Abstract
Our previous study has found that the abundance of peritumoral CD68+ macrophages was associated with poor prognosis in hepatocellular carcinoma (HCC) after resection. However, CD68 staining could not discriminate the protumoral or tumoricidal subpopulations from pan-macrophages. CD163 is a marker of alternatively activated macrophages. In this study, the clinical significance of CD163+ cells in tumors and peritumoral liver tissues was evaluated in a cohort of 295 patients with HCC after curative resection. We found that the density of CD163+ cells was well correlated with that of CD68+ cells in both tumors and peritumoral liver tissues but was much more. Immunostaining on consecutive sections and flow cytometry assay on surgical resected specimens further supported the findings that the CD163+ cells was more abundant than CD68+ cells. The density of peritumoral CD68+ cells was associated with poor recurrence-free survival (RFS) and poor overall survival (OS) (P = 0.004 and P = 0.001, respectively), whereas the CD163+ cells have no prognostic values either in tumors or in peritumoral liver tissues. In another cohort of 107 HCC patients, preoperative plasma concentration of soluble form of CD163 (sCD163) was associated with active hepatitis-related factors but not associated with the markers of tumor invasion. In conclusion, both the CD163+ cells local infiltration and plasma sCD163 were of limited significance in HCC, and they were more likely markers related to active hepatitis rather than tumor progression.
Background
Hepatocellular carcinoma (HCC), accounting for 70% to 85% of primary liver cancer, is one of the most prevalent cancers worldwide. It ranks as the second leading cause of cancer deaths for men and the sixth for women and half of these cases and deaths were estimated to occur in China. [1] Although hepatectomy is one of the best methods to provide long-term survival for patients with HCC, [2] a high recurrence rate after surgery is a major problem. Tumor microenvironment, especially macrophages, which play an important role in the initiation, progression, and metastasis of various tumors, has been intensively studied in recent years [3], [4].
Macrophages constitute a major component of the infiltrates in most solid tumors. In several solid tumors, for example, breast, prostate, endometrium, bladder, kidney, esophagus and lymphomas, the intratumoral infiltrated macrophages were associated with poor prognosis. [5]–[11] Our previous study also found that the abundance of CD68+ macrophages infiltrated in peritumoral liver tissue, but not in tumor, was associated with poor prognosis for the patients with HCC after curative liver resection. [12] Macrophages could be divided into M1 (or classically activated) and M2 (or alternatively activated) subpopulations according to their functional phenotypes [13]. It is now generally accepted that tumor-associated macrophages have an M2 phenotype with pro-tumoral effects. [14] However, CD68, the most widely used marker for macrophages, could not distinguish M1 or M2 subtypes from all the infiltrated macrophages (pan-macrophages). [15].
CD163, a member of the scavenger receptor cysteine-rich family, is a 130-kDa transmembrane protein. [16], [17] CD163 was reported to be expressed almost exclusively on circulating monocytes and macrophages. [18]–[20] CD163 was involved in anti-inflammatory functions and predominantly expressed on M2 macrophages. [21]–[24] Intratumoral CD163+ macrophages counts were reported to be associated with poor prognosis in various tumors, such as melanoma (in tumor stroma), [25] nongynecologic leiomyosarcoma, [26] and renal cell carcinoma [27]. Based on these reports, we hypothesized that CD163+ macrophages were a subpopulation of CD68+ pan-macrophages and may be more suitable to identify the tumor-promoting macrophages. Using the marker CD163, we could further discriminate the patients with different prognosis. In this study, the distribution and clinical significance of macrophages in tumor and peritumoral liver tissues were evaluated with the markers of CD163 and CD68, and the differences between these two markers were analyzed.
Furthermore, the soluble form of CD163 (sCD163) could be released from active macrophages and was found in serum when macrophages were activated. Overexpression of sCD163 could be a marker of a progressive inflammatory milieu. [28]–[30] Serum sCD163 was significantly elevated in patients with fulminant hepatic failure as compared with patients with hepatitis or healthy controls [31]; it was also reported to be a predictor of mortality for patients with acute liver failure [32]. Patients with HCC often complicated with hepatitis and/or liver cirrhosis. Therefore, the clinical relevance of CD163 expression in liver tissue and the sCD163 level in peripheral blood is of particular interest. In the current study, plasma sCD163 levels was also evaluated in another cohort of patients with HCC and their associations with the clinical features of tumor progression and active hepatitis were explored.
