Abstract
Purpose
To describe the frequency of MLH1 promoter methylation in colorectal cancer (CRC); to explore the associations between MLH1 promoter methylation and clinicopathological and molecular factors using a systematic review and meta-analysis.
Methods
A literature search of the PubMed and Embase databases was conducted to identify relevant articles published up to September 7, 2012 that described the frequency of MLH1 promoter methylation or its associations with clinicopathological and molecular factors in CRC. The pooled frequency, odds ratio (OR) and 95% confidence intervals (95% CI) were calculated.
Results
The pooled frequency of MLH1 promoter methylation in unselected CRC was 20.3% (95% CI: 16.8–24.1%). They were 18.7% (95% CI: 14.7–23.6%) and 16.4% (95% CI: 11.9–22.0%) in sporadic and Lynch syndrome (LS) CRC, respectively. Significant associations were observed between MLH1 promoter methylation and gender (pooled OR = 1.641, 95% CI: 1.215–2.215; P = 0.001), tumor location (pooled OR = 3.804, 95% CI: 2.715–5.329; P<0.001), tumor differentiation (pooled OR = 2.131, 95% CI: 1.464–3.102; P<0.001), MSI (OR: 27.096, 95% CI: 13.717–53.526; P<0.001). Significant associations were also observed between MLH1 promoter methylation and MLH1 protein expression, BRAF mutation (OR = 14.919 (95% CI: 6.427–34.631; P<0.001) and 9.419 (95% CI: 2.613–33.953; P = 0.001), respectively).
Conclusion
The frequency of MLH1 promoter methylation in unselected CRC was 20.3%. They were 18.7% in sporadic CRC and 16.4% in LS CRC, respectively. MLH1 promoter methylation may be significantly associated with gender, tumor location, tumor differentiation, MSI, MLH1 protein expression, and BRAF mutation.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies, representing the third most common cancer in men and the second in women worldwide [1]. One of the genetic pathways in the development of CRC is the microsatellite instability (MSI) [2].
Microsatellites are repeated DNA sequences which occur approximately every 50–100 Kb base pairs throughout the human genome [3], [4]. Multiple studies have indicated that about 90% of the Lynch Syndrome (LS) [5], [6] and 10% to 15% of sporadic CRC can be detected of MSI [3], [4]. MSI in LS and sporadic CRC occurs through two different mechanisms. In LS, MSI is mainly caused by germline mutation of mismatch repair genes [7]. MSI in sporadic CRC is commonly due to methylation induced silencing of the MLH1 gene [8].
DNA methylation refers to the presence of a methyl group on a cytosine residue [9]. DNA methylation of tumor suppressor genes leading to transcriptional inactivation has been identified as an important mechanism in human carcinogenesis [10], [11]. MLH1 gene, as a number of suppressor genes, is prone to be silenced by promoter methylation in CRC [8], [12], [13].
Since the first report of MLH1 promoter methylation in sporadic colon tumors [8], the prevalence of MLH1 promoter methylation have been widely studied not only in sporadic but also in LS CRC. However, the results are inconsistent. The frequency of MLH1 promoter methylation in sporadic CRC varied from 0.0% [14] to 66.9% [15]. It varied from 0.0% [16] to 21.4% [17] in LS CRC.
BRAF and KRAS are important members of RAS/RAF/MAPK signaling pathway, which regulates cell growth, proliferation, differentiation, and apoptosis in malignant and nonmalignant cells [18]. BRAF mutation has been shown to be associated with MLH1 promoter methylation [19], [20]. Whereas, MLH1 promoter methylation was few detected in KRAS mutant CRC [21]. The associations between MLH1 promoter methylation and BRAF and KRAS mutation in CRC have been widely studied with inconsistent results [15], [21], [22], [23]. The associations between MLH1 promoter methylation and other clinicopathological and molecular characteristics of CRC such as tumor location, tumor staging, tumor differentiation, family history, MSI, and MLH1 protein expression were also widely studied. However, the results are inconsistent. Therefore, we conducted a systematic review and meta-analysis to accurately estimate the frequency of MLH1 promoter methylation in LS and sporadic CRC, and the associations between MLH1 promoter methylation and clinicopathological/molecular characteristics of CRC.
Methods
Search Strategy and Selection Criteria
We conducted a systematic literature search using PubMed and Embase from January 1, 1997 to September 7, 2012 to identify all the relevant English-language articles. The following keywords were used: “methylation” and “MLH1” and “promoter” and “colorectal cancer” and/or “carcinoma” or “tumor” or “neoplasm”. We also hand-searched the reference lists of the retrieved articles and reviews for additional articles.
The inclusion/exclusion criteria were as follows: (1) papers on MLH1 promoter methylation in unselected CRC were included. In contrast, papers that selected subgroups were excluded (such as selected based on age, tumor staging and ulcerative colitis-associated CRC); (2) sporadic CRC and/or LS related CRC remained as specific selected groups, often stratified by MSI status and/or MLH1 expression loss; (3) data regarding the DNA methylation of tumor tissue of CRC were included in the pooled analysis, whereas data regarding the DNA methylation of normal colonic mucosa [24], [25], [26], serum [27], [28], and peripheral blood leukocyte [29], [30], [31] of CRC were excluded; (4) studies that investigated multiple CRCs were excluded [32], [33], [34]; (5) case reports were excluded; (6) repetitive reports were unified by using the latest or the largest edition; (7) paper with insufficient or duplicated data were excluded.
Data Extraction
Two authors (X. and X.P) independently conducted literature searches to identify all possible papers that met the inclusion criteria. Disagreements were settled by consensus or a third review (Y.B.N) for adjudication. The following information were extracted from every eligible study: authors, publication year, continent, country, patient source, sample size, methylation detecting method, positive frequency, gender, family history, tumor location (proximal and distal), tumor staging, and promoter regions.
Classification of Family History
Patients had no family history of cancer regardless of the onset age were categorized as sporadic CRC. LS was diagnosed if a patient with family history met either Amsterdam criteria (I or II) [35], [36] or Bethesda criteria (original or revised) [37], [38] or confirmed with germline mutation in a DNA mismatch repair gene [39], [40]. The unselected CRC tumors were defined as patients from nature population or hospital-based. The unselected CRC tumors included sporadic and LS CRC, which were defined as total CRC.
Tumor Staging and Differentiation
Tumor staging was categorized as I, II, III and IV stages based on the TNM classification (The Union for International Cancer Control [UICC]) [41]. Stage I: Cancer has begun to spread, but is still in the inner lining. Stage II: Cancer has spread to other organs near the colon or rectum. It has not reached lymph nodes. Stage III: Cancer has spread to lymph nodes, but has not been carried to distant parts of the body. Stage IV: Cancer has been carried through the lymph system to distant parts of the body. Differentiation was graded on a scale of poor, moderate or well differentiation.
