and one in the GF group wished there had been more counseling calls. When asked about recommendations for improving the acceptability of treatment, the only specific recommendations were to include more calls, to “give more information” and to provide “more simplistic information about the treatment.” The latter two comments came from the GF group. None of the participants in either group expressed ideations of fatalism, genetic determinism, or privacy concerns.
Logistical Feasibility
Study staff did not identify any barriers to treatment using the telephone-based GF or BC protocols. Biospecimen collection and genotyping went smoothly for the four smokers who had not been previously genotyped. The time between mailing biospecimen kits and receiving participants’ genotype ranged from 20 to 69 days, largely reflecting individual differences in the speed with which samples were returned to the lab. Once received at the lab, biospecimens were analyzed within at least 2 weeks. Results suggest that the number needed to recruit previously genotyped patients (7.4 screened for every one enrolled) was markedly lower than the number needed to recruit each smoker among those who had not been previously genotyped (68.5).
Psychological Impact
There was no evidence of adverse psychological effects caused by the GF. Groups reported similar levels of depression, self-efficacy for quitting, intention to quit, motivation to quit, fatalism about smoking, and perceived control over quitting at follow-up (Table 3).
Conclusions
Results from the Phase 1 formative interviews and the Phase 2 pilot study demonstrated the feasibility and acceptability of offering a comprehensive, genetically tailored intervention for smoking cessation. Smokers interviewed in Phase 1 were all interested in the option of genetically tailored treatment and participants in both phases had adequate genetic literacy for the intervention. Phase 2 pilot participants further found the intervention to be acceptable, as evidenced by their level of participation and satisfaction ratings. Despite disparities in recruitment between participants who had been previously genotyped and those who had not, the intervention was feasible to implement in our health care setting. Finally, there was no evidence of adverse psychological impacts on any of the qualitative or quantitative outcomes assessed. These results may not generalize to all smokers or settings but bode favorably for delivering pharmacogenetic interventions to insured health plan members in the future.
A number of lessons can be gleaned from this formative work to inform future research and treatment development efforts. For one, recruitment is clearly easier among smokers with biobanked data. Our data suggest that access to these data may facilitate future research or provision of pharmacogenetic treatment. It is unclear why smokers who had not been previously biobanked were less like to participate in this pilot trial, but this could reflect a selection bias since these participants had participated in prior research and, therefore, may have been more willing to participate in clinical trials; it could reflect the increased barrier to enrollment inherent in requiring smokers to first provide a biospecimen for genetic analysis; or it could reflect differences in the perceived benefit of participating in this study. Unfortunately, we cannot determine the degree to which possible selection bias accounted for differences in recruitment feasibility from our data, but the proportion of eligible smokers who refused participation was notably greater among those requiring genotyping (56%) than those for whom genotypes were already known (22%).
The results also demonstrate the ability to deliver a personalized medicine program without face-to-face contact. In our pilot trial, all counseling was done by phone and the biospecimen collection and medication delivery were done by mail. By centralizing these processes, it is possible to expand intervention reach on a population level. For example, based on our experience, it may be feasible to integrate a personalized medicine protocol into the structure of tobacco quitlines, such as those commonly offered in health plans or through state-sponsored programs.
Finally, it is notable that the GF did not appear to have any adverse outcomes on smokers’ motivation, self-efficacy, fatalism, treatment participation, treatment adherence, or other key psychological and behavioral outcomes of interest. Although not definitive, the results provide encouraging preliminary data in support of providing patients with pharmacogenetically tailored treatments for smoking cessation in the future.
Strengths and Limitations
The current study has a number of strengths that include its use of a mixed-methods study design, novel approach to treatment, and development of a replicable, patient-centered, treatment protocol for delivering a genetically tailored intervention to smokers. The major limitation of this study is the small sample size, as is the nature with all exploratory pilot trials; however, greater confidence can be placed in the results based on the alignment between qualitative and quantitative outcomes of interest. Additionally, all participants were volunteer research participants who were willing to be informed of their genotype. As such, the results may not generalize to all smokers. In fact, both genetic literacy and interest in genetic testing can vary depending on the sample of interest (Park et al., 2011; Wright et al., 2007).
Future Directions
Since the design and launch of the current study, a number of notable changes have occurred, including the use of predefined panels of thousands of polymorphisms identified from genome-wide association studies to predict “quit success” (Uhl, Drgon, Johnson, & Rose, 2009; Uhl et al., 2008), emergence of other, more efficacious pharmacological treatments with pharmacogenetic signals (e.g., varenicline and combination bupropion/NRT; King et al., 2012; Sarginson et al., 2011; Swan et al., 2012), and Internet-based, direct-to-consumer genotyping services offering pharmacogenomic advice. As a result, the single gene, single polymorphism approach to tailoring treatment that was used in this pilot trial may not be relevant to the future of personalized medicine. Nevertheless, the basic intervention framework established in this trial will remain relevant, and the lack of observable adverse psychological outcomes is somewhat reassuring as we seek to design future intervention studies of this kind.
