Abstract
Nucleoside reverse transcriptase inhibitors (NRTI) remain a cornerstone of current antiretroviral regimens in combinations usually with a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or an integrase inhibitor (INI). The antiretroviral efficacy and relative safety of current NRTI results from a tight and relatively specific binding of their phosphorylated nucleoside triphosphates (NRTI-TP) with the HIV-1 reverse transcriptase which is essential for replication. The intracellular stability of NRTI-TP produces a sustained antiviral response, which makes convenient dosing feasible. Lessons learned regarding NRTI pharmacology screening, development, and use are discussed. NRTI and prodrugs currently under clinical development are outlined.
Keywords: antiviral agents, pharmacology, nucleoside analogs, HIV
Nucleoside reverse transcriptase inhibitors (NRTI) have a well-established regulatory history with 11 drugs currently approved in by the US Food and Drug Administration (US FDA) for the treatment of human immunodeficiency virus (HIV), hepatitis B virus (HBV) or hepatitis C virus (HCV). A major key target for HIV-1 drug development is the viral reverse transcriptase (HIV-RT), a polymerase active early in the viral replication cycle which reverse transcribes the virus’ genetic information, stored as RNA, into DNA. This process does not occur with host RNA, making antiretroviral agents (ARV) that specifically target HIV-1 RT generally nontoxic to human cells.
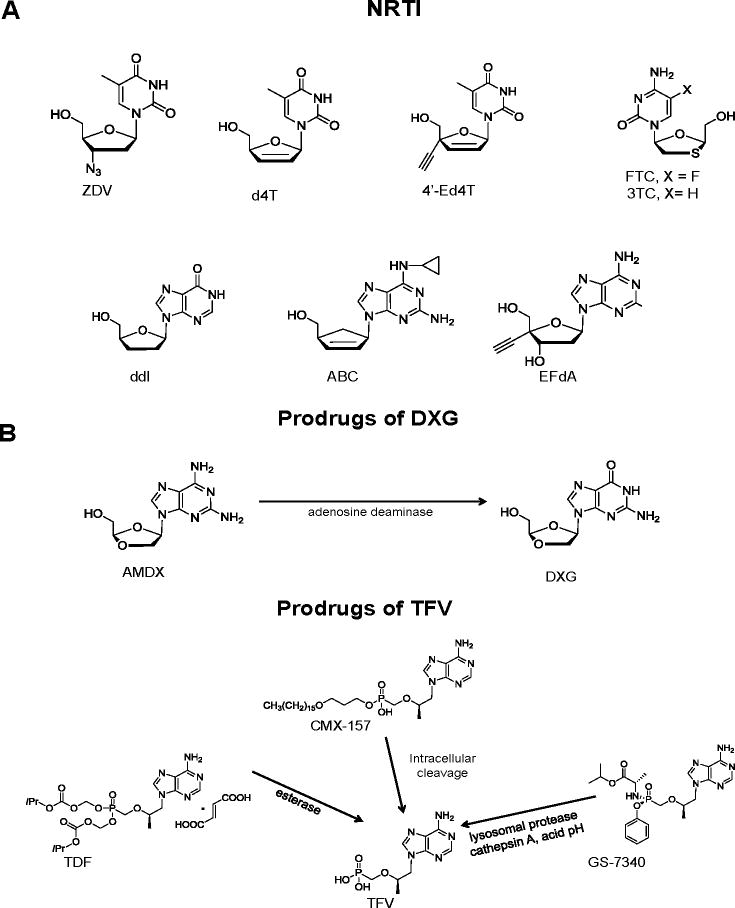
By 2012, several NRTI were approved by the US FDA for the treatment of HIV-1 including Zidovudine® (ZDV, AZT), Videx® (ddI, didanosine), Ziagen® (abacavir sulfate, ABC), Epivir® (3TC, lamivudine), Emtriva® (FTC, emtricitabine), and Viread® (tenofovir disoproxil fumarate, TDF). Hivid® (ddC, zalcitabine) was discontinued due to genotoxicity and Zerit® (d4T, stavudine) is rarely used in the US, but is still in use in resource poor settings [1]. These NRTI remain the cornerstone of current combination antiretroviral therapies (cART) [1,2]. cART regimens have markedly decreased mortality and morbidity from HIV-1 infections in the developed world [3]. Existing therapies cannot eradicate HIV infection due to the compartmentalization of the virus and its latent properties [4]. Therefore, chronic therapy remains the standard of care for the foreseeable future. Modern cART regimens are very effective clinically, but can fail, due primarily to lack of adherence to strict regimens, delayed toxicities and/or the emergence of drug-resistant HIV strains [5]. We will summarize lessons learned from the five potent US FDA approved and most commonly used NRTI, namely ZDV, 3TC, FTC, TDF and ABC, (Figure 1), which have been the focus of previous comprehensive reviews [2,6]. We will review potent NRTI and prodrugs undergoing clinical evaluation, including: Amdoxovir® [AMDX or DAPD, RFS Pharma LLC], a prodrug of dioxolane-G (DXG), the only guanosine analog currently under clinical development; CMX-157 (Chimerix), a highly lipophylic phosphoroamidate prodrug of TFV; GS-3740, a isopropylalaninyl monoamidate phenyl monoester prodrug of tenofovir; Festinavir®, a d4T analog with a 4′-ethynyl substitution (Festinavir, BMS, 4′-Ed4T); and EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine) recently licensed by Merck from Yamasa Corporation in Japan. Structures of NRTI and prodrugs are shown in Fig. 1.
Fig. 1.
Structures of agents mentioned in review: NRTI (A): ZDV (Zidovudine), d4T (stavudine), 4′-Ed4T (Festinavir), 3TC (lamivudine), FTC (emtricitabine), ddI (didanosine), ABC (abacavir), and EFdA. NRTI prodrugs (B): AMDX (amdoxovir), DXG (dioxolane G); TDF (tenofovir disoproxil fumarate), CMX-157 (hexadecyloxy-TFV), GS-7340 (isopropylalaninyl monoamidate-TFV), TFV (tenofovir).
Cellular pharmacology and antiviral potency of NRTI
NRTI are usually hydrophilic molecules that enter cells through a combination of passive diffusion and carrier mediated transport. Many of the over 350 solute carrier superfamily may contribute to the membrane transport of NRTI [7] The equilibrative and concentrative nucleoside transporters may be the dominant mediators of NRTI cellular uptake, because they function rapidly (~ millisecond equilibrative t1/2) and are present on the cell membranes of lymphocytes and other cells [8]. Carrier mediated transport may also be a factor in drug resistance, as certain NRTI and NRTI-TP are substrates for the membrane associated multi-drug cell efflux transporter MRP-4 (MRP4) present in some cells [9]. Once internalized, NRTI require phosphorylation by intracellular phosphotransferases and nucleoside kinases to their active fraudulent NRTI triphosphate (NRTI-TP) analog. Like natural nucleosides, NRTI have varying affinities to and are phosphorylated by different cellular kinases. Studies performed in vitro and humans suggest that it is preferable to combine NRTI that employ different initial kinases in regimens, to avoid competitive inhibition of their respective phosphorylation enzymes [10,11]. NRTI-TP undergo dephosphorylation by cellular 5′-nucleotidases and phosphatases which are enantiomer-specific in nature. This phenomenon was exploited in the design of NRTI with the L-conformation such as 3TC and (−)-FTC, which have prolonged cellular stability half lives (t½) compared to other NRTI, allowing them to be dosed once per day. The metabolism of NRTI has been reviewed in detail [12]. Thymidine kinase (TK1) and thymidylate kinase (TMPK) are present in larger amounts in dividing than in resting cells. ZDV and d4T are phosphorylated by these enzymes, so that higher concentrations of their respective nucleotides accumulate in active than in resting lymphocytes [13]. Transformed lymphocytic cell lines, e.g., MT2 and CEM, are more easily maintained in cell culture, and have been used to test the pharmacology of NRTI, including those that have cell cycle specific phosphorylation [14,15]. However, they are constitutively activated (dividing), as one of the hallmarks of cancer cells may include disrupted metabolism. Therefore, we prefer measuring NRTI phosphorylation and antiviral potency of NRTI using primary human mononuclear cells. The use of peripheral blood mononuclear (PBM) cells allows comparison of phosphorylation and antiviral potency before and after stimulation with phytohemaglutinin, so that cell cycle effects can be assessed. Furthermore, activated PBM cells are the primary substrate for HIV infection in vivo. However, even with primary PBM cell culture, it still remains difficult to perform a direct comparison between ZDV-TP and d4T-TP accumulation in vitro and in humans, due to different activation fractions of PBM cells in vitro and in vivo [13].
