Any two people on Earth may be connected by a chain of ‘a friend of a friend’ in six steps or less, dubbed as the ‘six degrees of separation’. In the world of plant systems, the degree of separation between the signaling ‘pathways’ of photoreceptor phytochromes and growth regulator phytohormones is smaller than previously expected, according to the recent studies. Plants control their growth by simultaneously sensing and responding to both the external environmental signals and the internal developmental signals. Taking the well-studied light inhibition of hypocotyl elongation of young seedlings as an example, this response is controlled by both the red/far-red light receptor phytochromes and phytohormones, including auxin, cytokinins (CK), gibberellins (GA), brassinosteriods (BR), ethylene, and Abscisic acid (ABA). How phytochromes and phytohormones interact to control growth has been a subject of plant physiology studies for decades (Neff et al., 2006), and it appears to be a favored subject for the systems biology studies nowadays.
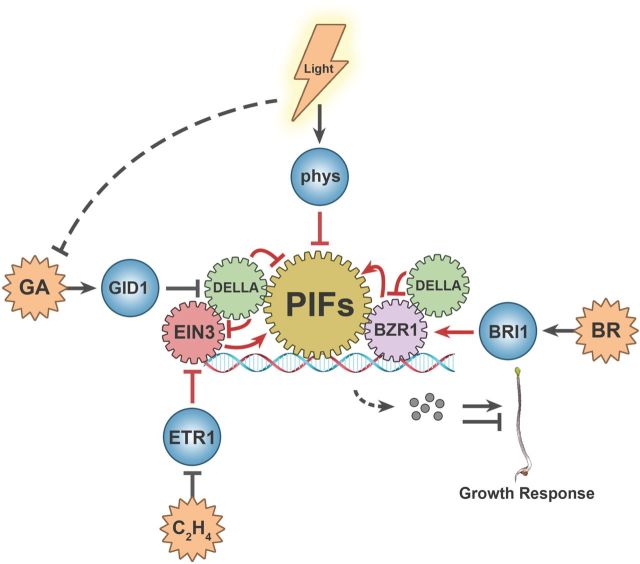
Results of various genetic studies suggest that light affects the cellular levels of at least some phytohormones and the signal transduction of presumably all phytohormones to modulate growth responses. Conversely, the cellular levels of phytohormones also affect photoreceptor signal transduction. For instance, mutations of the DET2 gene, encoding a brassinosteriods biosynthesis enzyme steroid 5α-reductase, result in constitutive photomorphogenesis (Li et al., 1996); mutations of the TAA1 gene encoding the auxin biosynthesis enzyme tryptophan aminotransferase impair the shade avoidance responses (Tao et al., 2008), whereas overexpression of a GA-inactivating enzyme GA2 oxidase also causes constitutive photomorphogenesis (Zhao et al., 2007). However, the molecular mechanism governing the functional interaction between the photoreceptor and phytohormone signal transduction remained unclear until the first example of molecular interaction was reported in 2008 (de Lucas et al., 2008; Feng et al., 2008). In these earlier studies, the GA signaling proteins DELLA were shown to physically interact with the phytochrome-interacting bHLH transcription factors PIF3 and PIF4 (Figure 1). The DELLA proteins interact with PIFs to prevent them from binding to the promoter DNA of light-regulated genes and from promoting cell elongation. In response to light, phytochromes interact with PIFs to trigger their degradation, resulting in inhibition of hypocotyl elongation. In response to the GA signal, DELLA proteins are ubiquitinated and degraded, releasing PIFs to bind to the target promoters and to modulate expression of genes associated with hypocotyl elongation.