Funding
This project was funded by the National Institute on Drug Abuse (NIDA; grant DA-027331, David, PI). Dr. David received additional support from DA017441 and the National Institute of General Medical Sciences grant (GM061374). Dr. Munafo greatly acknowledges support for his time from the UK Centre for Tobacco Control Studies, a UKCRC Public Health Research: Centre of Excellence and funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration. Finally, thanks to Group Health Cooperative for providing all study medication and pharmacy support for the randomized pilot trial.
Declaration of Interests
Drs. McClure, Munafò, Bergen, Fauver, and Niaura and Mss. Nishita and St. John have no relevant competing interests to declare. Dr. David has consulted for a one-day Pfizer-sponsored conference on behavioral treatments for smoking cessation and is a Scientific Advisor to Genophen, Inc. Dr. Swan consulted for one day to Pfizer to review data on varenicline. Dr. Javitz has conducted research sponsored by Pfizer, SmithKline Beecham, CV Therapeutics, Biogen, Berlex Laboratories, Johnson & Johnson, Ciba-Geigy, Angiotech, Merck & Co., Eli Lilly, and the Pharmaceutical Researchers and Manufacturers Association.
Acknowledgments
The authors would like to thank Dr. Alison Wright from King’s College, London, UK, for provision of prototypes of genetic feedback patient education materials, Dr. Debra Roter from the Johns Hopkins School of Public Health, Drs. Elyse Park and Nancy Rigotti from Harvard Medical School, and Drs. Rochelle and Rosen Belinda Borrelli from the Warren Alpert Medical School of Brown University for their assistance with the study design and expert panel participation. Additional thanks to Chester Pabiniak, Lisa Shulman, and Amy Mohelnitzky at Group Health Research Institute for their assistance implementing the study activities. Finally, thanks to Dr. Gary Swan for providing access to study participants from CA 071358 (Swan, PI) and access to existing genotype data on these individuals from U01 DA 020830 (Benowitz, PI).
References
- Allison M. (2008). Is personalized medicine finally arriving? Nature Biotechnology 26 509–517doi:10.1038/nbt0508–509 [DOI] [PubMed] [Google Scholar]
- Altman R. B. (2011). Pharmacogenomics: “Noninferiority” is sufficient for initial implementation Clinical Pharmacology and Therapeutics 89 348–350doi:10.1038/clpt.2010.310 [DOI] [PubMed] [Google Scholar]
- Baars M. J., Henneman L., Ten Kate L. P. (2005). Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: A global problem Genetics in Medicine 7 605–610doi:0.1097/01.gim.0000182895.28432.c7 [DOI] [PubMed] [Google Scholar]
- Breitling L. P., Müller H., Illig T., Rujescu D., Winterer G., Dahmen N, Brenner H., et al. (2011). Dopamine-related genes and spontaneous smoking cessation in ever-heavy smokers Pharmacogenomics 12 1099–1106 doi:10.2217/pgs.11.74 [DOI] [PubMed] [Google Scholar]
- Collier R. (2012). Genetic literacy poor in primary care CMAJ: Canadian Medical Association Journal 184 E467–E468doi:10.1503/cmaj.109-4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Green E. D., Guttmacher A. E., Guyer M. S.; U.S. National Human Genome Research Institute (2003). A vision for the future of genomics research Nature 422 835–847doi:10.1038/nature01626 [DOI] [PubMed] [Google Scholar]
- David S. P., Brown R. A., Papandonatos G. D., Kahler C. W., Lloyd-Richardson E. E., Munafò M. R., et al. (2007). Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation Nicotine & Tobacco Research 9 821–833doi:10.1080/14622200701382033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S. P., Gramling R. (2003). Teaching genetics in primary care through a transatlantic video conference Nature Reviews Genetics 4–2doi:10.1038/nrg845-c1 [Google Scholar]
- David S. P., Johnstone E. C., Churchman M., Aveyard P., Murphy M. F., Munafò M. R. (2011). Pharmacogenetics of smoking cessation in general practice: Results from the patch II and patch in practice trials Nicotine & Tobacco Research 13 157–167doi:10.1093/ntr/ntq246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S. P., Johnstone E. C., Murphy M. F., Aveyard P., Guo B., Lerman C., Munafò M. R. (2008). Genetic variation in the serotonin pathway and smoking cessation with nicotine replacement therapy: New data from the Patch in Practice trial and pooled analyses Drug and Alcohol Dependence 98 77–85doi:10.1016/j.drugalcdep.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S. P., Strong D. R., Munafò M. R., Brown R. A., Lloyd-Richardson E. E., Wileyto P. E., Niaura R., et al. (2007). Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: Analysis of pooled data from two clinical trials Nicotine & Tobacco Research 9 1251–1257doi:10.1080/14622200701705027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. E. (2009). Personalized medicine: Boon or budget-buster? The Annals of Pharmacotherapy 43 958–962doi:10.1345/aph.1L563 [DOI] [PubMed] [Google Scholar]