Promoter Regions
Promoter regions tested were noted as the A, B, C and D regions proposed by Deng et al. [42], where primer sequences were given. Promoter regions were checked against the sequence −1000 to −1, relative to the start codon of MLH1.
Molecular Classification
MSI is typically assessed by analyzing five microsatellite markers (BAT25, BAT26, D2S123, D5S346, and D17S250) suggested by the National Cancer Institute [43]. One study expanded this panel to ten markers, which made the diagnosis of MSI CRC easier [38]. In this meta-analysis, three categories of MSI status were defined according to the following criteria: two or more loci out of five loci with instability (or ≥30–40% of loci if a larger panel of markers was used) was defined as MSI-H; one locus with instability (or <30–40% of loci in larger panels) was defined as lower-level microsatellite instability (MSI-L); and no loci with instability (or no apparent instability in larger panels) was defined as microsatellite stable (MSS). For papers without detailed information about MSI-H and MSI-L, only two levels of microsatellite instability status could be categorized: MSI-positive (MSI) and MSI-negative (MSS). BRAF and KRAS status were classified into mutant and wild type. MLH1 protein expression status was defined as positive or negative.
Statistical Analysis
The pooled frequency of MLH1 promoter methylation and 95% confidence intervals (95% CI) were estimated. The frequency of MLH1 promoter methylation was compared in different tumor characteristics. Heterogeneity among studies was evaluated with Cochran’s Q test [44] and the I2 statistic [45], [46]. When heterogeneity was not an issue (I2 values <50%), a fixed effect model was used to calculate parameters. If there was substantial heterogeneity (I2 values ≥50%), a random-effects model was used to pool data and attempt to identify potential sources of heterogeneity based on subgroup analyses. The pooled OR was estimated for the association between MLH1 promoter methylation and clinicopathological, molecular features. P values tailed less than 0.05 were considered statistically significant.
Publication bias was evaluated with funnel plot, Begg’s rank correlation [47], and Egger’s regression [48]. If publication bias existed, the trim and fill method was used to adjust the pooled frequency, pooled OR and 95% CI [49]. Data were calculated with Comprehensive Meta-Analysis V2.
Results
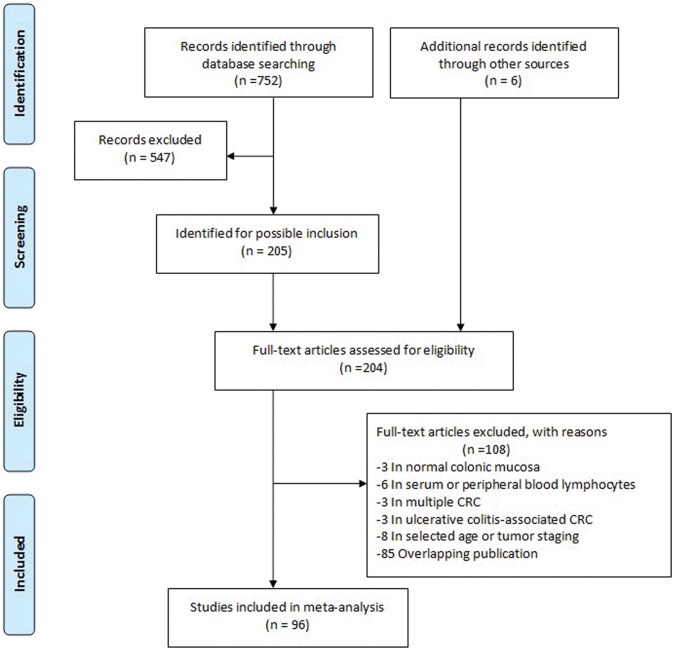
752 relevant articles were identified for initial review according to the inclusion and exclusion criteria. After screening, information for 10528 individuals from 96 studies was reviewed and included in the meta-analyses. Figure 1 showed the detailed selection process of articles.
Figure 1. Flow diagram of study selection.
Frequency of MLH1 Promoter Methylation in Total CRC Tumors
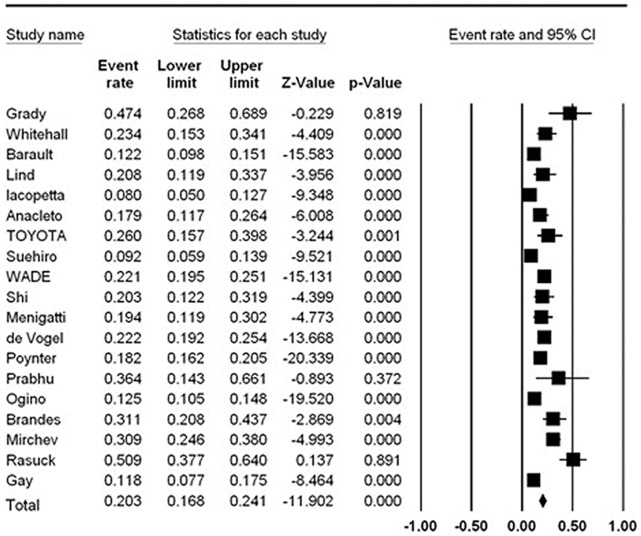
Overall, there were 19 studies with 5584 patients demonstrating MLH1 promoter methylation in total CRC tumors. The total frequency of MLH1 promoter methylation was 20.3% (95% CI: 16.8–24.1%) (Table 1, Figure 2). There was significant heterogeneity among the studies (I2 = 87.896%).
Table 1. Pooled frequency of MLH1 promoter methylation in colorectal cancer patients with different clinicopathological features.
