Abstract
Introduction:
We recently reported that certain amounts of the carcinogen N′-nitrosonornicotine (NNN) can be formed endogenously from nicotine and/or nornicotine in some users of oral nicotine replacement therapy products. Although the acidic environment of the stomach creates the most favorable conditions for nitrosation, this reaction could also occur in the oral cavity in the presence of bacteria that catalyze nitrosation at neutral pH.
Methods:
To test the hypothesis that endogenous formation of NNN could occur in the oral cavity, we investigated nitrosation of nicotine and nornicotine in human saliva. To specifically identify NNN as derived from precursors added to saliva, we incubated saliva samples with [pyridine-D4]nicotine and [pyridine-D4]nornicotine, with and without the addition of nitrite, and subsequently analyzed [pyridine-D4]NNN by liquid chromatography–tandem mass spectrometry.
Results:
Consistent with kinetic studies on nicotine and nornicotine nitrosation, incubation of saliva with [pyridine-D4]nornicotine alone produced detectable amounts of [pyridine-D4]NNN, whereas only traces of [pyridine-D4]NNN were found in samples incubated with [pyridine-D4]nicotine and sodium nitrite. Incubation of saliva samples from 10 nonsmoking volunteers with [pyridine-D4]nornicotine resulted in the formation of [pyridine-D4]NNN in 8 samples, with yields ranging from 0.003% to 0.051% of the added alkaloid.
Conclusion:
Our results demonstrate that NNN can be formed from nornicotine in human saliva without deliberate addition of any other substance. Therefore, nornicotine, as present in tobacco or in nicotine replacement products, is a carcinogen precursor.
Introduction
Tobacco use causes about one third of all cancer deaths in the United States and is the leading preventable cause of death among Americans (United States Department of Health and Human Services, 2004). The tobacco-specific nitrosamines N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) are among the most abundant and powerful carcinogens present in unburned tobacco and in smoke. Both NNN and NNK are formed from tobacco alkaloids during tobacco processing; therefore, human exposure to these carcinogens is believed to occur exclusively upon contact with tobacco products. However, we recently reported occasional significant increases in urinary NNN biomarkers in some users of oral nicotine replacement therapy (NRT) products such as nicotine gum or lozenge, compared with baseline smoking levels in the same subjects (Stepanov, Carmella, Briggs, et al., 2009; Stepanov, Carmella, Han, et al., 2009). NNN is believed to play an important role in the induction by tobacco products of cancers of the esophagus and oral cavity (Hecht, 1998), and along with NNK is classified by the International Agency for Research on Cancer as carcinogenic to humans (International Agency for Research on Cancer, 2007). We hypothesized that the observed occasional increases in urinary biomarkers of NNN in some NRT users are due to endogenous nitrosation of nicotine and/or nornicotine, the latter being metabolically formed from nicotine or originally present in NRT products. Furthermore, previous data suggest that endogenous formation of NNN might occur in some smokers (Stepanov, Carmella, Briggs, et al., 2009). This could contribute to the large interindividual variation in levels of urinary NNN biomarkers among smokers and to the remarkably strong association between the levels of urinary NNN biomarkers and risk of esophageal cancer in smokers (Yuan et al., 2011).
In rats, treatment with nicotine or nornicotine and sodium nitrite resulted in endogenous formation of NNN (Carmella, Borukhova, Desai, & Hecht, 1997; Porubin, Hecht, Li, Gonta, & Stepanov, 2007). In humans, endogenous formation of N-nitrosamines occurs through the reaction of dietary precursors with nitrosating agents supplied by diet (Bartsch, Ohshima, Pignatelli, & Calmels, 1989; Marletta, 1988; Mirvish, 1995; Shepard, Schlatter, & Lutz, 1987). Saliva of oral NRT users and smokers contain nicotine and potentially nornicotine (Rose, Levin, & Benowitz, 1993), as well as nitrite (Granli, Dahl, Brodin, & Bockman, 1989; Marletta, 1988). While the acidic environment of the stomach creates the most favorable conditions for NNN synthesis from the precursors delivered with the swallowed saliva (Mirvish, 1975), this reaction can also occur in the oral cavity in the presence of bacteria that catalyze nitrosation at neutral pH (Jiebarth, Spiegelhalder, & Bartsch, 1997). Thus, some studies indicated that additional amounts of NNN could be formed in saliva of smokeless tobacco users (Hoffmann & Adams, 1981).