Patients and Methods
Patients, Specimens, and Follow-up
Surgical specimens from 295 consecutive patients who underwent curative liver resection in our institute (Liver Cancer Institute, Fudan University) with pathologically confirmed HCC were collected as cohort 1. Plasma samples from another 107 patients with HCC (cohort 2) were also collected before surgery and stored at –80°C until use. Tumor stage was determined according to the 2009 International Union Against Cancer TNM classification system (7th edition). [33] Tumor differentiation was graded by the Edmondson grading system. Hepatitis B history was defined as ever detectable serum hepatitis B surface antigen. Microvascular invasion was defined as the presence of tumor cells within a vascular lumen lined by endothelium under microscopy. [34].
Follow-up procedures for the patients after surgery were described in our previous reports. [35], [36] Briefly, patients were monitored by abdominal ultrasonography, serum α-fetoprotein (AFP), and chest radiography with an interval of 2 to 6 months according to the postoperative time. If recurrence was suspected, computed tomography scanning or magnetic resonance imaging was performed immediately. Treatment modalities after relapse were administered according to a uniform guideline. [36]–[38] Overall survival (OS) or recurrence-free survival (RFS) was defined as the interval between surgery and death or disease recurrence, respectively. If recurrence was not diagnosed, patients were censored on the date of death or the last follow-up. This study was approved by the Zhongshan Hospital Research Ethics Committee. Informed consent was obtained from each patient according to the committee’s regulation. Participants provided their written informed consent to participate in this study.
Evaluation of Immunohistochemistry and Enzyme-linked Immunosorbent Assay
Two cores were taken from representative formalin-fixed paraffin-embedded tumor tissue and liver tissue from the patients of cohort 1 to construct tissue microarray slides (in collaboration with Shanghai Biochip Company, Shanghai, China). Tumor samples were taken from the nonnecrotic peripheral zone of the tumor. Liver samples were taken from the peritumoral liver tissue within a distance of 1 cm away from the tumor margin. Duplicate cylinders from 2 different areas, a total of 4 punches from each patient, were obtained. Sections were constructed with tumors and matched peritumoral samples. The immunohistochemistry protocols and the evaluation of the immunostaining were described in our previous studies. [35] Primary antibodies were mouse anti-human antibodies combined with CD163 (1∶100 dilution; AbD Serotec, Oxford, UK) and CD68 (1∶100 dilution; Abcam, Cambridge, MA). Under high-power magnification (×200), photographs of 2 representative fields of each punch were captured by a computerized image system composed of a Leica CCD camera DFC 500 connected to a Leica DM IRE2 microscope and Leica Qwin Plus v3 software (Leica Microsystems Imaging Solution, Cambridge, UK). Identical settings were used for all photographs. The area of positive staining was measured in pixels by Image-Pro Plus v6.2 software (Media Cybernetics, Bethesda, MD) as described. [12] The densities of CD163 and CD68 staining were expressed as the ratio of positive staining area to the total area of each photograph and the cell densities were taken as surrogate measures for the counts of CD163+ and CD68+ cells.
Plasma sCD163 levels of the patients from cohort 2 were measured by an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) according to the user’s manual.
Cell Suspension Solution Preparation and Flow Cytometric (FCM)Analysis of CD68 and CD163
Single cell suspension were obtained from three paired HCC tissue and surrounding non-tumoral liver tissue from surgical resection samples. Specimens were minced with scissors and digested by incubation for 1 h at 37°C in high-glucose DMEM containing 0.1% collagenase IV (Sigma-Aldrich, St. Louis, MO). After being washed in medium plus 10% fetal bovine serum, the cell suspension was forced through a graded series of meshes to separate the cell components from stroma and aggregates. After that, cells were fixed by 4% formaldehyde for 10 min, and then bursting of cell membranes by using 0.05% of the tritonX-100 for 15 min, after that, cells were incubated for 30 min at 4°C with CD163and CD68 or with a control in PBS containing 0.5% (w/v) BSA. Cells were analyzed on a fluorescent activated cell sorter (BD Biosciences, San Jose, CA). We used primary murine monoclonal antibodies against human CD68 conjugated to FITC, and human CD163conjugated to allophycocyanin (APC). FITC-CD68 and APC-CD163 antibodies were purchased from Biolegend (San Diego, CA).
Statistical Analysis
Analysis was performed with SPSS13.0 for Windows (SPSS). The Pearson χ2 test or Fisher exact test was used to compare qualitative variables, and quantitative variables were analyzed by the Student t test or Spearman correlation test. Kaplan-Meier analysis was used to determine the survival. The log-rank test was used to compare the survival between subgroups, and the Cox regression model was used to perform multivariate analysis. P<0.05 was considered statically significant.