| Classification | No. ofstudies | No. ofdetectedcases | No. ofmethylationcases | Pooled frequencyand 95%CI (%) | P | Heterogeneity | Publication bias | ||
| I2 (%) | P | Begg’stest P | Egger’stest P | ||||||
| CRC(total) | 19 | 5584 | 1005 | 20.3(16.8–24.1)* | 0.000 | 87.896 | 0.000 | 0.132 | 0.472 |
| Subject source** | |||||||||
| Hospital-based | 16 | 2335 | 449 | 20.5(15.6–26.5)* | 0.000 | 86.938 | 0.000 | 0.589 | 0.761 |
| Population-based | 4 | 3249 | 556 | 16.4(12.1–22.0)* | 0.000 | 93.166 | 0.000 | 0.497 | 0.244 |
| Family history | |||||||||
| LS | 8 | 243 | 31 | 16.4(11.9–22.0) | 0.000 | 15.564 | 0.165 | 0.048 | 0.001 |
| SCRC | 29 | 3583 | 739 | 18.7(14.7–23.6)* | 0.000 | 90.505 | 0.000 | 0.143 | 0.032 |
| Gender** | |||||||||
| Female | 6 | 555 | 124 | 20.8(15.6–27.2) | 0.000 | 48.843 | 0.082 | 0.851 | 0.255 |
| Male | 6 | 699 | 102 | 11.8(6.9–16.5)* | 0.000 | 75.322 | 0.001 | 0.348 | 0.136 |
| Location** | |||||||||
| Proximal | 6 | 474 | 139 | 29.6(20.4–40.8)* | 0.001 | 76.757 | 0.001 | 0.573 | 0.795 |
| Distal | 6 | 698 | 71 | 6.5(3.0–13.4)* | 0.000 | 74.442 | 0.002 | 0.348 | 0.029 |
| UICC stage** | |||||||||
| I & II | 4 | 160 | 34 | 22.4(15.6–31.0) | 0.000 | 20.902 | 0.285 | 0.174 | 0.028 |
| III & IV | 4 | 123 | 24 | 25.5(9.3–53.5)* | 0.083 | 82.686 | 0.001 | 1.000 | 0.807 |
| Differentiation** | |||||||||
| Poor | 6 | 182 | 56 | 31.0(24.6–38.1) | 0.000 | 0.000 | 0.637 | 0.188 | 0.540 |
| Moderate or Well | 6 | 769 | 130 | 17.6(11.9–25.3)* | 0.000 | 68.101 | 0.008 | 0.188 | 0.796 |
Abbreviations: CRC, colorectal cancer; LS, lynch syndrome; SCRC, sporadic colorectal cancer.
Random effect estimate.
It is only pooled data with total colorectal cancer.
Figure 2. The pooled frequency of MLH1 promoter methylation in CRC.
Family History
The frequency of MLH1 promoter methylation was 18.7% (95% CI: 14.7–23.6%) in 3583 sporadic CRC of 29 studies and 16.4% (95% CI: 11.9–22.0%) in 243 LS reported in eight studies (Table 1). The frequency of MLH1 promoter methylation in sporadic MSI-H and LS CRC MSI-H were 73.6% (95% CI: 67.3–79.0%) and 15.3% (95% CI: 8.8–25.4%), respectively (Table 2). For MSI-H CRC, significant association between MLH1 promoter methylation and family history was observed (pooled OR = 20.828, 95% CI: 4.056–106.950; P<0.001; I2 = 55.363%; Table 3), when pooled data on four studies [50], [51], [52], [53].
Table 2. Pooled frequency of MLH1 promoter methylation in colorectal cancer patients with different molecular features.
| Classification | No. ofstudies | No. ofdetectedcases | No. ofmethylationcases | Frequency and95%CI (%) | Heterogeneity | Publication bias | ||
| I2 (%) | P | Begg’stest P | Egger’stest P | |||||
| CRC(total) MSI Status | ||||||||
| MSI-H | 12 | 968 | 566 | 62.6(54.0–70.4)* | 78.288 | 0.000 | 0.411 | 0.049 |
| MSI-L | 4 | 344 | 24 | 12.2(3.0–38.2)* | 90.733 | 0.000 | 1.000 | 0.845 |
| MSI | 16 | 1325 | 576 | 55.8(45.2–65.8)* | 89.197 | 0.000 | 0.280 | 0.002 |
| MSS | 10 | 1791 | 155 | 5.2(2.2–11.6)* | 88.938 | 0.000 | 0.655 | 0.068 |
| LS- MSI-H | 5 | 95 | 11 | 15.3(8.8–25.4) | 0.000 | 0.517 | 0.050 | 0.160 |
| SCRC MSI Status | ||||||||
| MSI-H | 11 | 249 | 188 | 73.6(67.3–79.0) | 42.882 | 0.000 | 0.119 | 0.228 |
| MSI | 7 | 193 | 125 | 67.3(47.1–82.7)* | 79.255 | 0.000 | 0.293 | 0.253 |
| MSS | 4 | 264 | 51 | 17.5(10.0–29.0) | 35.109 | 0.202 | 0.174 | 0.014 |
| CRC(total) MLH1expression | ||||||||
| Positive | 2 | 67 | 7 | 11.9(1.5–53.8)* | 83.531 | 0.014 | – | – |
| Negative | 5 | 106 | 73 | 66.5(44.4–83.2)* | 73.534 | 0.004 | 1.000 | 0.981 |
| MSI-H- Loss of MLH1 protein | 5 | 247 | 201 | 80.8(75.3–85.3) | 0.000 | 0.644 | 0.624 | 0.321 |
| SCRC-MLH1 expression | ||||||||
| Positive | 6 | 308 | 25 | 9.8(3.4–25.2)* | 76.529 | 0.001 | 0.851 | 0.366 |
| Negative | 10 | 156 | 108 | 69.8(45.5–86.5)* | 78.786 | 0.000 | 0.089 | 0.083 |
| MSI-H- Loss of MLH1 protein | 3 | 64 | 56 | 86.3(75.3–92.9) | 0.000 | 0.618 | 0.602 | 0.782 |
| LS- Loss of MLH1 protein | 5 | 169 | 65 | 37.8 (25.3–52.1)* | 62.057 | 0.000 | 0.142 | 0.155 |
| CRC(total)BRAF situation | ||||||||
| Mutant | 3 | 138 | 70 | 53.2(27.7–77.2)* | 67.744 | 0.045 | 0.602 | 0.572 |
| Wild type | 3 | 764 | 113 | 13.7(5.1–32.0)* | 90.433 | 0.000 | 0.602 | 0.792 |
| CRC(total)KRAS situation | ||||||||
| Mutant | 3 | 353 | 41 | 14.0(10.2–19.0) | 9.722 | 0.330 | 0.117 | 0.119 |
| Wild type | 3 | 570 | 135 | 21.8(13.2–33.8)* | 77.209 | 0.012 | 0.602 | 0.699 |
Abbreviations: CRC, colorectal cancer; LS, lynch syndrome; MMR, mismatch repair; SCRC, sporadic colorectal cancer; MSI, Microsatellite instability; MSS, microsatellite stability; MSI-L, lower-level microsatellite instability; MSI-H, high-level microsatellite instability.
Random effect estimate.