To test the hypothesis that endogenous formation of NNN can occur in the oral cavity of NRT or tobacco users, we investigated nitrosation of nicotine and nornicotine in human saliva. To specifically identify NNN as derived from the precursors added to saliva, we used [pyridine-D4]nicotine and [pyridine-D4]nornicotine and subsequently analyzed [pyridine-D4]NNN by liquid chromatography–tandem mass spectrometry (LC-MS/MS).
Materials and Methods
Chemicals
[Pyridine-D4]nicotine, [pyridine-D4]nornicotine, and [pyridine- D4]N′-nitrosonornicotine ([pyridine-D4]NNN) were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada), and [2,2′,3,4,5,6-13C]NNN ([13C6]NNN) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). All other chemicals were obtained from Fisher Scientific (Pittsburgh, PA).
Subjects
Nonsmoking volunteers were recruited at the Masonic Cancer Center, University of Minnesota, and were asked to collect 2–5ml of their saliva via expectoration into sterile polypropylene tubes. All subjects were Caucasian, between 23 and 46 years old, and 6 out of 10 were male. Collection of samples was approved by the University of Minnesota Research Subjects’ Protection Programs Institutional Review Board: Human Subjects Committee.
Saliva Incubation and [pyridine-D4]NNN Analysis
Freshly collected saliva was placed on ice and treated within 1h of collection. As an initial test of nitrosation in saliva, 1ml aliquots of pooled saliva from three nonsmokers were incubated for 30min at 37 °C with either [pyridine-D4]nicotine or [pyridine-D4]nornicotine, with and without addition of sodium nitrite. The reaction was stopped by placing samples on ice and adding 20 µl 10 N NaOH (Mirvish, Wallcave, Eagen, & Shubik, 1972; Rao & McLennon, 1977). After addition of [13C6]NNN (internal standard), samples were promptly loaded on ChemElut cartridges and eluted with methylene chloride. This was followed by purification on Oasis MCX cartridges and BondElut cartridges as previously described for NNN analysis in urine (Stepanov, Carmella, Briggs, et al., 2009). The purified samples were analyzed for [pyridine-D4]NNN by LC-MS/MS as previously described (Stepanov & Hecht, 2008), with selected reaction monitoring for m/z 182 → m/z 152 for [pyridine-D4]NNN and m/z 184 → m/z 154 for [13C6]NNN.
Analysis of Nornicotine in Nicotine Gum and Lozenge
Nornicotine content in nicotine gum (Nicorette, 4mg nicotine) and lozenges (Commit, 2 and 4mg nicotine) was determined by gas chromatography–mass spectrometry as previously described (Stepanov, Jensen, Hatsukami, & Hecht, 2008).
Results
Optimization of Sample Purification
Various procedures for the postincubation sample purification were tested. Limiting sample purification to a single-step extraction with methylene chloride and concentration of the organic extract to dryness under a stream of N2 led to ion suppression—a phenomenon that affects analyte ionization causing a loss in response by the mass analyzer—and artifactual formation of [pyridine-D4]NNN. Ultimately, we subjected samples to the procedure described in the Materials and Methods section. No significant ion suppression was observed with this method. Because the procedure includes acidic conditions during MCX purification, which is favorable for nitrosation reactions, we conducted an additional experiment to test for potential artifactual formation of [pyridine-D4]NNN during sample processing. A saliva sample was split into two aliquots, and [pyridine-D4]nornicotine was added to one of the samples (aliquot A) prior to its incubation as described and to the other one (aliquot B) after its elution from ChemElut cartridges, prior to drying and acidification for the MCX step. Only a trace amount of [pyridine-D4]NNN was detected in aliquot B, comprising 0.6% of the [pyridine-D4]NNN yield in aliquot A.