Results
Demographics
Table 1 lists the demographic data of patients from cohorts 1 and 2. At the time of the last follow-up of cohort 1, 138 patients had tumor recurrence and 123 patients had died, including 35 patients who died of liver failure without tumor recurrence. The 1-, 3- and 5-year OS rates were 88%, 64%, and 58%, respectively, and the 1-, 3- and 5-year recurrence rates were 25%, 43%, and 47%, respectively. Because the median follow-up time for patients in cohort 2 was less than 12 months, follow-up data were not analyzed for this cohort.
Table 1. Clinicopathologic features of patients from cohort 1 and cohort 2.
| Features | Values/counts | |
| Cohort 1 (n = 295) | Cohort 2 (n = 107) | |
| Age, median (range), y | 52 (22–80) | 54 (28–77) |
| Gender, male/female | 247/48 | 91/16 |
| α-Fetoprotein, median (range), ng/dL | 185 (0–60,500) | 61.75 (1–60,500) |
| Liver cirrhosis, yes/no | 227/68 | 90/17 |
| Hepatitis B history, yes/no | 230/65 | 91/16 |
| Hepatitis B e antigen, positive/negative | 111/184 | 31/76 |
| Tumor size, mean ± SD, cm | 5.58±3.93 | 5.41±3.43 |
| Encapsulation, complete/none | 146/149 | 52/55 |
| Tumor differentiation, I–II/III–IV | 202/93 | 75/31 |
| Microvascular invasion, yes/no | 119/176 | 47/60 |
| TNM stage, I/II/IIIA | 157/118/20 | 72/8/27 |
Abbreviations: y, year.
Immunohistochemical Findings in Tissue Microarray
CD68 and CD163 staining appeared mainly in the cytoplasm of stroma cells in tumor and peritumoral liver tissue. Most tumor cells and hepatic cells were negatively stained, although sporadic positive staining on these cells could also be observed (Figure 1). The average densities of CD163 and CD68 staining were 0.023±0.034 and 0.011±0.023 in tumor, respectively, and 0.037±0.040 and 0.020±0.020 in peritumoral liver tissue, respectively (Figure 2A). The densities of CD68+ and CD163+ cells in peritumoral liver tissue were significantly higher than those within tumor (P<0.001 for both).
Figure 1. Representative immunostaining of CD163 and CD68 on consecutive sections of paired tumor tissue and peritumoral liver tissue.
The densities of intratumoral CD68+ cells (A) and CD163+ cells (C) were lower in tumor than in corresponding peritumoral liver tissue (B and D). The density of CD163+ cells was higher than that of CD68+ cells in both tumor and in peritumoral liver tissue. Scale bar, 200 µm.
Figure 2. The dot plots of the densities of CD163+ and CD68+ cells in tumor and in peritumoral liver tissue from the 295 patients (cohort 1).
(A) The dot plots of ratios of CD163 and CD68-expressing cells to total cells in 3 paired tumors and peritumoral liver tissues which were counted by flow cytometry (B). Error bars, S.D.
The Clinical Relevance of the CD163+ and CD68+ Macrophages
The density of intratumoral CD163+ macrophages was positively correlated with patient’s age and serum alkaline phosphatase (ALP) concentration, whereas the density of intratumoral CD68+ macrophages was only correlated with the patient’s age. Both the peritumoral CD163+ and CD68+ macrophages were associated with the hepatitis-related features, such as serum aspartate aminotransferase (AST) and γ-glutamyl transpeptidase (γ-GT), and the tumor-related features, including serum α-fetoprotein (AFP), tumor size, and presence of microvascular invasion; the density of peritumoral CD68+ macrophages was also associated high TNM stage (Tables 2 and 3).
Table 2. Relationships between intratumoral/peritumoral CD163 expression and clinicopathologic features.