Clinical studies with thymidine analogs typically report NRTI-TP contents of PBM cells, collected using a Ficoll gradient, without further enriching for activated CD4+ cells [16,17]. Because TK1 and TMPK expressions are cell cycle dependent, and the vast majority of PBM cells are non-activated in vivo, consideration of data from these measurements underestimates the ZDV-TP content in activated lymphocytes. Since HIV only infects PBM cells which are activated (CD4+), and the phosphorylation of ZDV and d4T is minimal compared to activated cells, a partial solution may be to scale NRTI-TP using “typical” activated PBM cell fractions measured in vitro and in vivo [18,19]; e.g., assuming activated PBM cell fractions of ~40 % in PHA stimulated cells and ~8 % in vivo [20,21]. However, the fraction of dividing cells may vary between HIV infected individuals, and tends to be elevated in untreated HIV infected individuals and may decrease towards more normal levels once the infection is stabilized using cART [22]. In vitro to in vivo correlations are further complicated by variations in TMPK activities in PHA stimulated PBM cells from different donors, as TMPK is the rate limiting enzyme for phosphorylating ZDV-MP to ZDV-TP.
NRTI-TP compete with their natural dNTP for incorporation by HIV-1 RT into the elongating proviral DNA. Because all current NRTI lack a 3′-hydroxyl group, incorporation results in chain termination. NRTI-TP competes with its natural dNTP each time it appears in the active site near the reverse-transcribing viral RNA chain, produces multiple opportunities for NRTI blockade during a single round of reverse transcription. The long intracellular half-life (t1/2) of many NRTI-TP can result in a significant post-plasma antibiotic effect, which may sometimes be exploited for designing more convenient dose regimens that are more forgiving when it comes to missed doses. This differs from NNRTI and PI, in which the antiviral effect is due to the unmetabolized drug, and where efficacy can be directly predicted by monitoring between dose trough plasma concentrations (Cmin) [23]. For NRTI, efficacy may be related to maximal plasma concentrations (Cmax), in order to “drive” cellular accumulation and phosphorylation of the NRTI to the long-lived triphosphate form, unless phosphorylation is rate limiting, as occurs with ZDV [16].
Cellular toxicities and adverse effects of NRTI
Symptoms and mechanisms of NRTI toxicity have been extensively reviewed, and are most commonly ascribed to mitochondrial toxicities, which may result in organ specific toxicities which may include myopathy, peripheral neuropathy, pancreatitis and lactic acidosis [24]. In brief, certain NRTI phosphates can act as chain terminators when incorporated into the replicating DNA of mitochondria (mtDNA) due to the action of DNA polymerase (mtDNA polymerase γ, mtDNA pol γ), which is less able to discriminate between NRTI-TP and NTP than the polymerase found in the nucleus. This results in a relative depletion of mtDNA, and disrupted oxidative phosphorylation, as evidenced by the accumulation of non-esterified fatty acids and dicarboxylic acids. A competitive inhibition of mtDNA pol γ by NRTI-TP could also inhibit the repair of the mtDNA damage, since mtDNA pol γ also serves as the sole enzyme responsible for the base excision repair of oxidative damage in mtDNA. The association between d4T and ZDV with lipodystrophy has resulted in a decrease in their use, in favor of less toxic NRT (e.g., (−)-FTC, 3TC, and TDF), which are less prone to produce mitochondrial toxicity [25]. Therefore, NRTI should be designed to target HIV-1 RT while minimizing interaction with mtDNA pol γ, as is the case with NRTI 3TC and (−)-FTC, which have a markedly improved safety profile than ZDV and d4T, which are associated with side effects such as bone-marrow toxicity, lipodystrophy, and peripheral neuropathy. Enzymatic methods are commonly used to rank the relative affinities of NRTI for mtDNA pol γ (Ki-DNA pol γ) [26]. A more direct enzymatic measurement for assessing mtDNA pol γ mediated toxicity related to the Ki-DNA pol γ is the in vitro toxicity index (TI), defined as the time required to replicate the mitochondrial genome, based on the rates of incorporation and removal of chain terminators [27]. Our laboratory prefers mitochondrial toxicity screening with a cell-based assay over 14 days, which takes into account the overall pharmacology of the NRTI analog including cellular uptake, phosphorylation, mitochondrial transport, and phosphorylation in addition to inhibition of mtDNA synthesis [28]. Other cell culture models are under development to evaluate for organ specific mitochondrial toxicity such as pancreatis [29].
TDF, is associated with an increased incidence of renal adverse events associated with markers of proximal tubule damage, including reduced creatinine clearance, proteinuria, glucosuria, phosphaturia, which may be enhanced in individuals with pre-existing renal disease [30]. The primary metabolite, TFV is a substrate for eflux transporters in the proximal renal tubules [31], and studies using a murine model demonstrated mitochondrial toxicity in proximal renal tubules after TFV administration [32]. The biologically active metabolite of ABC, carbovir-TP, is incorporated by mtDNA pol γ, and ABC is associated with changes in biomarkers of cardiovascular risk [33], although not all studies have been able to confirm that potential risk [34–36]. Hypersensitivity reactions occur in about 8% of individuals during clinical trials, which appears independent of mitochondrial toxicity, and is most prevalent in individuals with the HLA-B*5701 genotype [37]. Therefore, ABC is not recommended for individuals with this genotype. (http://www.viivhealthcare.com/products/ziagen.aspx?sc_lang=enpdf, accessed 06-29-2012).
In vivo PK studies in NRTI drug development
Current NRTI used for the treatment of HIV are orally administered. In vitro evaluation does not provide direct information on the extent and rate of oral absorption, distribution and elimination (ADME) or in vivo toxicity of NRTI. Therefore, it is important to conduct in vivo studies early in the drug development process. Precise mechanisms of NRTI absorption remain uncharacterized. However, nucleoside/nucleotide transporters are present in the GI tract [38]. Other mechanisms including fatty acid absorption processes may be involved for NRTI prodrugs such as CMX-157 (below), which are not recognized by these transporters. Because of economic constraints, early in vivo PK and toxicity studies are usually performed in rodents, with confirmation in larger animals (e.g., dogs and/or monkeys). The resulting preclinical data may be used to derive interspecies dose scaling parameters for first in human (Phase 1) studies [39]. The metabolism of NRTI has been reviewed in detail [12]. Briefly, most NRTI (e.g., ddC, 3TC, FTC and TFV) are eliminated unchanged in the urine. However, about 74% of the ZDV dosage is recovered in the urine after conversion to the 5-O-glucuronide, after metabolism by UDP-glucuronosyl-transferase (UGT), and a minor fraction is metabolized to a 3′-amino containing metabolite by non-specific cytochrome P450 and reductase enzymes, followed by renal filtration of the metabolites. In the case of ABC, only 1.4% of administered ABC is recovered unchanged in the urine, the remaining fraction being glucuronidated via UGT to the 5-O-glucuronide metabolite. The 5′-OH moeity on the carbocyclic ring of ABC is also a substrate for alcohol dehydrogenase (ADH). The heterocyclic bases of ddI and d4T are rapidly metabolized by depurination and depyrimidation, respectively.
TFV (a nucleoside phosphonate) and DXG (a dioxolane NRTI), are poorly absorbed in their parent form. Therefore, prodrugs of DXG (e.g., AMDX or DAPD) and TFV (e.g. TDF GS-7340 and CMX-157) (reviewed below), were developed to improve their ADME properties. Prodrug moieties may also influence tissue distribution. TDF is associated with a < 2% incidence of renal toxicity, which may present as a reduction in creatinine clearance, proteinuria, glucosuria, phosphaturia, and other symptoms [40]. However, unlike TFV, the highly lipophylic prodrug CMX-157 (featured below) is enzymatically cleaved intracellularly, so that plasma concentrations of TFV are low and CMX-157 does not undergo renal filtration and is not a substrate for OAT transporters, making it less likely to display renal toxicity [41].