Saliva Incubation with [Pyridine-D4]nicotine and [pyridine-D4]Nornicotine
Typical LC-MS/MS chromatograms obtained upon analysis of saliva incubated under various conditions are presented in Figure 1. [Pyridine-D4]NNN was not detected in the blank saliva aliquot that was used as a negative control (Figure 1A). Incubation of saliva with 50ng (0.33 nmol) of [pyridine-D4]nornicotine alone produced 38 pg (0.21 pmol) [pyridine-D4]NNN or 0.06% yield. Increasing the amount of [pyridine-D4]nornicotine to 200ng and adding 0.7mg of sodium nitrite produced 5.5ng [pyridine-D4]NNN, a nearly 40-fold increase in yield. Only traces of [pyridine-D4]NNN were detected in samples incubated with [pyridine-D4]nicotine.
Figure 1.
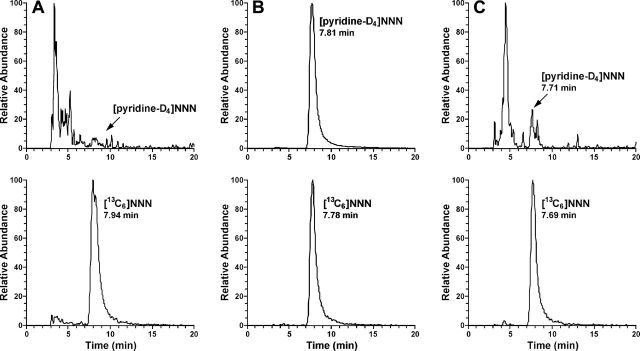
Examples of liquid chromatography–tandem mass spectrometry chromatograms obtained upon analysis of saliva incubated under various conditions: (A) Blank saliva (negative control), (B) saliva incubated with 50ng [pyridine-D4]nornicotine, and (C) saliva incubated with 5 µg [pyridine-D4]nicotine and 0.7mg NaNO2.
[Pyridine-D4]NNN Formation in Saliva of Nonsmoking Volunteers
Individual saliva samples from 10 nonsmoking volunteers were incubated with deuterium-labeled nicotine and nornicotine and analyzed for [pyridine-D4]NNN. The results are summarized in Table 1. Formation of [pyridine-D4]NNN was observed in 8 samples treated with [pyridine-D4]nornicotine, yields ranging from 0.003% to 0.051% of the added alkaloid.
Table 1.
Formation of [pyridine-D4]NNN Upon Incubation of Nonsmokers’ Saliva With [pyridine-D4]nornicotinea
| Subject | [pyridine-D4]NNN formed, pg | % yield from [pyridine-D4] nornicotine | ||
|---|---|---|---|---|
| 1 | 3.20 | 0.005 | ||
| 2 | LODb | N/Ac | ||
| 3 | 4.29 | 0.007 | ||
| 4 | LOD | N/A | ||
| 5 | 7.25 | 0.012 | ||
| 6 | 30.26 | 0.051 | ||
| 7 | 2.25 | 0.004 | ||
| 8 | 2.08 | 0.003 | ||
| 9 | 6.75 | 0.011 | ||
| 10 | 7.85 | 0.013 |
aSaliva samples from individual nonsmokers were incubated with 50ng (0.33 nmol) [pyridine-D4]nornicotine for 30min at 37°C.
bLOD, below the limit of detection (0.001 pmol [pyridine-D4]NNN).
cN/A = not applicable.
Nornicotine Analysis in Nicotine Lozenge and Gum
Commit lozenge (2mg nicotine) contained 4.4 µg nornicotine/piece; Commit lozenge (4mg nicotine) contained 5.5 µg nornicotine/piece; and Nicorette gum (4mg nicotine) contained 9.5 µg nornicotine/piece. The amount of nornicotine averaged 0.2% of nicotine content in these products.
Discussion
This study demonstrates for the first time that the minor tobacco alkaloid nornicotine can be easily nitrosated in human saliva to form NNN, a potent carcinogenic nitrosamine. Nornicotine is present in tobacco and cigarette smoke, and, as demonstrated here, in oral NRT products such as nicotine gum and lozenge. Nitrate is present in human saliva and is converted by oral microflora to nitrite. The findings of this study support our previous observation that NNN can be formed endogenously in users of oral NRT products and potentially in smokers and smokeless tobacco users.
