Abstract
We report the isolation and characterization of a cDNA clone encoding HSP47, a transformation-sensitive heat shock protein that binds to collagen. A cDNA library was prepared from total RNA isolated from heat-shocked chicken embryo fibroblasts and screened by using oligonucleotide mixtures prepared on the basis of the N-terminal amino acid sequence of biochemically purified HSP47. The cDNA insert contained 3,278 bp, which encoded a 15-amino-acid signal peptide and a mature protein coding region consisting of 390 amino acid residues; it also included part of the 5' noncoding region and a long 3' noncoding region. The deduced amino acid sequence revealed an RDEL sequence at the C terminus, which is a variant of the KDEL retention signal for retention of proteins in the endoplasmic reticulum. Northern (RNA) blot analyses and nuclear run-on assays established that the induction of HSP47 by heat shock and its suppression after transformation of chicken embryo fibroblasts by Rous sarcoma virus are regulated at the transcriptional level. A homology search revealed that this protein belongs to the serpin family, the superfamily of plasma serine protease inhibitors. Although structurally homologous to the serpins, HSP47 lacks the active site thought to be essential for the inhibition of proteases and does not appear to bind to intracellular proteases. HSP47 is the first heat shock protein found to be a member of the serpin superfamily. Conversely, it is the first serpin family member that is not secreted from cells, which could be explained by acquisition of the RDEL retention signal during evolution.
Full text
PDF



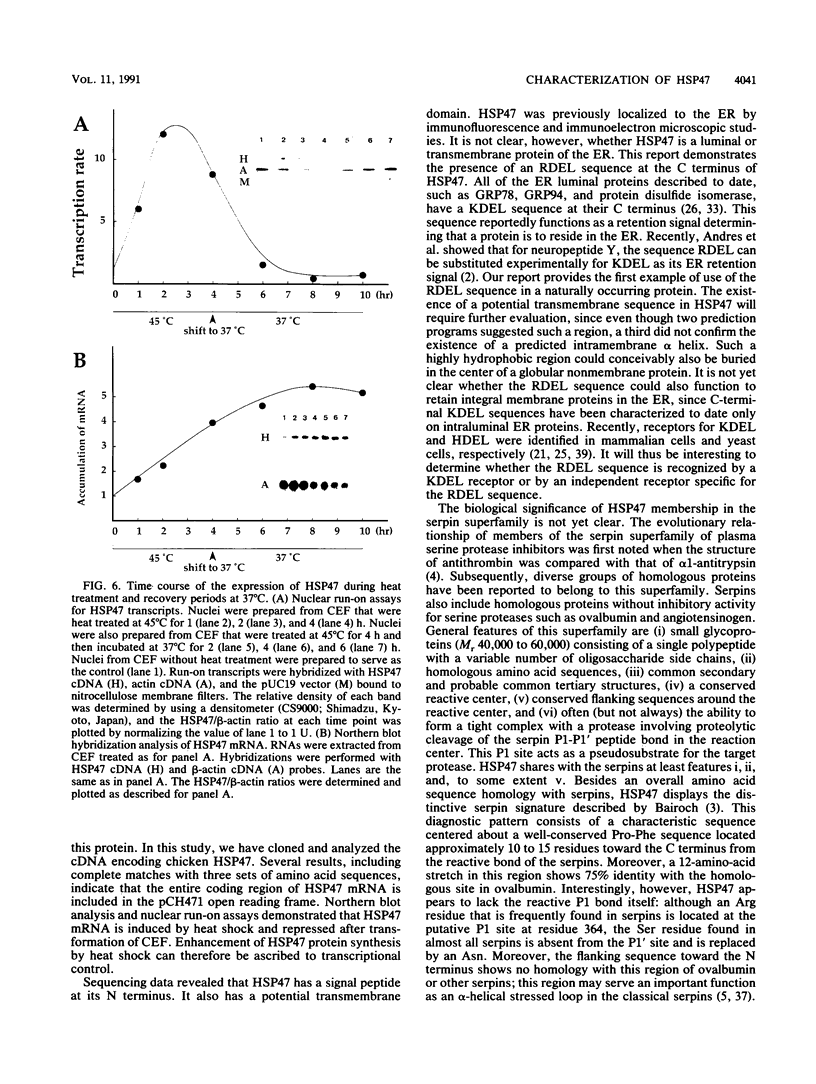



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres D. A., Dickerson I. M., Dixon J. E. Variants of the carboxyl-terminal KDEL sequence direct intracellular retention. J Biol Chem. 1990 Apr 15;265(11):5952–5955. [PubMed] [Google Scholar]
- Carrell R., Owen M., Brennan S., Vaughan L. Carboxy terminal fragment of human alpha-1-antitrypsin from hydroxylamine cleavage: homology with antithrombin III. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1032–1037. doi: 10.1016/0006-291x(79)91983-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Dente L., Cortese R. Cell-specific expression of a transfected human alpha 1-antitrypsin gene. Cell. 1985 Jun;41(2):531–540. doi: 10.1016/s0092-8674(85)80026-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flink I. L., Bailey T. J., Gustafson T. A., Markham B. E., Morkin E. Complete amino acid sequence of human thyroxine-binding globulin deduced from cloned DNA: close homology to the serine antiproteases. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7708–7712. doi: 10.1073/pnas.83.20.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hammond G. L., Smith C. L., Goping I. S., Underhill D. A., Harley M. J., Reventos J., Musto N. A., Gunsalus G. L., Bardin C. W. Primary structure of human corticosteroid binding globulin, deduced from hepatic and pulmonary cDNAs, exhibits homology with serine protease inhibitors. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5153–5157. doi: 10.1073/pnas.84.15.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Taylor A., Sage H., Hogan B. L. Distinct patterns of glycosylation of colligin, a collagen-binding glycoprotein, and SPARC (osteonectin), a secreted Ca2+-binding glycoprotein. Evidence for the localisation of colligin in the endoplasmic reticulum. Eur J Biochem. 