Population pharmacokinetic-pharmacodynamic (PopPK-PD) modeling and simulations
PopPK-PD (pharmacometrics) is an increasingly popular tool for the development of ARV including NRTI [42]. PopPK-PD models consider the different levels of variability in PK parameters which occur within and between individuals, and populations. An advantage of PopPK-PD compared to other PK methods is the ability to used fragmentary (missing values) or even unstructured data (e.g. from clinical visits), because this methodology makes use variance estimates to interpolate for “missing” data [43]. Subject covariates (e.g. gender, body weight, renal function, among others) may be included in models, and used to systematically analyze relationships with PK parameters, to determine whether uniform dosing is feasible, or to develop subject specific dosing protocols in silico. Thus, body weight and creatininine clearance have been used to predict NRTI clearance in individuals with renal failure [44]; and in pediatric individuals [45]. Variance models based on data from earlier studies may be used to test potential regimens in silico, making them more likely to be effective and/or reduce toxicity.
The impact of PopPK-PD on drug development is evidenced by the US FDA and European Medicines Agency issuing “Guidances for Industry” on PopPK modeling. (www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM133184.pdf; www.tga.gov.au/pdf/euguide/ewp18599006en.pdf, both accessed 07-24-2012).
PopPK-PD models can be expanded to include biochemical and/or disease processes. For example, ZDV is associated with dose limiting bone marrow toxicities, making its use problematic in resource limited settings where toxicity monitoring facilities are lacking. Mitochondrial toxicity of ZDV may be associated with the accumulation of ZDV-MP, while antiviral efficacy is related to the accumulation of ZDV-TP [46]. A clinical study suggested that the phosphorylation of ZDV to ZDV-DP, but not to ZDV-MP, may be saturated at clinical doses [16]. An in silico pseudo-mechanistic simulation study, using a PopPK-enzymatic model suggested the feasibility of ZDV dose reduction from 300 to 200 mg bid, to limit side effects (related to ZDV-MP), without compromising efficacy (related to ZDV-TP) [18]. Although this result was supported by a pilot clinical study, larger studies are warranted to validate this approach [47]. d4T is the only other thymidine NRTI currently approved for HIV-1, and is in the process of being phased out in resource-poor countries by the WHO due to a high incidence of slow onset lipodystrophy and peripheral neuropathy side effects. Although, retrospective and pilot clinical and modeling studies suggest that adequate cellular concentrations of d4T-TP are maintained for viral inhibition, and tolerability may be improved when d4T doses are reduced from 30 to 20 mg, bid [48], there are conflicting reports as to whether dose reduction is sufficient to adequately control toxicity [49]. Therefore, there is room for a safe thymidine NRTI with potent activity versus HIV-1 with the K65R mutation, to replace ZDV and/or d4T. Festinavir® (Oncolys Biopharma, licenced to Bristol-Myers Squibb) (featured below), is a new long-acting 4′-ethynyl derivative of d4T. However, this drug should be paired with some potent ARV that are not cross resistant to prevent the emergence of resistant virus.
The majority of HIV in the circulation is derived from productively infected CD4+ lymphocytes. Therefore, modeling viral depletion and CD4+ recovery during treatment requires suitable estimates of cell and infection dynamics. Pioneering research on CD4+ cell dynamics in vivo and their infection by HIV was performed by Novak, Perelson and others [50,51]. In 2006, Rosario, et al., published a model which combined a virus dynamics model with PopPK, which was used to streamline the phase 2 development of the CCR5 receptor blocking ARV maraviroc [52,53]. A similar PopPk virus dynamics model has been developed for 3TC, by superimposing phosphorylation to 3TC-TP, and binding with HIV-RT with viral infection kinetics, illustrating the feasibility of predicting viral depletion kinetics versus NRTI dose regimens during monotherapy [54]. This approach could be used for suggesting starting dosage regimens for novel NRTI under preclinical development.
Viral Reservoirs (genital tract, gut, macrophages, lymph nodes, CNS) and NRTI Prodrugs
cART can decrease serum HIV loads to below detectable levels, but are not curative, since HIV persists as latent infection (e.g., lymphocytes) or replicates in tissues shielded from antiretroviral agents by physiological barriers such as: the CNS which is shielded by the blood brain barrier (BBB), lymphoid tissue, and genital-urinary tract, shielded by endothelial cells surrounding the prostate and testes [55,56]. Because macrophage-like cells, including circulating monocytes or microglia can phosphorylate NRTI less well than CD4+ lymphocytes, concentrations of NRTI-TP may be sub-therapeutic, allowing these cells to potentially serve as viral reservoirs [57].
Penetration of NRTI into male and female genital tracts has been extensively reviewed, as they are important routes of infection for the majority of individuals [58]. NRTI tended to produce relatively greater exposures in seminal plasma of males and cervical vaginal fluids of females than other ARV. ZDV, 3TC, FTC, ddI and TFV penetrated both genital tracts at concentrations equal to or exceeding those in plasma, and above wild-type HIV-1 effective concentration (EC50) for at least part of the dose interval. Concentrations of ABC and d4T were reportedly high in semen plasma and low in cervical-vaginal secretions. Following a trial which demonstrated the efficacy and safety of using daily Truvada (FTC + TDF) to prevent the spread of HIV (HIV PrEP) in a cohort of men who have sex with men, other clinical trials are underway to evaluate the efficacy of various combinations of TDF and FTC to prevent HIV-1 infection among other high-risk populations [59]. These positive results were in agreement with PK measurements of extracellular AUC over 14 days (AUC0-14, ng.days per mL plasma, or per gram of solid tissue). AUC0-14 was 88.5, 49.8, 510, and 2989 for TFV in plasma, cervical (CV), vaginal (VT) and rectal tissue (RT), respectively, versus 60.1, 419, 2496 and 265 for FTC. Corresponding cellular AUC0-14 reported for active TFV-DP were 10,813; 2939; 441; and 4,884 fmol.days/106 in PBM cells, VT, CT and RT, respectively. AUC0-14 measured in PBM cells were 12,192 and 151 fmol.days/106 cells, indicating good drug exposures in these tissues [60]. In July 2012, Truvada was approved by the US FDA as HIV PrEP for high-risk individuals to reduce virus transmission (http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm312264.htm, accessed 07-202012).
Gut-associated lymphoid tissue (GALT) comprises the largest lymphoid organ infected by HIV-1, and is a viral reservoir and host-pathogen interface in infection [61]. GALT represents a heterogeneous microenvironment where multiple HIV-1 target cells co-exist (macrophages and lymphocytes), allowing for a complex interplay between infection and cell signaling. Severe CD4+ T cell depletion and HIV-1 reservoir seeding in the gut mucosa begins early in the infection process and continues with disease progression [62]. HIV-1 DNA levels in gut mucosa of individuals undergoing cART are associated with persistent immune activation and inflammation, altered distribution of mucosal natural killer (NK) cells, and microbial translocation [63]. There are conflicting reports regarding direct correlation with establishment and persistence of heterogeneous quasispecies in the GALT. A study reported on the detection of different ZDV resistant HIV-1 strains in the colon versus that observed in other gut tissues, or blood of individuals administered ZDV ddI or ZDV/ddI combinations. This suggested that particular gut tissues could select differentially for NRTI resistant genotypes [61]. By contrast, other studies on chronically infected individuals reported a lack of evidence for compartmentalization of HIV-1 quasispecies observed in biopsies of the colon and ilium versus peripheral blood, suggesting an early establishment and persistence of viral reservoirs in the GALT with minimal diversity. A mega HAART regimen reported clearing HIV-1 from the gut and sera of infected individuals to < 50 copies per ml, suggesting that it may be feasible to develop drugs and treatment regimens to clear virus from this important reservoir [62].