The use of deuterium-labeled precursors allowed us to specifically identify NNN as formed from the precursors added to saliva prior to incubation, thus eliminating concerns that NNN measured in saliva of our nonsmoking volunteers could have other sources, for example, exposure to secondhand smoke. We also took particular precautions to ensure that artifactual nitrosation did not take place after the incubation during sample preparation for LC-MS/MS analysis. The method used in this study is practically identical to the one that has been previously shown not to result in artifactual NNN formation (Stepanov & Hecht, 2005; Stepanov, Carmella, Briggs, et al., 2009).
Nitrosation of [pyridine-D4]nicotine during saliva incuba tion experiments was minimal, which is in agreement with kinetic studies showing that nornicotine is nitrosated to form NNN much more efficiently than nicotine (Mirvish, Sams, & Hecht, 1977). These results also suggest that nornicotine, and not nicotine, is the major precursor of endogenously synthesized NNN in some users of oral NRT products.
There was significant interindividual variation in the amount of [pyridine-D4]NNN formed upon incubation with [pyridine-D4]nornicotine of saliva collected from nonsmoking volunteers (Table 1). Endogenous formation of N-nitrosamines in humans can be greatly affected by various dietary and host factors. For example, ascorbic acid and vitamin E inhibit endogenous nitrosation in humans, whereas some phenolic compounds can both inhibit and catalyze N-nitroso compound formation, depending on a variety of factors (Bartsch, Ohshima, & Pignatelli, 1988). The time of nicotine or nornicotine contact with nitrosating agents relative to the time of food consumption is also critical: under fasting conditions, the concentration of nitrite in saliva varies between 10 and 1000 µmol/L, increasing 2- to 5-fold for several hours after the ingestion of nitrate-containing food (McColl, 2005). In addition, certain oral microflora can catalyze nitrosation reactions at neutral pH (Jiebarth et al., 1997), and the use of antiseptic mouthwash reduces endogenous nitrosamine formation by 62%–74% (Shapiro, Hotchkill, & Roe, 1991). The potential role of oral microflora in the formation of [pyridine-D4]NNN in this study is supported by the previous finding that NNN formation does not occur upon urine incubation with nornicotine (Stepanov, Carmella, Briggs, et al., 2009). Although the effect of diet, oral health status, and other factors on the salivary synthesis of [pyridine-D4]NNN is outside the scope of this report, the observed interindividual variation in the capacity for oral nitrosation is in agreement with our previous observation that only some oral NRT users form NNN endogenously.
About 0.4%–0.8% of a nicotine dose is metabolized to nornicotine in humans (Benowitz, Jacob, Fong, & Gupta, 1994; Hukkanen, Jacob, & Benowitz, 2005), and it is possible that similar to nicotine, metabolically formed nornicotine could be concentrated in saliva (Rose et al., 1993). However, metabolism of nicotine to nornicotine, even though potentially a contributing factor in the endogenous NNN synthesis in NRT users, is not likely to be a major determinant of the effectiveness of this process. If endogenous formation of NNN depended on the enzyme-regulated metabolism of nicotine to nornicotine, significant interindividual, but not intraindividual, differences in urinary excretion of NNN would be observed in our previous studies (Stepanov, Carmella, Briggs, et al., 2009; Stepanov, Carmella, Han, et al., 2009).
The most likely sources of nornicotine in saliva are cigarette smoke or extraction from oral tobacco or NRT products. We here found that a single piece of nicotine gum or lozenge contains 100 to 200 times higher amount of nornicotine than the amount used in our incubation experiments. Users of nicotine gum extract up to 70% of its nicotine content (Benowitz, Jacob, & Savanapridi, 1987), and the same is likely true for nornicotine. Thus, chewing several pieces of nicotine gum per day for prolonged periods of time can potentially expose NRT users to significant amounts of orally synthesized NNN, depending on dietary habits, oral health status, and other factors. Conditions in the stomach are even more favorable for the nitrosation reactions (Mirvish, 1975). There are no data available on intragastric NNN synthesis in humans.