Table 3. Pooled associations between MLH1 promoter methylation and clinicopathological and molecular features.
| Classification | No. of studies | OR and 95%CI | P | Heterogeneity | Publication bias | ||
| I2 (%) | P | Begg’s test P | Egger’s test P | ||||
| SCRC- MSI-H vs. LS- MSI-H | 4 | 20.828(4.056–106.950)* | 0.000 | 55.363 | 0.081 | 0.497 | 0.225 |
| Female vs. Male** | 6 | 1.641(1.215–2.215) | 0.001 | 33.819 | 0.183 | 0.851 | 0.438 |
| Proximal vs. Distal** | 6 | 3.804(2.715–5.329) | 0.000 | 46.541 | 0.096 | 0.348 | 0.042 |
| UICC stage (I & II vs. III & IV)** | 4 | 1.044(0.441–2.471) | 0.922 | 42.854 | 0.154 | 0.497 | 0.773 |
| Poor vs. Moderate or Well** | 6 | 2.131(1.464–3.102) | 0.000 | 0.000 | 0.674 | 0.573 | 0.430 |
| MSI vs. MSS** | 10 | 27.096(13.717–53.526)* | 0.000 | 59.001 | 0.000 | 0.531 | 0.438 |
| MSI-H vs. Non- MSI-H** | 4 | 17.061(3.850–75.610)* | 0.000 | 69.023 | 0.021 | 0.497 | 0.336 |
| MSI vs. MSS*** | 4 | 33.549(3.942–285.515)* | 0.001 | 81.145 | 0.001 | 0.497 | 0.297 |
| MLH1 expression*** | |||||||
| Negative vs. Positive | 6 | 14.919(6.427–34.631) | 0.000 | 35.469 | 0.171 | 0.573 | 0.455 |
| BRAF situation | |||||||
| Mutant vs. Wild type** | 3 | 9.419(2.613–33.953)* | 0.001 | 67.030 | 0.048 | 0.602 | 0.115 |
| CRC- MSI-H** | |||||||
| Mutant vs. Wild type | 3 | 37.615(10.011–141.311) | 0.000 | 0.000 | 0.913 | 0.117 | 0.251 |
| KRAS situation | |||||||
| Mutant vs. Wild type** | 3 | 0.476(0.322–0.703) | 0.000 | 49.293 | 0.139 | 0.117 | 0.161 |
| MSI- CRC** | |||||||
| Mutant vs. Wild type | 2 | 0.340(0.167–0.693) | 0.003 | 0.000 | 0.674 | – | – |
Abbreviations: CRC, colorectal cancer; LS, lynch syndrome; SCRC, sporadic colorectal cancer; MSI, Microsatellite instability; MSS, microsatellite stability;
MSI-H, high-level microsatellite instability.
Random effect estimate.
It is only pooled data with total colorectal cancer.
It is only pooled data with sporadic colorectal cancer.
Gender
Gender information was available for 6 of the 19 studies with a total of 555 female and 699 male patients [16], [21], [22], [54], [55], [56]. The MLH1 promoter methylation in female and male were 20.8% (95% CI: 15.6–27.2%) and 11.8% (95% CI: 6.9–16.5%) in total CRC group (pooled OR = 1.641, 95% CI = 1.215–2.215; P = 0.001; I2 = 33.819%) (Table 1 and Table 3).
Tumor Location
MLH1 promoter methylation was observed in 29.6% (95% CI: 20.4–40.8%) of the 474 proximal tumors and 6.5% (95% CI: 3.0–13.4%) of the 698 distal tumors in six studies (Table 1). Significant association was observed between MLH1 promoter methylation and tumor location (pooled OR = 3.804, 95% CI: 2.715–5.329; P<0.001; I2 = 46.541%) (Table 3). These data were based on six studies covering a total of 1172 patients (Table 1).
Tumor Staging
The pooled prevalence of MLH1 promoter methylation and pooled OR for the association between MLH1promoter methylation and the UICC stage were estimated in four studies [54], [56], [57], [58] (Table 1 and Table 3). The pooled prevalence of MLH1 promoter methylation in stages I & II and in stages III & IV were 22.4% (95% CI: 15.6–31.0%) and 25.5% (95% CI: 9.3–53.5%), respectively (Table 1). The association between MLH1 promoter methylation and tumor staging was not significant (pooled OR = 1.044, 95% CI: 0.441–2.471; P = 0.922; I2 = 42.854%) (Table 3).
Tumor Differentiation
Six studies [16], [21], [54], [55], [56], [57] addressed the frequency of MLH1 promoter methylation in total CRC according to tumor differentiation. The frequency of MLH1 promoter methylation was 31.0% (95% CI, 24.6–38.1%) in 182 poor-differentiated CRC and 17.6% (95% CI, 11.9–25.3%) in 769 moderate or well-differentiated CRC, respectively (Table 1). MLH1 promoter methylation in poor-differentiated CRC was significantly higher than in moderate or well-differentiated CRC (pooled OR = 2.131, 95% CI, 1.464–3.102; P<0.001; I2 = 0.000%) (Table 3).
Microsatellite Instability
For total CRC, MLH1 promoter methylation was detected in 62.6% (95% CI: 54.0–70.4%) of the 968 MSI-H CRC in 12 studies, 12.2% (95% CI: 3.0–38.2%) of the 344 MSI-L CRC in four studies, 55.8% (95% CI: 45.2–65.8%) of the 1325 MSI CRC in 16 studies, and 5.2% (95% CI: 2.2–11.6%) of the 1791 MSS CRC in 10 studies (P<0.001), respectively (Table 2). Significant differences were found between MSI vs. MSS, MSI-H vs. MSS, and MSI-H vs. MSI-L (P<0.001, P<0.001, and P<0.001, respectively). Whereas, no difference was observed between MSI-L and MSS (P = 0.380). For sporadic CRC, the pooled prevalence of MLH1 promoter methylation was 73.6% (95% CI: 67.3–79.0%) in MSI-H CRC, 67.3% (95% CI: 47.1–82.7%) in MSI CRC, and 17.5% (95% CI: 10.0–29.0%) in MSS CRC (P<0.001; Table 2). In addition, for the 10 studies [16], [22], [53], [54], [57], [59], [60], [61], [62], [63] that provided both MSI and MSS status in total CRC, the pooled OR for the association between MLH1 promoter methylation and MSI status (MSI vs. MSS) was 27.096 (95% CI: 13.717–53.526; P<0.001; I2 = 59.001%; Table 3, Figure S1).
MLH1 Protein Expression
In tumors with a loss of MLH1 protein expression, MLH1 promoter methylation was detected in 66.5% (95% CI: 44.4–83.2%) of the 106 total CRC, 80.8% (95% CI: 75.3–85.3%) of the 247 MSI-H CRC, 69.8% (95% CI: 45.5–86.5%) of the 156 sporadic CRC, and 37.8% (95% CI: 25.3–52.1%) of the 169 LS tumors (P<0.001). Significant differences were observed when comparing LS tumors vs. total CRC, LS tumors vs. sporadic CRC, and LS tumors vs. MSI-H CRC tumors (P = 0.032, P<0.001, and P = 0.026, respectively). For tumors with MLH1 protein expression, MLH1 promoter methylation was detected in 11.9% (95% CI: 1.5–53.8%) of the 67 total CRC and in 9.8% (95% CI: 3.4–25.2%) of the 308 sporadic CRC (P = 0.862; Table 2). Six studies [64], [65], [66], [67], [68], [69] provided expression status as a loss of MLH1 protein expression in 308 cases and MLH1 protein expression in 75 cases of sporadic CRC. The pooled analysis showed significantly association between MLH1 promoter methylation and MLH1 protein expression (OR = 14.919, 95% CI: 6.427–34.631%; P<0.001; I2 = 35.469%) (Table 3).