HIV infection persists in the CNS even when serum viral loads are maintained below limit of detection, where it contributes to inflammation and neurocognitive impairment (NCI) [64]. A semi-quantitative measure of CNS penetration (CPE score) considers the ratio of predicted concentration of ARV penetrating into the CNS relative to the EC50 measured using activated PBM cells. ZDV and d4T tend to have better penetration into the CNS than other NRTI [65]. It remains to be seen whether CMX-157 (below), a phospholipid prodrug of TFV delivers adequate levels of TFV into the CNS. Cross-sectional observation studies reported lower incidences of NCI with regimens with higher CPE, and improved HIV-1 RNA levels among previously ARV naïve individuals [65]. However, the only published randomized study evaluating the strategy of increasing CPE demonstrated an inferior response in the arm containing ARV with the higher CPE scores [66]. Other refinements to the CPE score have been proposed including measures of potency in cells which could be relevant to seeding and/or persistence of CNS infection, e,g., monocytes and macrophages [67]. Chronic infection within macrophage-like cells in the brain/CNS can increase the overall activation state of uninfected monocytes, priming them for permissive infection, while influencing pro-inflammatory/pro-HIV-1 cytokine microenvironments[68]. Additionally, peripherally infected CD14+/CD16+ monocytes function as “Trojan horses” by traversing the BBB during initial infection [69]. We have reported on initial work on RNA related NRTI which target viral infection in macrophages by exploiting the similar dNTP/RTP concentration ratios in macrophages [70].
Use of NRTI with complementary resistance patterns
ABC and TDF select for the K65R resistance mutation in the reverse transcriptase (RT) gene, which results from a single amino acid change from lysine to arginine in the RT gene, producing moderate resistance [71,72]. Similarly, 3TC which has relatively potent (2 log10) viral load reduction rapidly (within a few months) selects for a single base mutation at position 184 of RT (producing M184V), reducing susceptibility by 100- to 1,000-fold [73]. Although still a problem, M184V selection may be lower in individuals using TDF than in those using 3TC, in regimens composed of two NRTIs plus the NNRTI efavirenz [74]. However, HIV with the M184V mutation have increased susceptibility to ZDV, possibly as a result of a conformational change making it more favorable for the azido group on ZDV-TP to bind HIV-1 RT [75]. Other RT mutations, e.g. the TAMS specifically, D67N, K70R, T215Y, T219Q) develop more slowly [76]. DXG, the active metabolite of AMDX (featured below) selects for the K65R mutation as well, making it a candidate for co-administration with ZDV.
Adherence and fixed dose regimens
There is a trend towards replacing older formulations which required administration of multiple formulations multiple times per day, with fixed dose formulations which allow once or twice per day administration. Examples of formulations administered once per day include: Atripla® (efaverenz + TDF + FTC, Bristol-Myers Squibb and Gilead Sciences), Complera® (rilpivirine + TDF + FTC, Janssen Therapeutics and Gilead Sciences), Truvada® (TDF + FTC, Gilead Sciences). Although once per day regimens are preferred, the safety profiles of all components in the formulation should be considered. For example, Atripla®, was developed as a convenient one tablet per day HIV-1 treatment regimen. However, a study with 472 individuals demonstrated that about 21 % of individuals treated with this formulation had to discontinue Atripla® due to unpleasant CNS side effects, including nightmares and insomnia, which were associated with efaverenz, the potent long acting non-nucleoside RT inhibitor included in the formulation. Therefore, even though convenient to administer, Atripla® may not be suitable for use in first-line therapy for HIV [77]. However, once per day formulations are not always readily available, especially in resource-poor settings where HIV-1 is most prevalent. Examples of twice per day formulations include: Epzicom® (ABC+ 3TC, ViiV Healthcare) and Trizivir® (ABC + 3TC + ZDV, ViiV Healthcare), and Combivir® (ZDV + 3TC, ViiV Healthcare). Fixed dose formulations containing d4T are still used in resource poor settings (e.g., GPO-VIR S 30®, Thai Government Pharmaceutical Organization; Triomune-30®, Cipla Ltd). However d4T is associated with debilitating long term side effects, associated with mitochondrial dysfunction, which include hyper-lacteremia, lipodystrophy, neuropathies, and lactic acidosis, some of which are irreversible [78]. Therefore, the current World Health Organization as amended in 2010 was to reduce the d4T dosage of 30 mg bid (instead of 40), in all individuals regardless of body weight and replacement with safer and at lease equally effective ARV (e.g. TDF) when feasible (www.searo.who.int/LinkFiles/HIV-AIDS_Rapid_Advice_Adult_ART_Guidelines(web).pdf; Accessed 01-03-2011).
NRTI in Development
Amdoxovir® [(−)-β-D-2,6-diaminopurine dioxolane, AMDX, DAPD, RFS Pharma, LLC], is the only guanosine NRTI under clinical development for the treatment of HIV-1. AMDX is a water soluble prodrug of 9-(β-D-1,3-dioxolan-4-yl)-guanine (DXG), and is under Phase 2 development through a US-IND. AMDX has been safely administered to more than 200 subjects in seven Phase 1 and 2 clinical trials [47,79]. AMDX is rapidly absorbed and is deaminated by adenosine deaminase, an ubiquitous enzyme, which subsequently undergoes cellular phosphorylation to DXG-triphosphate (DXG-TP), a potent inhibitor of wild type and drug resistant forms of HIV-1 [80] and hepatitis B virus in human hepatocytes [81]. Drug resistant HIV mutants susceptible to DXG in vitro include viruses containing M184V/I and thymidine analog mutations (TAMs: M41L, D67N, K70R, L210W, T215Y/F, K219Q/E), and the 69SS double insert [82]. The t1/2 of DXG-TP was ~16 hr in activated primary human lymphocytes and ~9 hr (or 27 hr including a questionable 48 hr time point) in humans, suggesting that twice a day (bid) dosing should provide adequate therapeutic coverage [83]. AMDX and DXG did not affect the levels of mtDNA in human hepatoma cells (HepG2) treated for 14 days at 10 μM, and there was no increase in lactic acid production in these cells [84]. Resistance in vitro develops slowly, and is associated with mutations at K65R or L74V [85]. Viruses containing the K65R mutation show moderate cross-resistance to zalcitabine, didanosine, adefovir and lamivudine (3TC), but increased sensitivity to ZDV [85]. ZDV alone selected for a mixture of K70K/R mutations at week 25 in vitro and AMDX alone selected for a mixture of K65R or L74V mutations at week 20. When AMDX and ZDV were incubated with HIV-infected primary human lymphocytes, no drug resistant mutations were detected through week 28 [86]. Therefore, a proof of principle PK and virus dynamic study of AMDX/placebo with or without ZDV was performed in 24 randomized subjects administered oral AMDX 500 mg bid, AMDX 500 mg plus ZDV 200 or plus ZDV 300 mg bid or ZDV 200 or 300 mg bid for 10 days. The ZDV doses (200 or 300 mg, bid) were selected to test the feasibility of using a 200 mg bid dose of ZDV, as a strategy to limit ZDV toxicity while maintaining efficacy, as was suggested by an in silico study (refer to PopPK-PD modeling and simulations section). Co-administration of AMDX with ZDV did not change either of the plasma PK parameters or percent AMDX, DXG or ZDV/GZDV recovery in the urine significantly [79]. The mean decrease in log10( of viral load) were −0.69 with ZDV 200 mg, −0.55 with ZDV 300 mg, −1.09 with AMDX, −2.00 with AMDX plus ZDV (200 mg), −1.69 with AMDX plus ZDV (300 mg), compared to 0.10 with placebo. AMDX plus ZDV (200 mg) was significantly more potent than AMDX monotherapy in area under the viral load versus time curve (AUC(VL)) and mean VL decline (P = 0.019 and P = 0.021, respectively), suggesting at least additive efficacy. There was a marked decrease in the variability of viral loads in groups administered ZDV with AMDX, compared with those administered AMDX alone [47]. All adverse events were mild to moderate. This combined therapy, including the use of a lower ZDV dosage, warrants further assessment in larger clinical trials.