| Variables | Intratumoral CD163 expression | Peritumoral CD163 expression | ||
| Average level or correlationcoefficient* | P | Average level or correlationcoefficient* | P | |
| Age, y | 0.184 | 0.001 | 0.001 | 0.984 |
| Gender, male vs female | 0.025±0.036 vs. 0.017±0.024 | 0.155 | 0.037±0.040 vs. 0.033±0.040 | 0.499 |
| Hepatitis B history, yes vs. no | 0.023±0.032 vs. 0.025±0.040 | 0.665 | 0.037±0.043 vs. 0.037±0.027 | 0.963 |
| Hepatitis B e antigen, positive vs.negative | 0.023±0.035 vs. 0.023±0.034 | 0.933 | 0.040±0.036 vs.0.035±0.042 | 0.325 |
| ALT | −0.019 | 0.744 | 0.051 | 0.387 |
| AST | 0.047 | 0.429 | 0.136 | 0.021 |
| γ-GT | 0.048 | 0.409 | 0.139 | 0.017 |
| ALP | 0.125 | 0.033 | 0.103 | 0.079 |
| Liver cirrhosis, yes vs. no | 0.023±0.037 vs. 0.023±0.023 | 0.930 | 0.039±0.042 vs. 0.030±0.031 | 0.087 |
| α-Fetoprotein | 0.001 | 0.987 | 0.159 | 0.006 |
| Tumor size | 0.048 | 0.410 | 0.276 | <0.001 |
| Tumor encapsulation, complete vs. no | 0.024±0.033 vs. 0.022±0.035 | 0.692 | 0.033±0.032 vs. 0.040±0.046 | 0.165 |
| Microvascular invasion, yes vs. no | 0.022±0.028 vs. 0.024±0.037 | 0.615 | 0.043±0.047 vs. 0.033±0.034 | 0.037 |
| Tumor differentiation, I–II vs. III–IV | 0.023±0.034 vs. 0.023±0.033 | 0.973 | 0.036±0.039 vs. 0.038±0.042 | 0.642 |
| TNM stage, I vs. II/IIIA | 0.024±0.039 vs. 0.022±0.027 | 0.516 | 0.033±0.035 vs. 0.041±0.044 | 0.085 |
If the clinicopathologic variable is a quantitative one, Spearman correlation analysis was performed and the correlation coefficient was presented.
Abbreviations: y, year; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase.
Table 3. Relationships between intratumoral/peritumoral CD68 expression and clinicopathologic features.
| Variables | Intratumoral CD68 expression | Peritumoral CD68 expression | ||
| Average level or correlation coefficient* | P | Average level or correlation coefficient* | P | |
| Age | 0.148 | 0.011 | −0.029 | 0.623 |
| Gender, male vs. female | 0.012±0.025 vs. 0.008±0.010 | 0.297 | 0.020±0.018 vs. 0.021±0.028 | 0.733 |
| Hepatitis B history, yes vs. no | 0.012±0.024 vs. 0.010±0.023 | 0.590 | 0.020±0.021 vs. 0.021±0.017 | 0.503 |
| Hepatitis B e antigen, positive vs. negative | 0.011±0.026 vs. 0.012±0.022 | 0.822 | 0.021±0.024 vs. 0.019±0.018 | 0.449 |
| ALT | 0.013 | 0.825 | −0.013 | 0.823 |
| AST | 0.091 | 0.120 | 0.118 | 0.045 |
| γ-GT | 0.036 | 0.542 | 0.126 | 0.031 |
| ALP | 0.079 | 0.178 | 0.092 | 0.116 |
| Liver cirrhosis, yes vs. no | 0.012±0.026 vs. 0.008±0.010 | 0.225 | 0.021±0. 018 vs. 0.017±.0.025 | 0.123 |
| α-Fetoprotein | 0.062 | 0.285 | 0.183 | 0.002 |
| Tumor size | 0.039 | 0.505 | 0.389 | <0.001 |
| Tumor encapsulation,complete vs. no | 0.012±0.025 vs. 0.011±0.022 | 0.683 | 0.018±0.016 vs. 0.022±0.023 | 0.093 |
| Microvascular invasion, yes vs. no | 0.014±0.029 vs. 0.010±0.019 | 0.192 | 0.024±0.025 vs. 0.017±0.015 | 0.005 |
| Tumor differentiation, I–II vs.III–IV | 0.009±0.014 vs. 0.016±0.037 | 0.103 | 0.020±0.018 vs. 0.020±0.025 | 0.745 |
| TNM stage, I vs. II/IIIA | 0.010±0.020 vs. 0.013±0.027 | 0.309 | 0.017±0.015 vs. 0.024±0.024 | 0.004 |
If the clinicopathologic variable is a quantitative one, Spearman’s correlation analysis was performed and the correlation coefficient was presented.
Abbreviations: y, year; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase.
CD163+ Cells were More Abundant than CD68+ Cells
In cohort 1, the density of CD163+ cells was positively correlated with that of CD68+ cells in tumor tissue (r = 0.417, P<0.001) and peritumoral liver tissue (r = 0.565, P<0.001). However, the average density of CD163+ cells was 22.83-fold and 4.11-fold higher in tumor and peritumoral liver tissue, respectively, compared with that of CD68+ cells. Immunostaining on consecutive sections of tumor tissue and peritumoral liver tissue also showed that the density of CD163+ cells was much higher than that of the CD68+ cells both in tumor and in peritumoral liver tissue (Figure 1).
FCM analysis also found that the ratio of CD163-expressing cells to total cells were higher than CD68 from surgical HCC specimens and non-tumoral surrounding liver tissue (P = 0.011 and P = 0.033, respectively, Figure 2B).