In summary, our results demonstrate that NNN can be formed in human saliva in the presence of nornicotine, supporting the hypothesis that NNN can be synthesized endogenously in humans. Oral synthesis of NNN could potentially contribute to the overall intake of this carcinogen by some smokers and smokeless tobacco users, affecting their risk of developing cancer. Removal of nornicotine from NRT products should be considered in order to protect consumers from being exposed to this potent carcinogen.
Funding
This work was supported by the National Cancer Institute [CA-81301] and the National Institutes of Health [DA-13333].
Declaration of Interests
There are no competing interests.
Acknowledgment
We thank Bob Carlson for editorial assistance.
References
- Bartsch H., Ohshima H., Pignatelli B. (1988). Inhibitors of endogenous nitrosation. Mechanisms and implications in human cancer prevention. Mutation Research 202 307–324 doi:10.1016/0027-5107(88)90194-7 [DOI] [PubMed] [Google Scholar]
- Bartsch H., Ohshima H., Pignatelli B., Calmels S. (1989). Human exposure to endogenous N-nitroso compounds: Quantitative estimates in subjects at high risk for cancer of the oral cavity, oesophagus, stomach and urinary bladder. Cancer Surveys 8 335–362 [PubMed] [Google Scholar]
- Benowitz N. L., Jacob P., III, Fong I., Gupta S. (1994). Nicotine metabolic profile in man: Comparison of cigarette smoking and transdermal nicotine. Journal of Pharmacology and Experimental Therapeutics 268 296–303 [PubMed] [Google Scholar]
- Benowitz N. L., Jacob P., III, Savanapridi C. (1987). Determinants of nicotine intake while chewing nicotine pola crilex gum. Clinical Pharmacology and Therapeutics 41 467–473 doi:10.1038/clpt.1987.58 [DOI] [PubMed] [Google Scholar]
- Carmella S. G., Borukhova A., Desai D., Hecht S. S. (1997). Evidence for endogenous formation of tobacco-specific nitrosamines in rats treated with tobacco alkaloids and sodium nitrite. Carcinogenesis 18 587–592 doi:10.1093/carcin/18.3.587 [DOI] [PubMed] [Google Scholar]
- Granli T., Dahl R., Brodin P., Bøckman O. C. (1989). Nitrate and nitrite concentrations in human saliva: Variations with salivary flow-rate. Food and Chemical Toxicology 27 675–680 doi:10.1016/0278-6915(89)90122-1 [DOI] [PubMed] [Google Scholar]
- Hecht S. S. (1998). Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology 11 559–603 doi:10.1021/tx980005y [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Adams J. D. (1981). Carcinogenic tobacco- specific N-nitrosamines in snuff and in the saliva of snuff dippers. Cancer Research 41 4305–4308 [PubMed] [Google Scholar]
- Hukkanen J., Jacob P., III, Benowitz N. L. (2005). Metabolism and disposition kinetics of nicotine. Pharmacological Reviews 57 79–115 doi:10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2007). Smokeless tobacco and tobacco-specific nitrosamines. In IARC monographs on the evaluation of carcinogenic risks to humans. (Vol. 89). Lyon, France: IARC; [PMC free article] [PubMed] [Google Scholar]
- Jiebarth D., Spiegelhalder B., Bartsch H. (1997). N-nitrosation of medicinal drugs catalysed by bacteria from human saliva and gastro-intestinal tract, including Helicobacter pylori. Carcinogenesis 18 383–389 doi:10.1093/carcin/18.2.383 [DOI] [PubMed] [Google Scholar]
- Marletta M. A. (1988). Mammalian synthesis of nitrite, nitrate, nitric oxide, and N-nitrosating agents. Chemical Research in Toxicology 1 249–257 doi:10.1021/tx00005a001 [DOI] [PubMed] [Google Scholar]
- McColl K. E. L. (2005). When saliva meets acid: Chemical warfare at the oesophagogastric junction. Gut 54 1–3 doi:10.1136/gut.2004.047126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirvish S. S. (1975). Formation of N-nitroso compounds: Chemistry, kinetics, and in vivo occurrence. Toxicology and Applied Pharmacology 31 325–351 doi:10.1016/0041-008X(75)90255-0 [DOI] [PubMed] [Google Scholar]