CMX-157 (hexadecyloxy-TFV, Chimerix), is a lysolecithin-derived prodrug of the nucleoside phosponate TFV which is undergoing phase 2 testing. CMX-157 was recently licensed to Merck Pharmaceuticals for further development and commercialization (http://www.marketwatch.com/story/merck-inks-two-pacts-for-investigational-hiv-drug-2012-07-24, accessed 07-25-2012). The lysolecithin moeity allows CMX-157 to utilize natural lysolecithin uptake pathways in the gut, resulting in high oral absorption observed in animal models (rats and monkeys) [87]. CMX-157 accumulated > 30 fold higher cellular concentrations of TFV-TP than did TDF in human PBM cells at physiological concentrations [88]. Favorable results were reported in a Phase 1 randomized blinded, dose-escalation trial, which demonstrated safety, tolerability and linear single dose PK between 25 and 400 mg in healthy human volunteers. Active TFV-DP remained detectable for six days in the PBM cells of individuals administered a single 400 mg administration of CMX157, suggesting the possibility of a convenient, once per week dose regimen (Chimerix website: http://www.chimerix.com/news-and-resources/news-and-resources-details/chimerixs-antiviral-cmx157-demonstrates-positive-phase-1-clinical-results-w, accessed 06-21-2012). Antiviral data from experiments performed in cell culture demonstrated a > 2-log improvement in potency (EC50) versus HIV-1 compared with TDF [89]. It was proposed in addition to RT inhibition by TFV-DP, the improved potency may also involve binding of CMX-157 to cell-free virions through a direct insertion into viral envelope and subsequent facilitated delivery of TFV into infected cells [90]. Unlike the TDF, CMX-157 remains intact in the circulation and is cleaved intracellularly to TFV. CMX-157 is not a substrate for nucleoside transporters and may enter cells by diffusion through the cell membranes, as it is lipophylic in nature. CMX-157 is not a substrate for human OAT1 and OAT3 transporters found in renal tubules, and is eliminated mainly via hepatic metabolism [41,87]. Cellular accumulation was reduced by more than 90% in the presence of 20% serum in vitro, which may be even greater in vivo (100% serum). A major potential advantage of CMX-157 may include enhanced drug delivery to fatty tissues such as lymph nodes (refer to section on viral reservoirs). However, increased lipophylicity may lead to an increased tissue volume of distribution, and decreased plasma concentrations. Future clinical trials will reveal the relative contributions of a vastly improved antiviral potency and increased drug exposure to lipophylic viral reservoirs, versus decreased plasma concentrations due to increased tissue distribution, and significant binding to serum proteins.
GS-7340 [((9-[(R)-2-[[[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxy-phosphinyl]-methoxy]propyl]adenine); Gilead Sciences], is an orally absorbed isopropylalaninyl monoamidate phenyl monoester prodrug of TFV, which is undergoing phase 2 testing for the treatment of HIV [91,92]. GS-7340 is ~400-fold more potent than TFV versus HIV in human PBM cells [93]. The stability of GS-7340 is about 200 fold greater than TDF, so that levels are detectable in the circulation. GS-7340 is converted by lysosomal protease cathepsin A (CatA) in PBM cells to an alanine metabolite of TFV (TFV-Ala), which then degrades to TFV under acidic pH in lysosomes [94]. PK studies in beagle dogs demonstrated a rapid plasma clearance with 34-fold greater 24 hr TFV-DP exposures in PBM cells compared to TDF, and five to 15-fold greater levels of GS-7340 metabolite was observed in lymphatic tissues compared with TDF. Therefore, GS-7340 has potential for targeting lymphatic tissue. Elevated amounts of GS-7340 metabolites were also observed in other tissues and in bile [93].
A phase 1 monotherapy trial compared GS-7340 (50 or 150 mg) to TDF (300 mg), once per day for 14 days in previously untreated HIV-infected men (n = 9 per group) [91]. The decline in log10 HIV-1 RNA copies per ml of the GS-730 groups administered 50 and 100 mg per day were − 1.57 ± 0.53 and − 1.71 ± 0.24, and were statistically greater than that the −0.94 ± 0.49 decline observed for the TDF group (p = 0.0257 and 0.0010, respectively). Plasma concentrations of TFV were 56 % and 88 % lower, respectively, compared to the TDF group. The accumulation of TFV-DP in PBM cells measured for the respective doses of GS-7340 were 8 and 18-fold higher and 4 and 33-fold higher on days 3 and 14, compared with subjects receiving TDF. A follow up randomized dose-finding, 10-day monotherapy study was conducted in 38 subjects (97% male), to compare antiviral efficacy and PK of GS-7340 (8, 25, and 40 mg once daily) to open-label TDF (300 mg once daily) or placebo (n = 9, 8, 8, 6, and 7, respectively) [92]. The averaged 10 day decline in log10 HIV RNA/ml (DAVG10d) of the GS-7340 8 mg group was similar to the TDF group (−0.76 versus −0.48, P = 0.22), and superior to the placebo group (−0.76 versus -0.01, P = 0.0002). DAVG10d of the 25 and 40 mg GS-7340 groups exceeded the TDF group (−0.94 and −1.13 versus −0.48, P = 0.01 and 0.001, respectively). Median changes in HIV-1 log10 RNA/ml from baseline after 10 days were: placebo (0.003); TDF (−0.97); GS-7340 8 mg (−1.08); 25 mg (−1.59); 40 mg (−1.73), suggesting that a 25 mg dose may produce close to maximal antiviral response. Plasma exposures to TFV in the GS-7340 groups were 80 to 97 % lower than the TDF group. GS-7340 was well tolerated and no TDF resistance mutations were detected in either study. Therefore, GS-7340 administered at 40 mg per day was considered more efficacious and potentially safer than 300 mg TDF per day.
Festinavir® [1-[(2R,5R)-5-ethynyl-5-(hydroxymethyl)-2H-furan-2-yl]-5-methylpyrimidine-2,4-dione-ethynylthymidine, 4′-Ed4T, OBP-601, BMS-986001, Oncolys Biopharma, licenced to Bristol-Myers Squibb]. 4′-Ed4T, is a modified d4T derivative with a 4′-ethynyl moiety which is in Phase 2 clinical testing. In vitro studies demonstrate that 4′-Ed4T, which is 4–6 fold more potent versus HIV-1 and has much lower affinity for mtDNA pol γ than d4T, making it much less toxic to mitochondria than d4T [95]. 4′-Ed4T did not degrade mtDNA in long term primary cultures of cells isolated from human kidney, skeletal muscle and subcutaneous fat, or influence lactate levels in culture with muscle cells [96]. The accumulation of 4′-Ed4T-TP in CEM cells were ~ twice that of ZDV-TP and ~4 fold higher than d4T-TP. Although the accumulation of 4′-Ed4T-MP was lower than for ZDV-MP, levels were higher than d4T-MP, consistent with TMPK catalyzing the rate limiting phosphorylation step. The decay t1/2 of 4′-Ed4T-TP in CEM cells was similar to ZDV-TP (t1/2 ~8.3 versus ~8.7 hr, respectively) and 2-fold slower than d4T-TP [15]. 4′-Ed4T demonstrated similar antiviral potency in wild-type NL4-3 HIV-1 and in HIV-1 with K65R and Q151M RT, and enhanced potency in HIV-1 containing the K103N mutations. Although 4′-Ed4T was 4.5 to 17.5 fold less potent in a multi-drug resistant clinical isolate than against a strain isolated from a treatment naïve individual, concentrations of 4′-Ed4T needed to inhibit the resistant virus remained relatively low [14].