BRAF Mutation
The pooled prevalence of MLH1 promoter methylation in 138 BRAF-mutated and 764 BRAF wild type CRC was 53.2% (95% CI: 27.7–77.2%) and 13.7% (95% CI: 5.1–32.0%) in three studies (pooled OR = 9.419; 95% CI: 2.613–33.953; P = 0.001; I2 = 67.030%) (Table 2 and Table 3). For the three studies that provided both MSI-H and BRAF mutation status in CRC, the pooled OR for the association between BRAF mutation status and MLH1 promoter methylation was 37.615 in MSI-H CRC (95% CI: 10.011–141.311; P<0.001; I2 = 0.000%) (Table 3).
KRAS Mutation
The pooled frequency of MLH1 promoter methylation was 14.0% (95% CI: 10.2–19.0%) in 353 KRAS-mutated and 21.8% (95% CI: 13.2–33.8%) in 570 wild-type CRC, in three studies [15], [21], [22] (pooled OR = 0.476; 95% CI: 0.322–0.703; P<0.001; I2 = 49.293%) (Table 2 and Table 3). Moreover, a statistically significant association was observed between MLH1 promoter methylation and KRAS mutation in MSI CRC (OR = 0.340; 95% CI: 0.167–0.693; P = 0.003; I2 = 0.000%) (Table 3).
Publication Bias
For the frequency of MLH1 promoter methylation in MSI CRC and MSI-H CRC, the funnel plot seemed asymmetry (Figure S2 A and B). Funnel plot for the association between MLH1 promoter methylation and tumor location (proximal vs. distal) also seemed asymmetry (Figure S3). Begg’s rank correlation and Egger’s regression methods further supported the significant publication bias. With the trim and fill method, the adjusted frequency of MLH1 promoter methylation decreased from 55.8% to 36.7% in MSI CRC and from 62.6% to 53.5% in MSI-H CRC. The pooled OR for the association between MLH1 promoter methylation and tumor location decreased from 3.804 (95% CI: 2.715–5.329) to 3.172 (95% CI: 2.323–4.331).
Discussion
Our meta-analysis suggested that the frequency of MLH1 promoter methylation in total CRC was 20.3%. They were 18.7% in sporadic CRC and 16.4% in LS CRC, respectively; significant associations were observed between MLH1 promoter methylation and gender, tumor location, tumor differentiation, MSI, MLH1 protein expression, and BRAF mutation.
The pooled MLH1 promoter methylation frequencies were 16.4% and 20.5% in 4 population-based studies and 16 hospital-based studies (One study [53] included 1061 population-based and 172 hospital-based CRC). In total CRC, the frequency of the MLH1 promoter methylation between hospital-based and population-based studies was not significantly different (P = 0.279) (Table 1).
A, B, C and D regions in the MLH1 promoter were commonly tested for methylation. However, only one study tested the MLH1 promoter methylation in “A” region [69], three studies tested the MLH1 promoter methylation in “C” region [56], [57], [70], other 15 studies did not provide the specific A, B, C or D regions in total CRC. The MLH1 promoter methylation frequency in “A” region (66.9%) was significantly higher than in “C” region in CRC (26.4%; P = 0.001) (Table S1). It may be due to the variation of methylation status in different regions of the MLH1 promoter.
The MLH1 promoter methylation was detected in total of 12 studies with 968 MSI-H CRC with a frequency of 62.6%. After the adjustment by trim and fill method, the pooled frequency decreased to 53.5%. The pooled MLH1 promoter methylation in 249 sporadic MSI-H CRC was 73.6%, significantly higher than in 95 LS MSI-H CRC (15.3%). The following may explain our results: in sporadic CRC, MSI-H was mainly caused by MLH1 promoter methylation [13], [71]; whereas, in LS CRC, MSI-H was mainly caused by MMR inactivation because of germline mutation [72].
In sporadic CRC, our meta-analysis indicated that the MLH1 promoter methylation frequency in 308 CRC with MLH1 protein expression (9.8%), which was lower than in 156 CRC without MLH1 protein expression (69.8%, P<0.001). In CRC with loss of MLH1 protein expression, the MLH1 promoter methylation was significantly higher in sporadic CRC (69.8%) than in LS CRC (37.8%, P = 0.026). In sporadic MSI-H CRC with loss of MLH1 protein, the MLH1 promoter methylation frequency was 86.3%. MLH1 promoter methylation could explain more fraction of MLH1 gene silencing in sporadic CRC than that in LS CRC. In this systematic review and meta-analysis, we can see that the highest frequency of MLH1 promoter methylation was in MSI-H CRC with loss of MLH1 protein, the following in sporadic CRC without MLH1 protein expression, and the lowest in the sporadic CRC with MLH1 protein expression.
The frequency of MLH1 promoter methylation in BRAF mutated total CRC was 53.2%, significantly higher than in BRAF wild type total CRC 13.7% (P = 0.001). In contrast, the MLH1 promoter methylation frequency in KRAS mutated total CRC (14.0%) was significantly lower than in KRAS wild type total CRC (21.8%) (P<0.001). The largest population-based study [21] observed similar results, the frequencies of MLH1 promoter methylation were 46.8% (51/109) in BRAF mutated CRC and 17.4% (97/559) BRAF wild type CRC (P<0.001); whereas, they were 15.5% (40/258) in KRAS mutated CRC and 26.2% (112/428) KRAS wild type CRC (P = 0.001). In MSI CRC, similar results were also observed. The MLH1 promoter methylation frequency in BRAF mutated MSI-H CRC (94.5%) was significantly higher than in BRAF wild MSI-H CRC (28.2%) (P<0.001). Whereas, the MLH1 promoter methylation frequency in KRAS mutated MSI CRC (25.9%) was significantly lower than in KRAS wild MSI CRC (50.4%) (P = 0.003). The following may explain the results: colon tumors progress by distinct genes of the RAS/RAF/MAP kinase pathway, depending on the genetic/epigenetic event underlying MMR deficiency (mutation and loss induced by MLH1 promoter methylation). MSI-H tumors with MMR gene mutations (hereditary and sporadic forms) my preferentially target KRAS, whereas, MSI-H tumors with MLH1 promoter methylation may preferentially target the BRAF gene [73]. In addition, the methylation of MGMT was associated with KRAS mutant CRC but not of BRAF mutant CRC could also support the results of our meta-analysis [20].