Prognostic Significance of CD163 and CD68 Staining
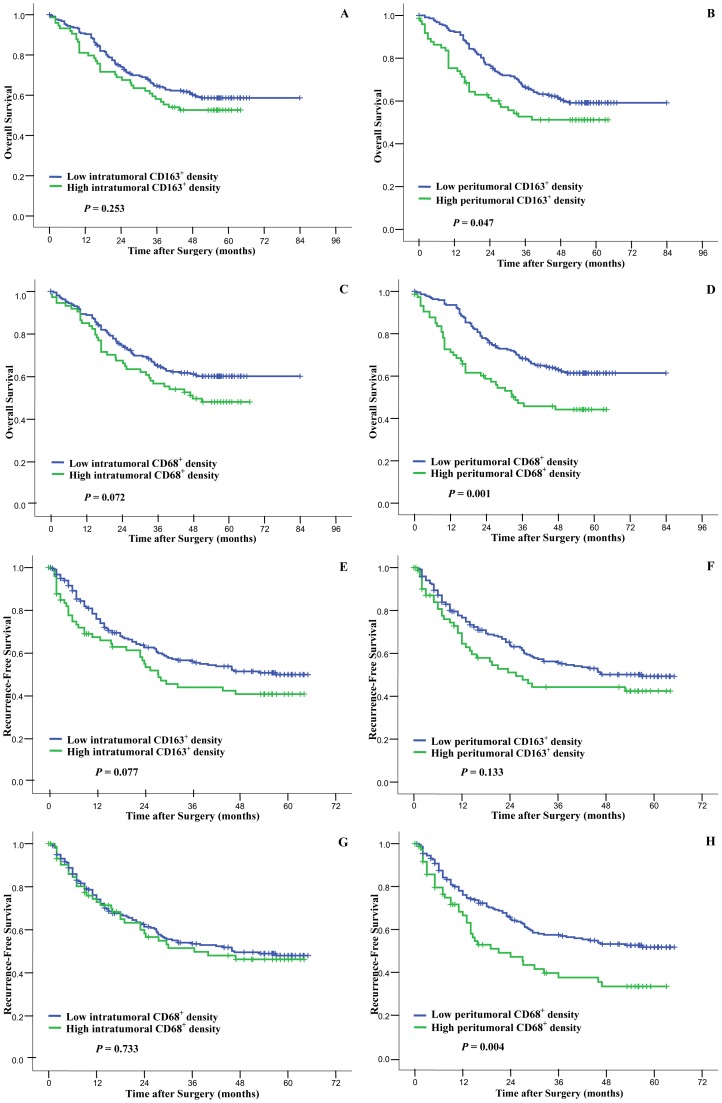
As shown in Table 4, the prognostic value of each clinicopathologic feature was analyzed in univariate and multivariate analysis. In the univariate analysis, serum AFP>200 ng/dL, serum γ-GT >50 U/L, tumor size >5 cm, presence of microvascular invasion, and advanced TNM stage were risk factors for both OS and RFS. Poor tumor differentiation, AST >75 U/L and ALP>140 U/L were also associated with poor OS. Using the 75th percentile value of the densities of CD163+ cells or CD68+ cells in cohort 1, we divided the patients into subgroups with a high or low macrophage density. Intratumoral CD163 expression was not associated with OS (P = 0.253, Figure 3A) or RFS (P = 0.077, Figure 3E). CD163+ macrophage infiltration in peritumoral liver tissue was associated with poor OS (P = 0.047; median OS for patients with high and low CD163+ macrophages infiltration were 56.4 months and 64.7 months, respectively) but not RFS (P = 0.133, Figure 3B and 3F). In consistence with our previous study, [12] patients with high CD68+ cells infiltration in peritumoral liver tissue had a poor prognosis for both OS and RFS (P = 0.001 and P = 0.004, respectively), and the intratumoral CD68+ cell density had no prognostic significance (Figure 3 and Table 4).
Table 4. Univariate and multivariate analyses of factors associated with survival and recurrence.