4′-Ed4T was well tolerated in a phase 1 study in healthy individuals administered 100 to 900 mg once per day. Plasma Cmax was reached in one to two hr, and remained above the in vitro EC50 and EC90 versus wild type HIV-1 (0.475 and 5 ng/ml, respectively). Plasma concentrations were unaffected by food and increased linearly with dose, and declined with a median t1/2 of 2.3 to 3.7 hr [97]. A phase 1b/2a monotherapy study was conducted in 32 HIV treatment experienced individuals, administered 100, 200, 300 or 600 mg doses over a 10 day period. Average log10 HIV-1 RNA reductions after 10 days were −0.87, −0.98, −1.36, and −1.2, respectively, comared to −0.07 for the placebo group. Median increases in CD4+ cell count were 70, 180, 130, and 120 cells/ml, respectively. One individual on the 600 mg dose had a thymidine analog mutation (TAM) on day 1 (T215/S), which was not observed on days 11 or 17. Two subjects in the 300 mg cohort had TAMS on day 17, which was not present at days one or 11. However, all three individuals had log viral load reductions > 1 on day 11 [98,99]. The apparent maximal viral load reduction and increase in CD4+ cell counts may suggest that similar to ZDV, phosphorylation may be saturated at clinically achievable doses, as was observed in the cell culture studies. The number of individuals tested was insufficient for statistical analysis.
EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine, Merck), is a potent purine nucleoside that is in advanced preclinical studies. An in vitro study demonstrated a much slower onset of resistance for EFdA in comparison with 40 -Ed4T or TDF, versus a cocktail of 11-HIV-1 clinical isolates incubated with MT4 cells. Furthermore, EFdA remained active against the resistant strains selected for by 4′-Ed4T or TFV. ZDV, 3TC and (−)-FTC which were inactive against the HIV-1 cocktail used in this experiment, suggesting that EFdA may have a different resistance profile than the other NRTI tested. [100]. A pre-steady-state enzyme kinetic study demonstrated that EFdA-TP was the first analog to be preferred over native nucleotides by HIV-1 RT, but to experience negligible incorporation by wild type (WT) pol γ, suggesting it may produce an ideal balance between high antiretroviral efficacy and minimal host toxicity. WT pol γ could discriminate Ed4T-TP from the natural dTTP analog 12,000-fold better than RT. A structurally related NRTI, 2′,3′-didehydro-3′-deoxythymidine (stavudine, d4T), was reported to be the only other analog favored by RT over native nucleotides, but it exhibited only a 13-fold difference (compared with 12,000-fold for Ed4T) in discrimination between the two enzymes. It was proposed that the 4′-ethynyl group of Ed4T serves as an enzyme selectivity moiety, crucial for discernment between RT and WT pol γ [101].
EFdA demonstrated three orders of magnitude greater potency than TFV, ZDV and (−)-FTC in blocking replication of SIV in macaque PBM cells. A study in macaques administered EFdA 0.4 mg/kg, subcutaneously twice per day, demonstrated a three to four log decreases in plasma virus that subsequently became undetectable. After treatment the rebound virus contained the M184V/I mutation, but remained fully susceptible to EFdA [102]. Enzymatic studies indicate that EFdA is poor substrate for mtDNA pol γ, so that it is unlikely to have mitochondrial toxicity [103]. At the time of this writing, EFDA has not undergone clincial testing.
Conclusions
The field of nucleoside chemistry and biology continues to produce numerous highly effective ARV for the treatment of HIV, HBV and hepatitis C virus that has prolonged the lives of millions of infected persons. Although no perfect NRTI exists yet, data contained in this review suggest that the field of nucleoside chemistry and biology is expanding and that we have made great strides in terms of potency, clinical efficacy and safety, thus providing improved treatment options for persons infected with HIV.
Acknowledgments
This work was supported in part by NIH grant 5P30-AI-50409 (CFAR), and by the Department of Veterans Affairs. Dr. Schinazi is an inventor of DAPD/DXG and may receive future royalties from the sale of these drugs. The authors acknowledge Dr. Ghazia Asif for many helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Research. 2010;85 (1):1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cihlar T, Ray AS. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 2010;85 (1):39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Este JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antiviral Res. 2010;85 (1):25–33. doi: 10.1016/j.antiviral.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Siliciano JD, Siliciano RF. The latent reservoir for HIV-1 in resting CD4+ T cells: a barrier to cure. Curr Opin HIV AIDS. 2006;1 (2):121–128. doi: 10.1097/01.COH.0000209582.82328.b8. [DOI] [PubMed] [Google Scholar]
- 5.Pereira CF, Paridaen JT. Anti-HIV drug development--an overview. Curr Pharm Des. 2004;10 (32):4005–4037. doi: 10.2174/1381612043382459. [DOI] [PubMed] [Google Scholar]
- 6.Schinazi RF, et al. Pharmacology of current and promising nucleosides for the treatment of human immunodeficiency viruses. Antiviral Res. 2006;71 (2–3):322–334. doi: 10.1016/j.antiviral.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hediger MA, et al. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins Introduction. Pflugers Arch. 2004;447 (5):465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 8.Plagemann PG, et al. Nucleoside and nucleobase transport in animal cells. Biochim Biophys Acta. 1988;947 (3):405–443. doi: 10.1016/0304-4157(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 9.Schuetz JD, et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5 (9):1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 10.Bethell R, et al. In vitro interactions between apricitabine and other deoxycytidine analogues. Antimicrob Agents Chemother. 2007;51 (8):2948–2953. doi: 10.1128/AAC.01204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havlir DV, et al. In vivo antagonism with zidovudine plus stavudine combination therapy. J Infect Dis. 2000;182 (1):321–325. doi: 10.1086/315683. [DOI] [PubMed] [Google Scholar]
- 12.Ray AS, Hitchcock MJM. Metabolism of antiviral nucleosides and nucleotides. In: Lafemina RL, editor. Antiviral Research: Strategies in Antiviral Drug Discovery. ASM Press; 2009. [Google Scholar]
- 13.Jacobsson B, et al. Decrease in thymidylate kinase activity in peripheral blood mononuclear cells from HIV-infected individuals. Biochem Pharmacol. 1998;56 (3):389–395. doi: 10.1016/s0006-2952(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 14.Nitanda T, et al. Anti-human immunodeficiency virus type 1 activity and resistance profile of 2,3 ′-didehydro-3 ′-deoxy-4 ′-ethynylthymidine in vitro. Antimicrobial Agents and Chemotherapy. 2005;49 (8):3355–3360. doi: 10.1128/AAC.49.8.3355-3360.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paintsil E, et al. Intracellular metabolism and persistence of the anti-human immunodeficiency virus activity of 2′,3′-didehydro-3′-deoxy-4′-ethynylthymidine, a novel thymidine analog. Antimicrob Agents Chemother. 2007;51 (11):3870–3879. doi: 10.1128/AAC.00692-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry MG, et al. The effect of zidovudine dose on the formation of intracellular phosphorylated metabolites. AIDS. 1996;10 (12):1361–1367. doi: 10.1097/00002030-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Becher F, et al. Monitoring of didanosine and stavudine intracellular trisphosphorylated anabolite concentrations in HIV-infected patients. Aids. 2004;18 (2):181–187. doi: 10.1097/00002030-200401230-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz SJ, et al. Development of an optimized dose for coformulation of zidovudine with drugs that select for the K65R mutation using a population pharmacokinetic and enzyme kinetic simulation model. Antimicrob Agents Chemother. 2008;52 (12):4241–4250. doi: 10.1128/AAC.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurwitz SJ, Schinazi RF. In silico study supports the efficacy of a reduced dose regimen for stavudine. Antiviral Res. 2011;92 (2):372–377. doi: 10.1016/j.antiviral.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsson B, et al. Decreased thymidine kinase levels in peripheral blood cells from HIV-seropositive individuals: implications for zidovudine metabolism. AIDS Res Hum Retroviruses. 1995;11 (7):805–811. doi: 10.1089/aid.1995.11.805. [DOI] [PubMed] [Google Scholar]