Several studies published recently, especially in this year, further illustrate how protein–protein and protein–DNA interactions of the phytochrome-responsive transcription regulators PIFs and GATAs and the phytohormone-responsive transcription regulators DELLAs, BZR1, and EIN3 may interact to affect growth responses (Tao et al., 2008; Luo et al., 2010; Bai et al., 2012; Fan et al., 2012; Gallego-Bartolome et al., 2012; Hao et al., 2012; Oh et al., 2012; Zhong et al., 2012) (Figure 1). The first report of this series of fascinating studies identified a long-sought-after transcription factor that binds to GATAA, the core sequence of a well-known light-regulated promoter element called I box (Terzaghi and Cashmore, 1995; Luo et al., 2010). The presumed GATA factor associated with the GATAA DNA element of the light-regulated promoters has remained elusive for more than two decades. It was noticed that two closely related GATA factors, GATA2 and GATA4, are BR-responsive genes, and the BR signaling factor BZR1 suppresses their transcription. GATA2 apparently acts as a negative regulator of hypocotyl elongation downstream from BZR1 and the light-responsive E3 ubiquitin ligase COP1. It was further shown that BZR1 binds to the promoter of GATA2 to mediate BR repression of GATA2 transcription, whereas COP1 facilitates degradation of the GATA2 protein in the absence of light. The dual control of GATA2 expression by BZR1 and COP1 maintains a relatively low level of the GATA2 protein in the dark or in response to BR, resulting in the release of growth inhibition. In addition to acting as a BR-dependent transcription suppressor of GATA2 transcription, BZR1 also interacts with the PIF4 protein to positively regulate transcription of hundreds of other genes, including the PRE class of bHLH transcription factors that are positive regulators of growth (Oh et al., 2012). ChIP-seq experiments demonstrate that BZR1 and PIF4 co-regulate hundreds of light- and BR-responsive genes by co-occupying the overlapping genomic targets. This is because the PIF4 target sequence G-box (CACGTG) happens to contain the core motif of the BZR1-binding sequence, CGTG. Such an interactive co-occupancy mechanism allows the BZR1–PIF4 complex to co-regulate the same target genes and to affect plant growth and development in response to light and the BR hormone signaling.
In addition to light and BR, GA also modulates transcription of the PRE genes, and the GA control of PRE transcription is dependent on the activity of DELLA and its physical interaction with BZR1 (Bai et al., 2012; Gallego-Bartolome et al., 2012). It was discovered that the dephosphorylated form of BZR1, resulting from BR activation, preferentially interacts with the DELLA proteins, and that BR activation of BZR1 is required for GA promotion of cell elongation. These findings suggest that DELLA may affect the transcription regulatory activity of BZR1 to control PRE transcription. Indeed, DELLA suppresses the BZR1–DNA interaction, which is reminiscent of how DELLA affects PIF activity described previously. Taken together, these results suggest a complex co-regulation of the PRE genes by three different factors—light, BR, and GA—which act through the physical interactions between the PIF4, BZR1, and DELLA proteins. In this scenario, the PIF4–BZR1 complex activates transcription of PRE to promote growth; BR activates growth by dephosphorylation and activation of BZR1. The activated BZR1 interacts with DELLA to become inactivated, but the DELLA-dependent inactivation of PIF4 and BZR1 can be released by GA. In response to light, phytochromes counteract the growth promotion activity of BR and GA by interacting with PIF4 to confer its degradation, suppressing hypocotyl elongation.
Figure 1.
A Simplified View of the Interactive Signaling Network of Phytochromes and Phytohormones. The multiple-pointed stars (orange) represent hormonal signals, spheres (blue) represent receptors, and gears represent signal-responsive transcription regulators associated with growth regulation. The arrows and bars represent stimulatory or inhibitory effect, respectively.