| Features | Overall survival | Recurrence-free survival | ||||
| P * | Multivariate | P * | Multivariate | |||
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age, ≤52 vs. >52 y | 0.932 | NA | 0.888 | NA | ||
| Gender, female vs. male | 0.265 | NA | 0.193 | NA | ||
| Hepatitis B history, yes vs. no | 0.395 | NA | 0.827 | NA | ||
| Liver cirrhosis, yes vs. no | 0.169 | NA | 0.163 | NA | ||
| α-Fetoprotein, >200 vs. ≤200 ng/dL | 0.033 | 1.078 (0.733–1.586) | 0.703 | 0.046 | 1.073 (0.752–1.531) | 0.696 |
| ALT, >75 vs. ≤75 U/L | 0.408 | NA | 0.380 | NA | ||
| AST, >75 vs. ≤75 U/L | <0.001 | 1.860 (1.105–3.130) | 0.019 | 0.186 | NA | |
| γ-GT, >50 vs. ≤50 U/L | <0.001 | 1.636 (1.090–2.456) | 0.018 | 0.001 | 1.396 (0.973–2.004) | 0.070 |
| ALP, >140 vs. ≤140 U/L | <0.001 | 1.635 (0.994–2.690) | 0.053 | 0.911 | NA | |
| Tumor size, >5 vs. ≤5 cm | <0.001 | 1.906 (1.270–2.861) | 0.002 | <0.001 | 1.523 (1.052–2.256) | 0.026 |
| Tumor differentiation, III–IV vs. I–II | 0.008 | 1.552 (1.075–2.241) | 0.019 | 0.072 | NA | |
| Tumor encapsulation, complete vs. none | 0.624 | NA | 0.448 | NA | ||
| Microvascular invasion, yes vs. no | <0.001 | 1.045 (0.559–1.954) | 0.889 | <0.001 | 1.022 (0.560–1.867) | 0.943 |
| TNM stage, I vs. II vs IIIA | <0.001 | 1.918 (1.437–2.561) | <0.001 | <0.001 | 1.858 (1.411–2.4446) | <0.001 |
| Peritumoral CD68, high vs. low | 0.001 | 1.518 (1.024–2.251) | 0.038 | 0.004 | 1.602 (1.087–2.362) | 0.017 |
| Intratumoral CD68, high vs. low | 0.072 | NA | 0.733 | NA | ||
| Peritumoral CD163, high vs. low | 0.047 | 0.800 (0.512–1.249) | 0.326 | 0.133 | NA | |
| Intratumoral CD163, high vs. low | 0.253 | NA | 0.077 | NA | ||
univariate analysis.
Abbreviations: y, year; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; NA, not adopted.
Figure 3. Cumulative overall survival (OS) and recurrence-free survival (RFS) curves of patients with high and low densities of CD163+ (panels A, B, E, F) or CD68+ (panels C, D, G, H) cells infiltrated in tumor and peritumoral liver tissue.
Intratumoral densities of CD68+ cells and CD163+ cells could not discriminate patient with different OS and RFS (panels A, C, E, G). In peritumoral liver tissue, the densities of CD163+ and CD68+ cells were both associated with poor OS, and the density of CD68+ cells was also associated with RFS (panels B, D, F, H).
The above factors with a P<0.05 in the univariate analysis were further analyzed in multivariate Cox proportional hazard analysis. Peritumoral CD68+ cell density was an independent risk factor for OS and RFS (P = 0.038 and P = 0.017, respectively); whereas the CD163+ cell density was not (Table 4).
Plasma sCD163 was a Marker of Hepatitis More than a Marker of Tumor Progression
The average level of preoperative plasma sCD163 was 74.43±30.01 ng/mL in the patients from cohort 2. High level of plasma sCD163 (using the 75th percentile level as the cutoff value) was significantly associated with hepatitis-related factors, including serum alanine aminotransferase (ALT), AST, γ-GT, and ALP, but not tumor-related factors, such as tumor size, microvessel invasion, and TNM stage (Table 5).
Table 5. Relationships between plasma sCD163 and clinicopathologic features.
| Variables | Average level or correlation coefficient* | P |
| Age, y | 0.229 | 0.018 |
| Gender, male/female | 73.49±30.87 vs. 79.78±24.74 | 0.442 |
| Hepatitis B history, yes/no | 79.10±16.57 vs. 73.61±31.79 | 0.502 |
| Hepatitis B e antigen, positive/negative | 73.99±28.43 vs. 74.61±30.82 | 0.923 |
| ALT | 0.327 | 0.001 |
| AST | 0.491 | <0.001 |
| γ-GT | 0.421 | <0.001 |
| ALP | 0.421 | <0.001 |
| Liver cirrhosis, yes/no | 76.79±31.21vs. 61.97±18.79 | 0.062 |
| α-Fetoprotein | 0.181 | 0.062 |
| Tumor size | 0.192 | 0.048 |
| Tumor encapsulation, complete/no | 71.91±31.04 vs. 76.82±29.09 | 0.401 |
| Microvascular invasion, yes/no | 78.95±31.24 vs. 70.89±28.78 | 0.174 |
| Tumor differentiation, I–II/III–IV | 71.74±30.63 vs. 79.84±27.94 | 0.192 |
| TNM stage, I/II–IIIA | 71.62±29.34 vs. 80.47±30.99 | 0.167 |
If the clinicopathologic variable is a quantitative one, Spearman’s correlation analysis was performed and the correlation coefficient was presented.