1987 Feb 16;163(1):57–65. doi: 10.1111/j.1432-1033.1987.tb10736.x. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Pelham H. R. A human homologue of the yeast HDEL receptor. Nature. 1990 Nov 8;348(6297):162–163. doi: 10.1038/348162a0. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Sweet D. J., Pelham H. R. The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell. 1990 Jun 29;61(7):1359–1363. doi: 10.1016/0092-8674(90)90699-f. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
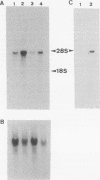
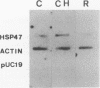
- Nagata K., Hirayoshi K., Obara M., Saga S., Yamada K. M. Biosynthesis of a novel transformation-sensitive heat-shock protein that binds to collagen. Regulation by mRNA levels and in vitro synthesis of a functional precursor. J Biol Chem. 1988 Jun 15;263(17):8344–8349. [PubMed] [Google Scholar]
- Nagata K., Saga S., Yamada K. M. A major collagen-binding protein of chick embryo fibroblasts is a novel heat shock protein. J Cell Biol. 1986 Jul;103(1):223–229. doi: 10.1083/jcb.103.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Saga S., Yamada K. M. Characterization of a novel transformation-sensitive heat-shock protein (HSP47) that binds to collagen. Biochem Biophys Res Commun. 1988 May 31;153(1):428–434. doi: 10.1016/s0006-291x(88)81242-7. [DOI] [PubMed] [Google Scholar]
- Nagata K., Yamada K. M. Phosphorylation and transformation sensitivity of a major collagen-binding protein of fibroblasts. J Biol Chem. 1986 Jun 5;261(16):7531–7536. [PubMed] [Google Scholar]
- Nakai A., Hirayoshi K., Nagata K. Transformation of BALB/3T3 cells by simian virus 40 causes a decreased synthesis of a collagen-binding heat-shock protein (hsp47). J Biol Chem. 1990 Jan 15;265(2):992–999. [PubMed] [Google Scholar]
- Nakai A., Hirayoshi K., Saga S., Yamada K. M., Nagata K. The transformation-sensitive heat shock protein (hsp47) binds specifically to Fetuin. Biochem Biophys Res Commun. 1989 Oct 16;164(1):259–264. doi: 10.1016/0006-291x(89)91711-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Saga S., Nagata K., Chen W. T., Yamada K. M. pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J Cell Biol. 1987 Jul;105(1):517–527. doi: 10.1083/jcb.105.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza J. C., Hardwick K. G., Dean N., Pelham H. R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990 Jun 29;61(7):1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Stein P. E., Leslie A. G., Finch J. T., Turnell W. G., McLaughlin P. J., Carrell R. W. Crystal structure of ovalbumin as a model for the reactive centre of serpins. Nature. 1990 Sep 6;347(6288):99–102. doi: 10.1038/347099a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Deyashiki Y., Nishioka J., Kurachi K., Akira M., Yamamoto S., Hashimoto S. Characterization of a cDNA for human protein C inhibitor. A new member of the plasma serine protease inhibitor superfamily. J Biol Chem. 1987 Jan 15;262(2):611–616. [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. A retinoic acid-inducible mRNA from F9 teratocarcinoma cells encodes a novel protease inhibitor homologue. J Biol Chem. 1990 Sep 15;265(26):15818–15822. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]