Our study suggested that the frequency of MLH1 promoter methylation was higher in female, proximal tumor location, and poor differentiation. Study reported that MSI CRC had a very distinct clinicopathological phenotype, which was commonly mucinous, poorly differentiated, presenting at earlier Dukes’ stage, and in the proximal side of the colon [74]. MSI CRC was also commonly female and older at diagnosis. Moreover, in sporadic CRC, MSI was mainly caused by MLH1 promoter methylation. Therefore, MSI CRC and MLH1 promoter methylation CRC may have similar clinicopathological phenotype. However, the underlying mechanisms need to be investigated.
Heterogeneity persisted in our meta-analysis. The followings may explain the sources of heterogeneity. Firstly, MLH1 promoter methylation was tested in different promoter regions. One study tested in “A” region; three studies tested in “C” region; other 15 studies did not supply specific regions tested. Additionally, various ages of the study subjects may also explain the heterogeneity. Genes of individual are progressively methylated with aging due to chromosomal instability [75]. However, only three of 19 studies provided the information of age of study subjects [16], [54], [55].
Although this meta-analysis provides some robust results, limitations also existed like all the meta-analysis. Firstly, dietary factors, smoking and drinking alcohol may affect the MLH1 promoter methylation. Lack of these original data of the studies reviewed limited our further evaluation of their effect on MLH1 promoter methylation [76], [77], [78]. Secondly, lacking of the original data limited our further evaluation of the interactions between the clinicopathological and molecular variables in CRC. Thirdly, the prevalence of MLH1 promoter methylation in CRC may increase with aging. However, the majority of studies did not provide this information, which limited our further evaluate their effect on MLH1 promoter methylation.
In summary, this systematic review and meta-analysis yield some conclusions: the MLH1 methylation in total CRC was 20.3%; they were 18.7% in sporadic CRC and 16.4% in LS CRC, respectively; MLH1 promoter methylation may be significantly associated with gender, tumor location, tumor differentiation, MSI, MLH1 protein expression, and BRAF mutation.
Supporting Information
Forest figure for association of MLHI promoter methylation and MSI status in CRC tumors (MSI vs. MSS).
(DOC)
Funnel plot of the log (event rate) versus its standard error, for the frequency of MLH1 promoter methylation in CRC tumors: (A) MSI and (B) MSI-H.
(DOC)
Funnel plot of the log odds ratio versus its standard error, for the frequency of MLH1 promoter methylation in CRC tumors: Proximal vs. Distal.
(DOC)
Pooled frequency of MLH1 promoter methylation in colorectal cancer patients with other subgroup analysis.
(DOC)
PRISMA Checklist.
(DOC)
Review protocol.
(DOC)
Funding Statement
This study was supported by grants from National Natural Science Foundation of China for Priority Areas (NSFC 30972538), Education Bureau of Heilongjiang Province (11531100), and the Graduate Foundation, supported by the Scientific Research of Heilongjiang Province (YJSCX2009-224HLJ) and Harbin Medical University (HCXB2009009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.GLOBOCAN 2008 website. Cancer Fact Sheet. International Agency for Research on Cancer: World Health Organization. Available: http://globocan.iarc.fr/factsheets/cancers/colorectal.asp.
- 2. Lengauer C, Kinzler KW, Vogelstein B (1997) Genetic instability in colorectal cancers. Nature 386: 623–627. [DOI] [PubMed] [Google Scholar]
- 3. Thibodeau SN, Bren G, Schaid D (1993) Microsatellite instability in cancer of the proximal colon. Science 260: 816–819. [DOI] [PubMed] [Google Scholar]
- 4. Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M (1993) Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363: 558–561. [DOI] [PubMed] [Google Scholar]
- 5. Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, et al. (1993) Clues to the pathogenesis of familial colorectal cancer. Science 260: 812–816. [DOI] [PubMed] [Google Scholar]
- 6. Tannergard P, Liu T, Weger A, Nordenskjold M, Lindblom A (1997) Tumorigenesis in colorectal tumors from patients with hereditary non-polyposis colorectal cancer. Hum Genet 101: 51–55. [DOI] [PubMed] [Google Scholar]
- 7. Jass JR, Walsh MD, Barker M, Simms LA, Young J, et al. (2002) Distinction between familial and sporadic forms of colorectal cancer showing DNA microsatellite instability. Eur J Cancer 38: 858–866. [DOI] [PubMed] [Google Scholar]
- 8. Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, et al. (1997) Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 57: 808–811. [PubMed] [Google Scholar]
- 9. Bird A (1992) The essentials of DNA methylation. Cell 70: 5–8. [DOI] [PubMed] [Google Scholar]
- 10. Kondo Y, Issa JP (2004) Epigenetic changes in colorectal cancer. Cancer Metastasis Rev 23: 29–39. [DOI] [PubMed] [Google Scholar]
- 11. Issa JP (2004) CpG island methylator phenotype in cancer. Nat Rev Cancer 4: 988–993. [DOI] [PubMed] [Google Scholar]
- 12. Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, et al. (1998) Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A 95: 6870–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, et al. (1998) Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 58: 3455–3460. [PubMed] [Google Scholar]
- 14. Belshaw NJ, Elliott GO, Williams EA, Bradburn DM, Mills SJ, et al. (2004) Use of DNA from human stools to detect aberrant CpG island methylation of genes implicated in colorectal cancer. Cancer Epidemiol Biomarkers Prev 13: 1495–1501. [PubMed] [Google Scholar]
- 15. Kumar K, Brim H, Giardiello F, Smoot DT, Nouraie M, et al. (2009) Distinct BRAF (V600E) and KRAS mutations in high microsatellite instability sporadic colorectal cancer in African Americans. Clin Cancer Res 15: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menigatti M, Di Gregorio C, Borghi F, Sala E, Scarselli A, et al. (2001) Methylation pattern of different regions of the MLH1 promoter and silencing of gene expression in hereditary and sporadic colorectal cancer. Genes Chromosomes Cancer 31: 357–361. [DOI] [PubMed] [Google Scholar]