- Mirvish S. S. (1995). Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Letters 93 17–48 doi:10.1016/0304-3835(95)03786-V [DOI] [PubMed] [Google Scholar]
- Mirvish S. S., Sams J., Hecht S. S. (1977). Kinetics of nornicotine and anabasine nitrosation in relation to N′-nitrosonornicotine occurrence in tobacco and to tobacco-induced cancer. Journal of the National Cancer Institute 59 1211–1213 [DOI] [PubMed] [Google Scholar]
- Mirvish S. S., Wallcave L., Eagen M., Shubik P. (1972). Ascorbate-nitrite reaction: Possible means of blocking the formation of carcinogenic N-nitroso compounds. Science (New York, N.Y.) 177 65–67 doi:10.1126/science.177.4043.65 [DOI] [PubMed] [Google Scholar]
- Porubin D., Hecht S. S., Li Z. Z., Gonta M., Stepanov I. (2007). Endogenous formation of N′-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. Journal of Agricultural and Food Chemistry 55 7199–7204 doi:10.1021/jf0712191 [DOI] [PubMed] [Google Scholar]
- Rao G. S., McLennon D. A. (1977). High-pressure liquid chromatographic analysis of carcinogenic N-nitroso derivatives of piperazine resulting from drug-nitrite interactions. Journal of Analytical Toxicology 1 43–45 [Google Scholar]
- Rose J. E., Levin E. D., Benowitz N. (1993). Saliva nicotine as an index of plasma levels in nicotine skin patch users. Therapeutic Drug Monitoring 15 431–435 doi:10.1097/ 00007691-199310000-00012 [DOI] [PubMed] [Google Scholar]
- Shapiro K. B., Hotchkill J. H., Roe D. A. (1991). Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food and Chemical Toxicology 29 751–755 doi:10.1016/0278-6915(91)90183-8 [DOI] [PubMed] [Google Scholar]
- Shepard S. E., Schlatter C., Lutz W. K. (1987). Assessment of the risk of formation of carcinogenic N-nitroso compounds from dietary precursors in the stomach. Food and Chemical Toxicology 25 91–108 doi:10.1016/0278-6915(87)90311-5 [DOI] [PubMed] [Google Scholar]
- Stepanov I., Carmella S. G., Briggs A., Hertsgaard L., Lindgren B., Hatsukami D., Hecht S. S. (2009a). Presence of the carcinogen N′-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Research 69 8236–8240 doi:10.1158/0008-5472.CAN-09-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Carmella S. G., Han S., Pinto A., Strasser A. A., Lerman C., Hecht S. S. (2009b). Evidence for endogenous formation of N′-nitrosonornicotine in some long term nicotine patch users. Nicotine & Tobacco Research 11 95–105 doi:10.1093/ntr/ntn004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Hecht S. S. (2005). Tobacco-specific nitrosamines and their N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiology, Biomarkers & Prevention 14 885–891 doi:10.1158/1055–9965.EPI-04-0753 [DOI] [PubMed] [Google Scholar]
- Stepanov I., Hecht S. S. (2008). Detection and quantitation of N′-nitrosonornicotine in human toenails by liquid chromatography-electrospray ionization-tandem mass spectrometry. Cancer Epidemiology, Biomarkers & Prevention 17 945–948 doi:10.1158/1055–9965.EPI-07-2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Jensen J., Hatsukami D., Hecht S. S. (2008). New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine & Tobacco Research 10 1773–1782 doi:10.1080/14622200802443544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2004). The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general Washington, DC: U.S. Government Printing Office; [Google Scholar]
- Yuan J. M., Knezevich A. D., Wang R., Gao Y. T., Hecht S. S., Stepanov I. (2011). Urinary levels of the tobacco-specific carcinogen N′-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 32 1366–1371 doi:10.1093/carcin/bgr125 [DOI] [PMC free article] [PubMed] [Google Scholar]