- 21.Hellerstein M, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5 (1):83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 22.Mohri H, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001;194 (9):1277–1287. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher CV. Pharmacologic considerations for therapeutic success with antiretroviral agents. Ann Pharmacother. 1999;33 (9):989–995. doi: 10.1345/aph.19075. [DOI] [PubMed] [Google Scholar]
- 24.Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen. 2007;48 (3–4):166–172. doi: 10.1002/em.20223. [DOI] [PubMed] [Google Scholar]
- 25.Carr A, et al. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14 (3):F25–32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 26.Feng JY, et al. Relationship between antiviral activity and host toxicity: comparison of the incorporation efficiencies of 2′,3′-dideoxy-5-fluoro-3′-thiacytidine-triphosphate analogs by human immunodeficiency virus type 1 reverse transcriptase and human mitochondrial DNA polymerase. Antimicrob Agents Chemother. 2004;48 (4):1300–1306. doi: 10.1128/AAC.48.4.1300-1306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson AA, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276 (44):40847–40857. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- 28.Stuyver LJ, et al. Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob Agents Chemother. 2002;46 (12):3854–3860. doi: 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassit L, et al. Comparasin of two human pancreatic cell lines for predicting mitochondrial toxicity by nucleoside analogs. HIV DART, 2008, Abstract 50. Global Antiviral Journal. 2008;4(Supplement 1):56. [Google Scholar]
- 30.Gallant JE, et al. The 3-year renal safety of a tenofovir disoproxil fumarate vs. a thymidine analogue-containing regimen in antiretroviral-naive patients. AIDS. 2008;22 (16):2155–2163. doi: 10.1097/QAD.0b013e3283112b8e. [DOI] [PubMed] [Google Scholar]
- 31.Cihlar T, et al. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides & Nucleic Acids. 2001;20 (4–7):641–648. doi: 10.1081/NCN-100002341. [DOI] [PubMed] [Google Scholar]
- 32.Kohler JJ, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009;89 (5):513–519. doi: 10.1038/labinvest.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kristoffersen US, et al. Changes in biomarkers of cardiovascular risk after a switch to abacavir in HIV-1-infected individuals receiving combination antiretroviral therapy. HIV Med. 2009;10 (10):627–633. doi: 10.1111/j.1468-1293.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabin CA, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D : A : D study: a multi-cohort collaboration. Lancet. 2008;371 (9622):1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohler JJ, et al. Absence of mitochondrial toxicity in hearts of transgenic mice treated with abacavir. Cardiovasc Toxicol. 2010;10 (2):146–151. doi: 10.1007/s12012-010-9070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundgren JD, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22 (14):F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin AM, et al. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proceedings of the National Academy of Sciences of the United States of America. 2004;101 (12):4180–4185. doi: 10.1073/pnas.0307067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward JL, Tse CM. Identification of the nucleoside transporters in human small intestine and colonic epithelial cell lines. Gastroenterology. 1998;114 (4):A431–A431. [Google Scholar]
- 39.Hurwitz SJ, et al. Comparative pharmacokinetics of Racivir, (+/−)-beta-2′,3′-dideoxy-5-fluoro-3′-thiacytidine in rats, rabbits, dogs, monkeys and HIV-infected humans. Antivir Chem Chemother. 2005;16 (2):117–127. doi: 10.1177/095632020501600204. [DOI] [PubMed] [Google Scholar]
- 40.Hall AM, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57 (5):773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Tippin TK, et al. Lipid conjugates of cidofovir and tenofovir are not substrates of human organic ion transporters hOAT1 and hOAT3. Abstract #T3396. World Congress and American Association of Pharmaceutical Scientists.2010. [Google Scholar]
- 42.Neely MN, Rakhmanina NY. Pharmacokinetic optimization of antiretroviral therapy in children and adolescents. Clin Pharmacokinet. 2011;50 (3):143–189. doi: 10.2165/11539260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Anderson BJ, et al. Population clinical pharmacology of children: general principles. Eur J Pediatr. 2006;165 (11):741–746. doi: 10.1007/s00431-006-0188-y. [DOI] [PubMed] [Google Scholar]
- 44.Jullien V, et al. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrob Agents Chemother. 2005;49 (8):3361–3366. doi: 10.1128/AAC.49.8.3361-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao W, et al. Population pharmacokinetics and maximum a posteriori probability Bayesian estimator of abacavir: application of individualized therapy in HIV-infected infants and toddlers. Br J Clin Pharmacol. 2012;73 (4):641–650. doi: 10.1111/j.1365-2125.2011.04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sales SD, et al. Zidovudine phosphorylation and mitochondrial toxicity in vitro. Toxicol Appl Pharmacol. 2001;177 (1):54–58. doi: 10.1006/taap.2001.9288. [DOI] [PubMed] [Google Scholar]
- 47.Murphy RL, et al. Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals. Antivir Ther. 2010;15 (2):185–192. doi: 10.3851/IMP1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casiro AD. The evolving role of stavudine as part of initial antiretroviral therapy in resource-constrained settings. Clinical Care Options. 2011;2011 [Google Scholar]
- 49.Sinxadi PZ, et al. Lack of association between stavudine exposure and lipoatrophy, dysglycaemia, hyperlactataemia and hypertriglyceridaemia: a prospective cross sectional study. AIDS Res Ther. 2010;7:23. doi: 10.1186/1742-6405-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perelson AS. Modelling viral and immune system dynamics. Nat Rev Immunol. 2002;2 (1):28–36. doi: 10.1038/nri700. [DOI] [PubMed] [Google Scholar]
- 51.Markowitz M, et al. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo. J Virol. 2003;77 (8):5037–5038. doi: 10.1128/JVI.77.8.5037-5038.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosario MC, et al. A pharmacokinetic-pharmacodynamic model to optimize the phase IIa development program of maraviroc. J Acquir Immune Defic Syndr. 2006;42 (2):183–191. doi: 10.1097/01.qai.0000220021.64115.37. [DOI] [PubMed] [Google Scholar]
- 53.Bonhoeffer S, et al. Virus dynamics and drug therapy. Proc Natl Acad Sci U S A. 1997;94 (13):6971–6976. doi: 10.1073/pnas.94.13.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurwitz SJ, et al. Development of a population simulation model for HIV monotherapy virological outcomes using lamivudine. Antivir Chem Chemother. 2007;18 (6):329–341. doi: 10.1177/095632020701800605. [DOI] [PubMed] [Google Scholar]
- 55.Chun TW, Fauci AS. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS. 2012;26 (10):1261–1268. doi: 10.1097/QAD.0b013e328353f3f1. [DOI] [PubMed] [Google Scholar]
- 56.Smith MZ, et al. HIV reservoirs and strategies for eradication. Curr HIV/AIDS Rep. 2012;9 (1):5–15. doi: 10.1007/s11904-011-0108-2. [DOI] [PubMed] [Google Scholar]
- 57.Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antivir Chem Chemother. 2009;20 (2):63–78. doi: 10.3851/IMP1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Else LJ, et al. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther. 2011;16 (8):1149–1167. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 59.Heneine W, Kashuba A. HIV Prevention by Oral Preexposure Prophylaxis. Cold Spring Harb Perspect Med. 2012;2 (3):a007419. doi: 10.1101/cshperspect.a007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patterson K, et al. Exposure of extracellular and intracellular tenofovir and emtricitabine in mucosal tissues after a single of fixed-dose TDF/FTC: implications for pre-exposure HIV prophylaxis (PrEP). XVIII International AIDS Conference.2010. [Google Scholar]
- 61.van Marle G, et al. Higher levels of zidovudine resistant HIV in the colon compared to blood and other gastrointestinal compartments in HIV infection. Retrovirology. 2010;7:74. doi: 10.1186/1742-4690-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ananworanich J, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7 (3):e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sips M, et al. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5 (1):30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75 (23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Letendre S, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65 (1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marra CM, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23 (11):1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shikuma CNB, et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17(7):1233–1242. doi: 10.3851/IMP2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer-Smith T, et al. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14 (4):318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennedy EM, et al. Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages. J Biol Chem. 2010;285 (50):39380–39391. doi: 10.1074/jbc.M110.178582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruane PJ, Luber AD. K65R-associated virologic failure in HIV-infected patients receiving tenofovir-containing triple nucleoside/nucleotide reverse transcriptase inhibitor regimens. MedGenMed. 2004;6 (2):31. [PMC free article] [PubMed] [Google Scholar]