Abbreviations: y, year; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase.
Discussion
The results from our previous studies and others have indicated that the abundance of peritumoral infiltrated CD68+ macrophages was associated with a poor prognosis for patients with HCC who underwent curative liver resection. [12], [39] In the present study, we had planned to further discriminate patients with different prognosis using CD163, a relatively specific marker for the M2 macrophages but found that the peritumoral CD163+ cells was only associated with a poor OS in Kaplan-Meier survival analysis and its predictive value was not better than that of CD68. Therefore, CD163 is not a good biomarker to discriminate patients’ prognosis when used in immunohistochemistry studies. In other words, the CD163+ macrophages infiltration may play a limited role in the progression of HCC.
In glioma, the proportions of CD163+ macrophages among CD68+ macrophages, which would reflect the proportion of macrophages polarized to the M2 phenotype, were reported to be associated with histologic grade. [40] In the present study, the CD163/CD68 ratio showed the limited clinical significance. Furthermore, CD163 did not seem to be a marker of a subpopulation of macrophages if CD68 could be deemed as a surface of pan-macrophages because the CD163+ cell density was much higher than CD68+ cell density in tumor and peritumoral liver tissue. Immunostaining of consecutive sections and FCM analysis also showed that the number of CD163+ cells was much higher than that of CD68+ cells both in tumor and in peritumoral liver tissues. Therefore, we can conclude that CD163 could not be used as a marker of subpopulation of CD68+ cells either in liver cancer or in liver tissue.
Both plasma sCD163 and peritumoral infiltrated CD163+ cells could be the indicators of chronic hepatitis. Previous studies reported that sCD163 was elevated in acute liver failure and was a predictor of mortality for the patients with acute liver failure. [31], [32] In accordance with these reports, we found that plasma sCD163 was associated with inflammatory markers of hepatitis, such as serum ALT, γ-GT and ALP. The densities of peritumoral infiltrated CD163+ cells were also positively correlated with these markers. Plasma sCD163 level and peritumoral CD163 expression was not statistically significant difference between the patients with and without cirrhosis, which is consistent with another report showing that the sCD163 level was higher in patients with acute liver failure than in those with stable liver cirrhosis or healthy controls [32].
Tumor cells were also reported to express CD163. Shabo et al reported that cancer cells expressing CD163 were associated with poor prognosis in patients with breast cancer [41] and rectal cancer, [42] and the CD163+ cancer cells were deemed as the fusion of cancer cells and macrophages and could be more radiation resistant or more aggressive. We also studied the expression of CD163 on tumor cells, but found few CD163-expressing tumor cells in HCC.
Taken together, the present results showed that the CD163+ cell density is of limited significance to predict the prognosis of the HCC patients. Plasma sCD163 and peritumoral CD163+ cell infiltration was more likely a maker of active hepatitis rather than a marker of tumor progression. And the marker CD163 itself could not be used as an indicator for the determination of the subpopulation of M2-macrophages in liver and in liver cancer tissue.
Acknowledgments
We thank Mr. Wei-De Zhang for assistance in collecting patients’ data.