- 17. Park IJ, Kim HC, Yoon YS, Yu CS, Jang SJ, et al. (2007) Clinicopathological characteristics of colorectal cancer with family history: an evaluation of family history as a predictive factor for microsatellite instability. J Korean Med Sci 22 Suppl: S91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peyssonnaux C, Eychene A (2001) The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell 93: 53–62. [DOI] [PubMed] [Google Scholar]
- 19. Bettstetter M, Dechant S, Ruemmele P, Grabowski M, Keller G, et al. (2007) Distinction of hereditary nonpolyposis colorectal cancer and sporadic microsatellite-unstable colorectal cancer through quantification of MLH1 methylation by real-time PCR. Clin Cancer Res 13: 3221–3228. [DOI] [PubMed] [Google Scholar]
- 20. Nagasaka T, Sasamoto H, Notohara K, Cullings HM, Takeda M, et al. (2004) Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. J Clin Oncol 22: 4584–4594. [DOI] [PubMed] [Google Scholar]
- 21. de Vogel S, Weijenberg MP, Herman JG, Wouters KA, de Goeij AF, et al. (2009) MGMT and MLH1 promoter methylation versus APC, KRAS and BRAF gene mutations in colorectal cancer: indications for distinct pathways and sequence of events. Ann Oncol 20: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 22. Iacopetta B, Grieu F, Li W, Ruszkiewicz A, Caruso M, et al. (2006) APC gene methylation is inversely correlated with features of the CpG island methylator phenotype in colorectal cancer. Int J Cancer 119: 2272–2278. [DOI] [PubMed] [Google Scholar]
- 23. Deng G, Bell I, Crawley S, Gum J, Terdiman JP, et al. (2004) BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res 10: 191–195. [DOI] [PubMed] [Google Scholar]
- 24. Ahlquist T, Lind GE, Costa VL, Meling GI, Vatn M, et al. (2008) Gene methylation profiles of normal mucosa, and benign and malignant colorectal tumors identify early onset markers. Mol Cancer 7: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakagawa H, Nuovo GJ, Zervos EE, Martin EW Jr, Salovaara R, et al. (2001) Age-related hypermethylation of the 5′ region of MLH1 in normal colonic mucosa is associated with microsatellite-unstable colorectal cancer development. Cancer Res 61: 6991–6995. [PubMed] [Google Scholar]
- 26. Menigatti M, Truninger K, Gebbers JO, Marbet U, Marra G, et al. (2009) Normal colorectal mucosa exhibits sex- and segment-specific susceptibility to DNA methylation at the hMLH1 and MGMT promoters. Oncogene 28: 899–909. [DOI] [PubMed] [Google Scholar]
- 27. Wallner M, Herbst A, Behrens A, Crispin A, Stieber P, et al. (2006) Methylation of serum DNA is an independent prognostic marker in colorectal cancer. Clin Cancer Res 12: 7347–7352. [DOI] [PubMed] [Google Scholar]
- 28. Leung WK, To KF, Man EP, Chan MW, Bai AH, et al. (2005) Quantitative detection of promoter hypermethylation in multiple genes in the serum of patients with colorectal cancer. Am J Gastroenterol 100: 2274–2279. [DOI] [PubMed] [Google Scholar]
- 29. Zhou HH, Yan SY, Zhou XY, Du X, Zhang TM, et al. (2008) MLH1 promoter germline-methylation in selected probands of Chinese hereditary non-polyposis colorectal cancer families. World J Gastroenterol 14: 7329–7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyakura Y, Sugano K, Akasu T, Yoshida T, Maekawa M, et al. (2004) Extensive but hemiallelic methylation of the hMLH1 promoter region in early-onset sporadic colon cancers with microsatellite instability. Clin Gastroenterol Hepatol 2: 147–156. [DOI] [PubMed] [Google Scholar]
- 31. Gazzoli I, Loda M, Garber J, Syngal S, Kolodner RD (2002) A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the MLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res 62: 3925–3928. [PubMed] [Google Scholar]
- 32. Lawes DA, Pearson T, Sengupta S, Boulos PB (2005) The role of MLH1, MSH2 and MSH6 in the development of multiple colorectal cancers. Br J Cancer 93: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dykes SL, Qui H, Rothenberger DA, Garcia-Aguilar J (2003) Evidence of a preferred molecular pathway in patients with synchronous colorectal cancer. Cancer 98: 48–54. [DOI] [PubMed] [Google Scholar]
- 34. Velayos FS, Lee SH, Qiu H, Dykes S, Yiu R, et al. (2005) The mechanism of microsatellite instability is different in synchronous and metachronous colorectal cancer. J Gastrointest Surg 9: 329–335. [DOI] [PubMed] [Google Scholar]
- 35. Vasen HF, Mecklin JP, Khan PM, Lynch HT (1991) The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 34: 424–425. [DOI] [PubMed] [Google Scholar]
- 36. Vasen HF, Watson P, Mecklin JP, Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116: 1453–1456. [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, et al. (1997) A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 89: 1758–1762. [DOI] [PubMed] [Google Scholar]
- 38. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, et al. (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, et al. (2005) Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352: 1851–1860. [DOI] [PubMed] [Google Scholar]
- 40. Ward RL, Turner J, Williams R, Pekarsky B, Packham D, et al. (2005) Routine testing for mismatch repair deficiency in sporadic colorectal cancer is justified. J Pathol 207: 377–384. [DOI] [PubMed] [Google Scholar]
- 41. Sobin LH, Fleming ID (1997) TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80: 1803–1804. [DOI] [PubMed] [Google Scholar]
- 42. Deng G, Chen A, Hong J, Chae HS, Kim YS (1999) Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res 59: 2029–2033. [PubMed] [Google Scholar]
- 43. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, et al. (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58: 5248–5257. [PubMed] [Google Scholar]
- 44. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 45. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DerSimonian R (1996) Meta-analysis in the design and monitoring of clinical trials. Stat Med 15: 1237–1248; discussion 1249–1252. [DOI] [PubMed]
- 47. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 48. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duval S, Tweedie R (2000) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 50. Yamamoto H, Min Y, Itoh F, Imsumran A, Horiuchi S, et al. (2002) Differential involvement of the hypermethylator phenotype in hereditary and sporadic colorectal cancers with high-frequency microsatellite instability. Genes Chromosomes Cancer 33: 322–325. [DOI] [PubMed] [Google Scholar]
- 51.Julie C, Tresallet C, Brouquet A, Vallot C, Zimmermann U, et al.. (2008) Identification in daily practice of patients with Lynch syndrome (hereditary nonpolyposis colorectal cancer): revised Bethesda guidelines-based approach versus molecular screening. Am J Gastroenterol 103: 2825–2835; quiz 2836. [DOI] [PubMed]