- 72.Winston A, et al. The prevalence and determinants of the K65R mutation in HIV-1 reverse transcriptase in tenofovir-naive patients. AIDS. 2002;16 (15):2087–2089. doi: 10.1097/00002030-200210180-00018. [DOI] [PubMed] [Google Scholar]
- 73.Schinazi RF, et al. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37 (4):875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McColl DJ, et al. Reduced emergence of the M184V/I resistance mutation when antiretroviral-naive subjects use emtricitabine versus lamivudine in regimens composed of two NRTIs plus the NNRTI efavirenz. HIV Clin Trials. 2011;12 (2):61–70. doi: 10.1310/hct1202-61. [DOI] [PubMed] [Google Scholar]
- 75.Schinazi RF, et al. Selection and characterization of HIV-1 with a novel S68 deletion in reverse transcriptase. Antimicrob Agents Chemother. 2011;55 (5):2054–2060. doi: 10.1128/AAC.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner D, et al. Multiple effects of the M184V resistance mutation in the reverse transcriptase of human immunodeficiency virus type 1. Clin Diagn Lab Immunol. 2003;10 (6):979–981. doi: 10.1128/CDLI.10.6.979-981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scourfield A, et al. Discontinuation of Atripla as first-line therapy in HIV-1 infected individuals. AIDS. 2012;26 (11):1399–1401. doi: 10.1097/QAD.0b013e328353b047. [DOI] [PubMed] [Google Scholar]
- 78.McComsey G, Lonergan JT. Mitochondrial dysfunction: patient monitoring and toxicity management. J Acquir Immune Defic Syndr. 2004;37(Suppl 1):S30–35. doi: 10.1097/01.qai.0000137004.63376.27. [DOI] [PubMed] [Google Scholar]
- 79.Hurwitz SJ, et al. Lack of pharmacokinetic interaction between amdoxovir and reduced- and standard-dose zidovudine in HIV-1-infected individuals. Antimicrob Agents Chemother. 2010;54 (3):1248–1255. doi: 10.1128/AAC.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng JY, et al. Anabolism of amdoxovir: phosphorylation of dioxolane guanosine and its 5′-phosphates by mammalian phosphotransferases. Biochem Pharmacol. 2004;68 (9):1879–1888. doi: 10.1016/j.bcp.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 81.Seigneres B, et al. Inhibitory activity of dioxolane purine analogs on wild-type and lamivudine-resistant mutants of hepadnaviruses. Hepatology. 2002;36 (3):710–722. doi: 10.1053/jhep.2002.35070. [DOI] [PubMed] [Google Scholar]
- 82.Mewshaw JP, et al. Dioxolane guanosine, the active form of the prodrug diaminopurine dioxolane, is a potent inhibitor of drug-resistant HIV-1 isolates from patients for whom standard nucleoside therapy fails. J Acquir Immune Defic Syndr. 2002;29 (1):11–20. doi: 10.1097/00042560-200201010-00002. [DOI] [PubMed] [Google Scholar]
- 83.Kewn S, et al. Enzymatic assay for measurement of intracellular DXG triphosphate concentrations in peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 2003;47 (1):255–261. doi: 10.1128/AAC.47.1.255-261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui L, et al. Effect of beta-enantiomeric and racemic nucleoside analogues on mitochondrial functions in HepG2 cells. Implications for predicting drug hepatotoxicity. Biochem Pharmacol. 1996;52 (10):1577–1584. doi: 10.1016/s0006-2952(96)00562-x. [DOI] [PubMed] [Google Scholar]
- 85.Parikh UM, et al. In vitro activity of structurally diverse nucleoside analogs against human immunodeficiency virus type 1 with the K65R mutation in reverse transcriptase. Antimicrob Agents Chemother. 2005;49 (3):1139–1144. doi: 10.1128/AAC.49.3.1139-1144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rapp KL, et al. The combination of zidovudine and amdoxovir prevents the selection of thymidine analog mutations in primary human lymphocytes. XVI International HIV Drug Resistance Workshop; 2007. p. S130. [Google Scholar]
- 87.Trost LC, et al. Preclinical evaluation of CMX157: a lipid-conjugated nucleotide analog for the treatment of HIV. 49th SOT Meeting Abstract # 1057–216.2010. [Google Scholar]
- 88.Lanier ER, et al. Development of Hexadecyloxypropyl Tenofovir (CMX157) for Treatment of Infection Caused by Wild-Type and Nucleoside/Nucleotide-Resistant HIV. Antimicrobial Agents and Chemotherapy. 2010;54 (7):2901–2909. doi: 10.1128/AAC.00068-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Painter GR, et al. Evaluation of hexadecyloxypropyl-9-R-[2-(Phosphonomethoxy)propyl]-adenine, CMX157, as a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrob Agents Chemother. 2007;51 (10):3505–3509. doi: 10.1128/AAC.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lanier R, et al. Hexadecyloxypropyl tenofovir associates directly with HIV and subsequently inhibits viral replication in untreated cells. 16th Conference on retroviruses and Opportunistic Infections; Montreal, Canada. 2009. p. Abstract 556. [Google Scholar]
- 91.Markowitz M, et al. GS-7340 Demonstrates Greater Declines in HIV-1 RNA than Tenofovir Disoproxil Fumarate During 14 Days of Monotherapy in HIV-1 Infected Subjects. 18th Conference on Retroviruses and Opportunistic Infections; 2011. p. Abstract # 568. [Google Scholar]
- 92.Ruane P, et al. GS-7340 25 mg and 40 mg demonstrate superior efficacy to tenofovir 300 mg in a 10-day monotherapy study of HIV-1+patients. Abstract # 103. 18th Conference on Retroviruses and Opportunistic Infections; 2012. p. Abstract # 568. [Google Scholar]
- 93.Lee WA, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49 (5):1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Birkus G, et al. Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases. Mol Pharmacol. 2008;74 (1):92–100. doi: 10.1124/mol.108.045526. [DOI] [PubMed] [Google Scholar]
- 95.Dutschman GE, et al. Novel 4′-substituted stavudine analog with improved anti-human immunodeficiency virus activity and decreased cytotoxicity. Antimicrob Agents Chemother. 2004;48 (5):1640–1646. doi: 10.1128/AAC.48.5.1640-1646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levin J, et al. The HIV NRTI BMS-986001 does not degrade mitochondrial DNA in long term primary cultures of cells isolated from human kidney, muscle and subcutaneous fat. 19th International AIDS Conference; 2012. p. Abstract # TUPE042. [Google Scholar]
- 97.Paintsil E, et al. A single-dose escalation study to evaluate the safety, tolerability, and pharmacokinetics of OBP-601, a novel NRTI, in healthy subjects. 16th Conference on Retroviruses and Opportunistic Infections; 2009. p. Abstract # 568. [Google Scholar]
- 98.Cotte L, et al. A phase Ib/2a dose escalation study of OBP-601 (4′ethynyl-d4T, festinavir) in treatment experienced, HIV-1-infected patients. 50th International Conference on Antimicrobial Agents and Chemotherapy; 2010. p. Abstract # H933. [Google Scholar]
- 99.Hwang C, et al. Antiviral activity, exposure-response, and resistance analyses of monotherapy with the novel HIV nucleoside reverse transcriptase inhibitor (NRTI) BMS-986001 in antiretroviral treatment-experienced subjects. 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012. p. Abstract #O-06. [Google Scholar]
- 100.Maeda K, et al. Delayed emergence of HIV-1 variants resistant to 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA): comparative sequential passage with tenofovir, emtricitabine and festinavir. AIDS. 2012;2012:Abstract # TUPE017. doi: 10.3851/IMP2697. [DOI] [PubMed] [Google Scholar]
- 101.Sohl CD, et al. Balancing Antiviral Potency and Host Toxicity: Identifying a Nucleotide Inhibitor with an Optimal Kinetic Phenotype for HIV-1 Reverse Transcriptase. Mol Pharmacol. 2012;82 (1):125–133. doi: 10.1124/mol.112.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphey-Corb M, et al. Response of SIV to the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) in vitro and in vivo. Antimicrob Agents Chemother. 2012;56(9):4707–12. doi: 10.1128/AAC.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sohl CD, et al. Mechanism of interaction of human mitochondrial DNA polymerase gamma with the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine indicates a low potential for host toxicity. Antimicrob Agents Chemother. 2012;56 (3):1630–1634. doi: 10.1128/AAC.05729-11. [DOI] [PMC free article] [PubMed] [Google Scholar]