- Erby L. H., Roter D., Larson S., Cho J. (2008). The rapid estimate of adult literacy in genetics (REAL-G): A means to assess literacy deficits in the context of genetics American Journal of Medical Genetics. Part A 146A 174–181doi:10.1002/ajmg.a.32068 [DOI] [PubMed] [Google Scholar]
- Fiore M. C., Jaen C. R., Baker T. B., Bailey W. C., Benowitz N. L., Curry S. J., Wewers M. E., et al. (2008). Treating tobacco use and dependence: 2008 update Rockville, MD: U.S. Department of Health and Human Services; [Google Scholar]
- Gold A. B., Lerman C. (2012). Pharmacogenetics of smoking cessation: Role of nicotine target and metabolism genes Human Genetics, 131, 857–876. doi:10.1007/s00439-012-1143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith L., Jackson L., O’Connor A., Skirton H. (2012). Direct-to-consumer genomic testing: Systematic review of the literature on user perspectives European Journal of Human Genetics, 20, 811–816. doi:10.1038/ejhg.2012.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg M. A., Collins F. S. (2010). The path to personalized medicine The New England Journal of Medicine 363 301–304doi:10.1056/NEJMp1006304 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire British Journal of Addiction 86 1119–1127doi:10.1111/j.1360-0443. 1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Keely J. P., Niaura R. S., Ossip-Klein D. J., Richmond R. L., Swan G. E. (2003). Measures of abstinence in clinical trials: Issues and recommendations Nicotine & Tobacco Research 5 13–25doi:10.1093/ntr/5.1.13 [PubMed] [Google Scholar]
- Hukkanen J., Jacob P., 3rd, Benowitz N. L. (2005). Metabolism and disposition kinetics of nicotine Pharmacological Reviews 57 79–115doi:10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- Johnstone E., Brown K., Saunders C., Roberts K., Drury M., Walton R., Murphy M. (2004). Level of nicotine replacement during a quit-smoking attempt Nicotine & Tobacco Research 6 377–379doi:10.1080/14622200410001676440 [DOI] [PubMed] [Google Scholar]
- Johnstone E. C., Yudkin P. L., Hey K., Roberts S. J., Welch S. J., Murphy M. F., et al. (2004). Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch Pharmacogenetics 14 83–90doi:10.1097/00008571-200402000-00002 [DOI] [PubMed] [Google Scholar]
- King D. P., Paciga S., Pickering E., Benowitz N. L., Bierut L. J., Conti D. V., et al. (2012). Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials Neuropsychopharmacology 37 641–650doi:10.1038/npp.2011.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortmann G. L., Dobler C. J., Bizarro L., Bau C. H. (2010). Pharmacogenetics of smoking cessation therapy American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 153B 17–28doi:10.1002/ajmg.b.30978 [DOI] [PubMed] [Google Scholar]
- Kroenke K., Strine T. W., Spitzer R. L., Williams J. B., Berry J. T., Mokdad A. H. (2009). The PHQ-8 as a measure of current depression in the general population Journal of Affective Disorders 114 163–173doi:10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Lerman C., Shields P. G., Wileyto E. P., Audrain J., Hawk L. H., Jr, Pinto A., et al. (2003). Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial Health Psychology 22 541–548doi:10.1037/0278-6133.22.5.541 [DOI] [PubMed] [Google Scholar]
- Marteau T. M., Aveyard P., Munafò M. R., Prevost A. T., Hollands G. J., Armstrong D, Kinmonth A. L., et al. (2012). Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: A randomised controlled trial PLoS ONE 7 e35249doi:10.1371/journal.pone.0035249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau T. M., Weinman J. (2006). Self-regulation and the behavioural response to DNA risk information: A theoretical analysis and framework for future research Social Science & Medicine 62 1360–1368doi:10.1016/j.socscimed.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Morisky D. E., Green L. W., Levine D. M. (1986). Concurrent and predictive validity of a self-reported measure of medication adherence Medical Care 24 67–74doi:10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- Park E. R., Green I., Rakowski W., Ostroff J., Perry K., Rigotti N. (2007). Risk perceptions among participants of the national lung cancer screening trial Annals of Behavioral Medicine, 33(Suppl.), S138doi:10.1007/s12160-009-9112-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. R., Kleimann S., Youatt E. J., Lockhart A., Campbell E. G., Levy D. E, Shields A. E. (2011). Black and White adults’ perspectives on the genetics of nicotine addiction susceptibility Addictive Behaviors 36 769–772doi:10.1016/j.addbeh.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe B. D. (1995). Fatalism among elderly African Americans. Effects on colorectal cancer screening Cancer Nursing 18 385–392doi:10.1097/00002820-199510000-00008 [PubMed] [Google Scholar]