The complexity of a biological system partially results from multifaceted actions of biological molecules. For example, the phytohormone ethylene can suppress hypocotyl elongation in the absence of light, but promote hypocotyl elongation in response to light. Another recent study provides an explanation to this puzzle (Zhong et al., 2012). It was found that the ethylene-responsive transcription factor EIN3 binds to the promoter of the PIF3 gene to activate its transcription. PIF3 is ubiquitinated and degraded in light, but EIN3-dependent activation of PIF3 transcription would compensate for the light-dependent loss of the PIF3 protein, resulting in ethylene and EIN3-dependent promotion of growth in light-grown plants. Moreover, EIN3 also targets and activates the transcription of another gene, ERF1, which encodes an AP2-type ethylene-responsive transcription factor that suppresses growth. In contrast to PIF3, the ERF1 protein is stabilized by light. Therefore, ethylene simultaneously activates two opposite pathways: one being PIF3-dependent promotion of growth in light and the other being ERF1-dependent growth suppression in dark. The final output would depend on the relative strength of light and hormonal signals. Similarly to other studies highlighted here, the EIN3 study demonstrates once again that the signal-dependent protein degradation is a general mechanism allowing plants to adjust cellular concentration of different growth regulators and to adapt to different environmental conditions.
We expect discoveries of many more transcription regulators and protein degradation regulators that act and counteract on individual cellular processes in plants that expose simultaneously to multiple signals. We further speculate that the principles governing other responses may be similar to those reported for the phytochrome and phytohormone responses highlighted here, and the next-generation technologies will enable systematic revelations of the ‘context-dependent’ systems information concerning protein function and regulation.
Funding
The authors’ lab is funded in part by the National Institutes of Health (GM56265 to C.L.).
ACKNOWLEDGMENTS
No conflict of interest declared.
References
- Bai M.Y., Shang J.X., Oh E., Fan M., Bai Y., Zentella R., Sun T.P., Wang Z.Y. (2012). Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis . Nature Cell Biol 14, 810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucas M., Daviere J.M., Rodriguez–Falcon M, Pontin M, Iglesias–Pedraz J.M, Lorrain S, Fankhauser C, Blazquez M.A., Titarenko E, Prat S. (2008). A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484 [DOI] [PubMed] [Google Scholar]
- Fan X.Y., Sun Y., Cao D.M., Bai M.Y., Luo X.M., Yang H.J., Wei C.Q., Zhu S.W., Chong K., Wang Z.Y. (2012). BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol. Plant 5, 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Martinez C., Gusmaroli G., Wang Y., Zhou J., Wang F., Chen L., Yu L., Iglesias-Pedraz J. M., Kircher S., et al. (2008). Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451, 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego–Bartolome J., Minguet E.G., Grau–Enguix F., Abbas M., Locascio A., Thomas S.G., Alabadi D., Blazquez M.A. (2012). Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis . Proc. Natl Acad. Sci. U S A 109, 13446–13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Oh E., Choi G., Liang Z., Wang Z.Y. (2012). Interactions between HLH and bHLH factors modulate light-regulated plant development. Mol. Plant 5, 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Nagpal P., Vitart V., McMorris T.C., Chory J. (1996). A role for brassinosteroids in light-dependent development of Arabidopsis . Science 272, 398–401 [DOI] [PubMed] [Google Scholar]
- Luo X.M., et al. (2010). Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis . Developmental Cell 19, 872–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M.M., Street I.H., Turk E.M., Ward J.M. (2006). Interaction of light and hormone signalling to mediate photomorphogenesis. In Photomorphogenesis in Plants and Bacteria Schäfer E., Nagy F., eds (DordrechtNetherlands: Springer), pp. 439–473 [Google Scholar]
- Oh E., Zhu J.Y., Wang Z.Y. (2012). Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nature Cell Biol 14, 802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., et al. (2008). Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi W.B., Cashmore A.R. (1995). Light-regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol 46, 419–444 [Google Scholar]
- Zhao X., et al. (2007). A study of Gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol 145, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Shi H., Xue C., Wang L., Xi Y., Li J., Quail P., Deng X., Guo H. (2012). A molecular framework of light-controlled phytohormone action in Arabidopsis . Curr. Biol 22, 1530 [DOI] [PMC free article] [PubMed] [Google Scholar]