Funding Statement
This study was jointly supported by National Natural Science Foundation of China (30872504, 81020108025), and China National “211” Project for Higher Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362: 1907–1917. [DOI] [PubMed] [Google Scholar]
- 3. Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66: 605–612. [DOI] [PubMed] [Google Scholar]
- 4. Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124: 263–266. [DOI] [PubMed] [Google Scholar]
- 5. Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, et al. (2005) Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 106: 2169–2174. [DOI] [PubMed] [Google Scholar]
- 6. Hamada I, Kato M, Yamasaki T, Iwabuchi K, Watanabe T, et al. (2002) Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer Res 22: 4281–4284. [PubMed] [Google Scholar]
- 7. Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, et al. (2000) Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 7: 263–269. [DOI] [PubMed] [Google Scholar]
- 8. Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S (2004) Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol 99: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 9. Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, et al. (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17: 445–451. [DOI] [PubMed] [Google Scholar]
- 10. Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, et al. (2004) Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res 24: 3335–3342. [PubMed] [Google Scholar]
- 11. Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, et al. (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14: 425–431. [PubMed] [Google Scholar]
- 12. Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, et al. (2008) High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 26: 2707–2716. [DOI] [PubMed] [Google Scholar]
- 13. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555. [DOI] [PubMed] [Google Scholar]
- 14. Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 15. Falini B, Flenghi L, Pileri S, Gambacorta M, Bigerna B, et al. (1993) PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol 142: 1359–1372. [PMC free article] [PubMed] [Google Scholar]
- 16. Hogger P, Dreier J, Droste A, Buck F, Sorg C (1998) Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163). J Immunol 161: 1883–1890. [PubMed] [Google Scholar]
- 17. Law SK, Micklem KJ, Shaw JM, Zhang XP, Dong Y, et al. (1993) A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol 23: 2320–2325. [DOI] [PubMed] [Google Scholar]
- 18. Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, et al. (2001) Identification of the haemoglobin scavenger receptor. Nature 409: 198–201. [DOI] [PubMed] [Google Scholar]
- 19. Lau SK, Chu PG, Weiss LM (2004) CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol 122: 794–801. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen TT, Schwartz EJ, West RB, Warnke RA, Arber DA, et al. (2005) Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol 29: 617–624. [DOI] [PubMed] [Google Scholar]
- 21. Ritter M, Buechler C, Langmann T, Orso E, Klucken J, et al. (1999) The scavenger receptor CD163: regulation, promoter structure and genomic organization. Pathobiology 67: 257–261. [DOI] [PubMed] [Google Scholar]
- 22. Buechler C, Ritter M, Orso E, Langmann T, Klucken J, et al. (2000) Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol 67: 97–103. [PubMed] [Google Scholar]
- 23. Moestrup SK, Moller HJ (2004) CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med 36: 347–354. [DOI] [PubMed] [Google Scholar]
- 24. Komohara Y, Hirahara J, Horikawa T, Kawamura K, Kiyota E, et al. (2006) AM-3K, an anti-macrophage antibody, recognizes CD163, a molecule associated with an anti-inflammatory macrophage phenotype. J Histochem Cytochem 54: 763–771. [DOI] [PubMed] [Google Scholar]
- 25. Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, et al. (2009) Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol 27: 3330–3337. [DOI] [PubMed] [Google Scholar]
- 26. Lee CH, Espinosa I, Vrijaldenhoven S, Subramanian S, Montgomery KD, et al. (2008) Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin Cancer Res 14: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 27. Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, et al. (2011) Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci 102: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 28. Matsushita N, Kashiwagi M, Wait R, Nagayoshi R, Nakamura M, et al. (2002) Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin Exp Immunol 130: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez C, Domenech N, Vazquez J, Alonso F, Ezquerra A, et al. (1999) The porcine 2A10 antigen is homologous to human CD163 and related to macrophage differentiation. J Immunol 162: 5230–5237. [PubMed] [Google Scholar]
- 30. Sulahian TH, Hintz KA, Wardwell K, Guyre PM (2001) Development of an ELISA to measure soluble CD163 in biological fluids. J Immunol Methods 252: 25–31. [DOI] [PubMed] [Google Scholar]
- 31. Hiraoka A, Horiike N, Akbar SM, Michitaka K, Matsuyama T, et al. (2005) Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol 40: 52–56. [DOI] [PubMed] [Google Scholar]
- 32. Moller HJ, Gronbaek H, Schiodt FV, Holland-Fischer P, Schilsky M, et al. (2007) Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol 47: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(2009) TNM Classification of Malignant Tumours; Sobin LH, Gospodarowicz MK, Wittekind C, editors. Oxford: Wiley-Blackwell. [Google Scholar]
- 34. Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, et al. (2009) A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137: 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qian YB, Zhang JB, Wu WZ, Fang HB, Jia WD, et al. (2006) P48 is a predictive marker for outcome of postoperative interferon-alpha treatment in patients with hepatitis B virus infection-related hepatocellular carcinoma. Cancer 107: 1562–1569. [DOI] [PubMed] [Google Scholar]
- 36. Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH, et al. (2007) Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol 47: 684–690. [DOI] [PubMed] [Google Scholar]
- 37. Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, et al. (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 38. Sun HC, Zhuang PY, Qin LX, Ye QH, Wang L, et al. (2007) Incidence and prognostic values of lymph node metastasis in operable hepatocellular carcinoma and evaluation of routine complete lymphadenectomy. J Surg Oncol 96: 37–45. [DOI] [PubMed] [Google Scholar]
- 39. Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, et al. (2009) Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 206: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Komohara Y, Ohnishi K, Kuratsu J, Takeya M (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 216: 15–24. [DOI] [PubMed] [Google Scholar]
- 41. Shabo I, Stal O, Olsson H, Dore S, Svanvik J (2008) Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer 123: 780–786. [DOI] [PubMed] [Google Scholar]
- 42. Shabo I, Olsson H, Sun XF, Svanvik J (2009) Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int J Cancer 125: 1826–1831. [DOI] [PubMed] [Google Scholar]