- Quaak M., Smerecnik C., van Schooten F. J., de Vries H., van Schayck C. P. (2012). Knowledge, attitudes and preferences regarding genetic testing for smoking cessation. A cross-sectional survey among Dutch smokers BMJ Open 2 e000321doi:10.1136/bmjopen-2011-000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population Applied Psychological Measurement 1 385–401doi:10.1177/014662167700100306 [Google Scholar]
- Sarginson J. E., Killen J. D., Lazzeroni L. C., Fortmann S. P., Ryan H. S., Schatzberg A. F., Murphy G. M., Jr (2011). Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 156B 275–284doi:10.1002/ajmg.b.31155 [DOI] [PubMed] [Google Scholar]
- Schneider J., Kaplan S. H., Greenfield S., Li W., Wilson I. B. (2004). Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection Journal of General Internal Medicine 19 1096–1103doi:10.1111/j.1525-1497.2004.30418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M., Woloshin S., Black W. C., Welch H. G. (1997). The role of numeracy in understanding the benefit of screening mammography Annals of Internal Medicine 127 966–972 [DOI] [PubMed] [Google Scholar]
- Secretary’s Advisory Committee on Genetics Health and Society (2008). Realizing the promise of pharmacogenomics: Opportunities and challenges—Report of the secretary’s advisory committee on genetics, health, and society Washington, DC: Department of Health and Human Services. [Google Scholar]
- Stapleton J. A., Sutherland G., O’Gara C., Spirling L. I., Ball D. (2011). Association between DRD2/ANKK1 Taq1A genotypes, depression and smoking cessation with nicotine replacement therapy Pharmacogenetics and Genomics 21 447–453doi:10.1097/FPC.0b013e328347473a [DOI] [PubMed] [Google Scholar]
- Suther S., Goodson P. (2003). Barriers to the provision of genetic services by primary care physicians: A systematic review of the literature Genetics in Medicine 5 70–76doi:10.1097/01.GIM.0000055201.16487.61 [DOI] [PubMed] [Google Scholar]
- Swan G. E., Jack L. M., Valdes A. M., Ring H. Z., Ton C. C., Curry S. J., & McAfee T. (2007). Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR Health Psychology 26 361–368doi:10.1037/0278-6133.26.3.361 [DOI] [PubMed] [Google Scholar]
- Swan G. E., Javitz H. S., Jack L. M., Wessel J., Michel M., Hinds D. A., Bergen A.W., et al. (2012). Varenicline for smoking cessation: Nausea severity and variation in nicotinic receptor genes Pharmacogenomics Journal, 12, 349–358. doi:10.1038/tpj.2011.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan G. E., McClure J. B., Jack L. M., Zbikowski S. M., Javitz H. S., Catz S. L., et al. (2010). Behavioral counseling and varenicline treatment for smoking cessation American Journal of Preventive Medicine 38 482–490doi:10.1016/j.amepre.2010.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G. R., Drgon T., Johnson C., Rose J. E. (2009). Nicotine abstinence genotyping: Assessing the impact on smoking cessation clinical trials The Pharmacogenomics Journal 9 111–115doi:10.1038/tpj.2008.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G. R., Drgon T., Johnson C., Walther D., David S. P., Aveyard P., Munafò M. R. (2010). Genome-wide association for smoking cessation success: Participants in the Patch in Practice trial of nicotine replacement Pharmacogenomics 11 357–367doi:10.2217/pgs.09.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G. R., Liu Q. R., Drgon T., Johnson C., Walther D., Rose J. E., Lerman C, et al. (2008). Molecular genetics of successful smoking cessation: Convergent genome-wide association study results Archives of General Psychiatry 65 683–693doi:10.1001/archpsyc.65.6.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. J., Aveyard P., Guo B., Murphy M., Brown K., Marteau T. M. (2007). Is attributing smoking to genetic causes associated with a reduced probability of quit attempt success? A cohort study Addiction (Abingdon, England) 102 1657–1664doi:10.1111/j.1360-0443.2007.01937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. J., French D. P., Weinman J., Marteau T. M. (2006). Can genetic risk information enhance motivation for smoking cessation? An analogue study Health Psychology 25 740–752doi:10.1037/0278-6133.25.6.740 [DOI] [PubMed] [Google Scholar]
- Yudkin P., Hey K., Roberts S., Welch S., Murphy M., Walton R. (2003). Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch BMJ 327 28–29doi:10.1136/bmj.327.7405.28 [DOI] [PMC free article] [PubMed] [Google Scholar]