- 52. Potocnik U, Glavac D, Golouh R, Ravnik-Glavac M (2001) Causes of microsatellite instability in colorectal tumors: implications for hereditary non-polyposis colorectal cancer screening. Cancer Genet Cytogenet 126: 85–96. [DOI] [PubMed] [Google Scholar]
- 53. Poynter JN, Siegmund KD, Weisenberger DJ, Long TI, Thibodeau SN, et al. (2008) Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev 17: 3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lind GE, Thorstensen L, Lovig T, Meling GI, Hamelin R, et al. (2004) A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol Cancer 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shi X, Li J, Zhao C, Lv S, Xu G (2006) Methylation analysis of hMLH1 gene promoter by a bisulfite-sensitive single-strand conformation polymorphism-capillary electrophoresis method. Biomed Chromatogr 20: 815–820. [DOI] [PubMed] [Google Scholar]
- 56. Gay LJ, Arends MJ, Mitrou PN, Bowman R, Ibrahim AE, et al. (2011) MLH1 promoter methylation, diet, and lifestyle factors in mismatch repair deficient colorectal cancer patients from EPIC-Norfolk. Nutr Cancer 63: 1000–1010. [DOI] [PubMed] [Google Scholar]
- 57. Grady WM, Rajput A, Lutterbaugh JD, Markowitz SD (2001) Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res 61: 900–902. [PubMed] [Google Scholar]
- 58. Brandes JC, van Engeland M, Wouters KA, Weijenberg MP, Herman JG (2005) CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis 26: 1152–1156. [DOI] [PubMed] [Google Scholar]
- 59. Anacleto C, Leopoldino AM, Rossi B, Soares FA, Lopes A, et al. (2005) Colorectal cancer “methylator phenotype”: fact or artifact? Neoplasia 7: 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, et al. (2005) Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology 129: 837–845. [DOI] [PubMed] [Google Scholar]
- 61. Shannon BA, Iacopetta BJ (2001) Methylation of the hMLH1, p16, and MDR1 genes in colorectal carcinoma: associations with clinicopathological features. Cancer Lett 167: 91–97. [DOI] [PubMed] [Google Scholar]
- 62. Kuismanen SA, Holmberg MT, Salovaara R, Schweizer P, Aaltonen LA, et al. (1999) Epigenetic phenotypes distinguish microsatellite-stable and -unstable colorectal cancers. Proc Natl Acad Sci U S A 96: 12661–12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yiu R, Qiu H, Lee SH, Garcia-Aguilar J (2005) Mechanisms of microsatellite instability in colorectal cancer patients in different age groups. Dis Colon Rectum 48: 2061–2069. [DOI] [PubMed] [Google Scholar]
- 64. Huang Q, Huang JF, Zhang B, Baum L, Fu WL (2012) Methylation variable position profiles of hMLH1 promoter CpG islands in human sporadic colorectal carcinoma. Diagn Mol Pathol 21: 24–33. [DOI] [PubMed] [Google Scholar]
- 65. Fox EJ, Leahy DT, Geraghty R, Mulcahy HE, Fennelly D, et al. (2006) Mutually exclusive promoter hypermethylation patterns of hMLH1 and O6-methylguanine DNA methyltransferase in colorectal cancer. J Mol Diagn 8: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vilkin A, Niv Y, Nagasaka T, Morgenstern S, Levi Z, et al. (2009) Microsatellite instability, MLH1 promoter methylation, and BRAF mutation analysis in sporadic colorectal cancers of different ethnic groups in Israel. Cancer 115: 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee KH, Lee JS, Nam JH, Choi C, Lee MC, et al. (2011) Promoter methylation status of hMLH1, hMSH2, and MGMT genes in colorectal cancer associated with adenoma-carcinoma sequence. Langenbecks Arch Surg 396: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 68. Arnold CN, Goel A, Compton C, Marcus V, Niedzwiecki D, et al. (2004) Evaluation of microsatellite instability, hMLH1 expression and hMLH1 promoter hypermethylation in defining the MSI phenotype of colorectal cancer. Cancer Biol Ther 3: 73–78. [DOI] [PubMed] [Google Scholar]
- 69. Strazzullo M, Cossu A, Baldinu P, Colombino M, Satta MP, et al. (2003) High-resolution methylation analysis of the hMLH1 promoter in sporadic endometrial and colorectal carcinomas. Cancer 98: 1540–1546. [DOI] [PubMed] [Google Scholar]
- 70.Mirchev M, Kotzev I, Kahl P, Büttner R, Angelova L, et al.. (2007) Epigenetic silencing of MLH1 and p16INK and their relation to certain clinicopathological features in patients with colorectal cancer. Journal of IMAB - Annual Proceeding (Scientific Papers): 95–96.
- 71. Raedle J, Trojan J, Brieger A, Weber N, Schafer D, et al. (2001) Bethesda guidelines: relation to microsatellite instability and MLH1 promoter methylation in patients with colorectal cancer. Ann Intern Med 135: 566–576. [DOI] [PubMed] [Google Scholar]
- 72. Peltomaki P, Vasen HF (1997) Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology 113: 1146–1158. [DOI] [PubMed] [Google Scholar]
- 73. Oliveira C, Westra JL, Arango D, Ollikainen M, Domingo E, et al. (2004) Distinct patterns of KRAS mutations in colorectal carcinomas according to germline mismatch repair defects and hMLH1 methylation status. Hum Mol Genet 13: 2303–2311. [DOI] [PubMed] [Google Scholar]
- 74. Ward R, Meagher A, Tomlinson I, O’Connor T, Norrie M, et al. (2001) Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 48: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Issa JP (1999) Aging, DNA methylation and cancer. Crit Rev Oncol Hematol 32: 31–43. [DOI] [PubMed] [Google Scholar]
- 76. Aguilera O, Fernandez AF, Munoz A, Fraga MF (2010) Epigenetics and environment: a complex relationship. J Appl Physiol 109: 243–251. [DOI] [PubMed] [Google Scholar]
- 77. Mathers JC, Strathdee G, Relton CL Induction of epigenetic alterations by dietary and other environmental factors. Adv Genet 71: 3–39. [DOI] [PubMed] [Google Scholar]
- 78.Garagnani P, Pirazzini C, Franceschi C (2012) Colorectal Cancer Microenvironment: among Nutrition, Gut Microbiota, Inflammation and Epigenetics. Curr Pharm Des. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest figure for association of MLHI promoter methylation and MSI status in CRC tumors (MSI vs. MSS).
(DOC)
Funnel plot of the log (event rate) versus its standard error, for the frequency of MLH1 promoter methylation in CRC tumors: (A) MSI and (B) MSI-H.
(DOC)
Funnel plot of the log odds ratio versus its standard error, for the frequency of MLH1 promoter methylation in CRC tumors: Proximal vs. Distal.
(DOC)
Pooled frequency of MLH1 promoter methylation in colorectal cancer patients with other subgroup analysis.
(DOC)
PRISMA Checklist.
(DOC)
Review protocol.
(DOC)