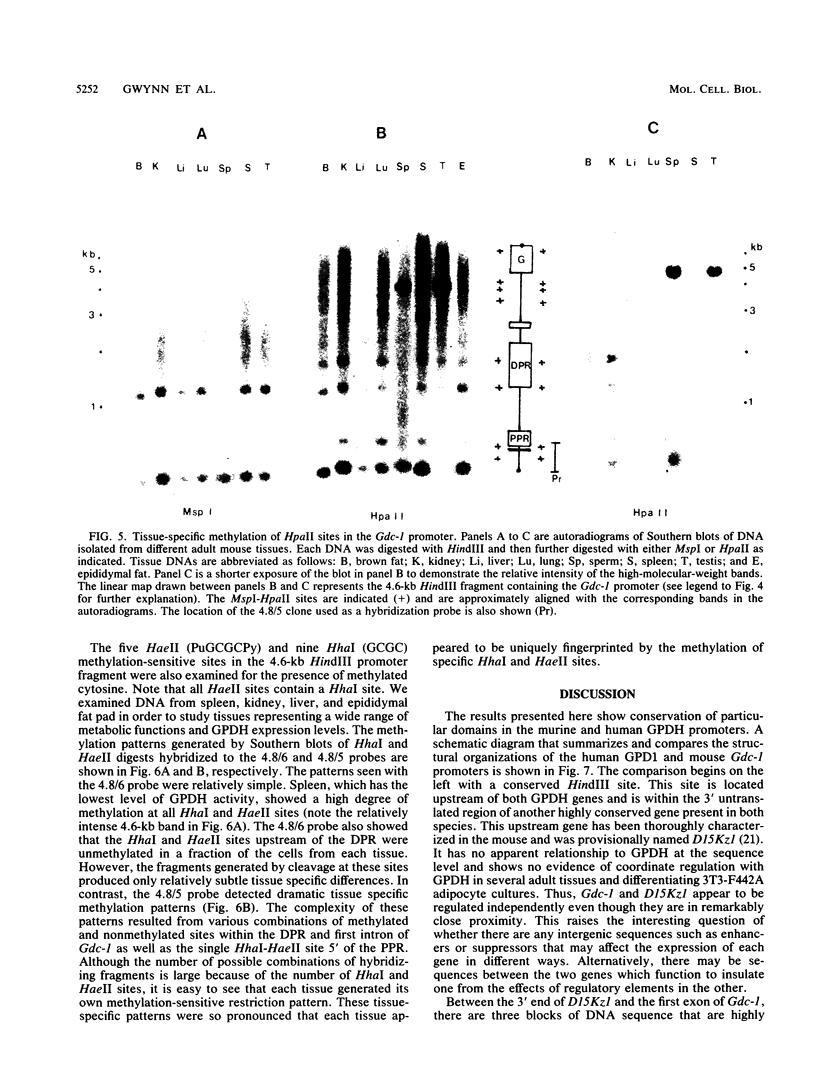
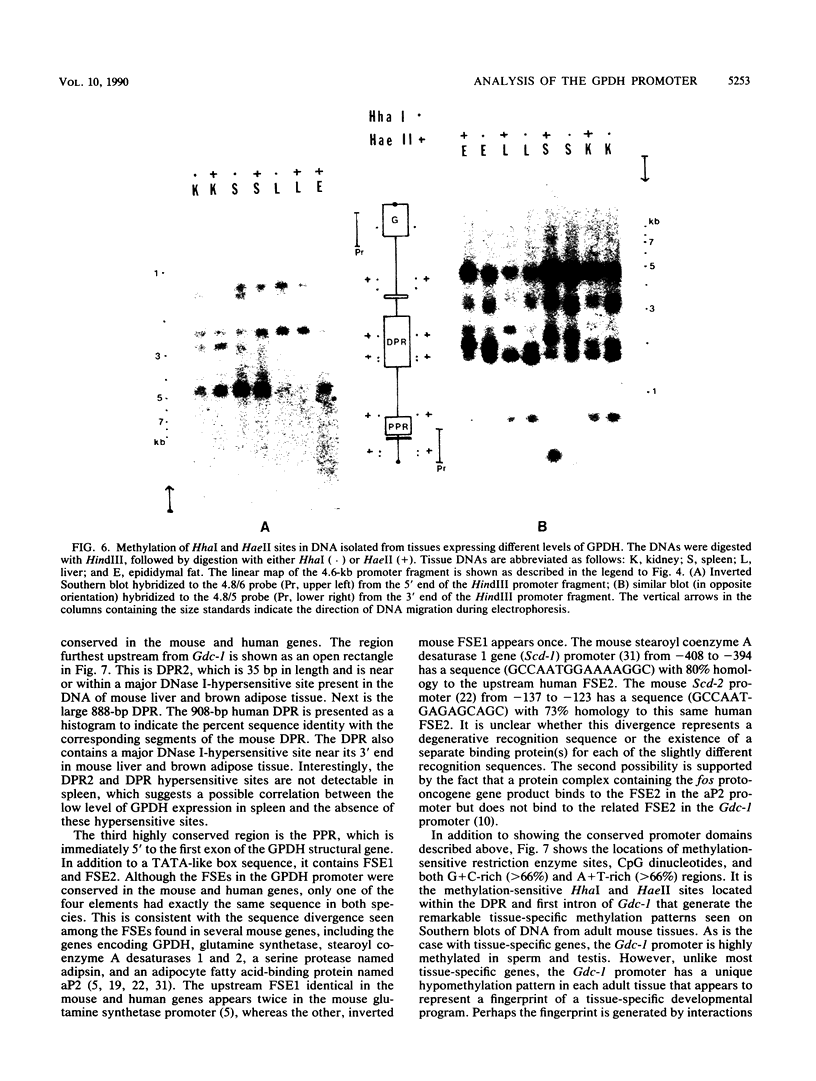
Abstract
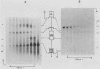
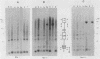
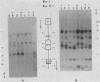
Cloned segments of the mouse glycerol-3-phosphate dehydrogenase (GPDH) gene, Gdc-1, were used to screen a human library. Human clones obtained spanned 25 kilobases of genomic DNA containing the human GPDH gene, GPD1. The 4 kb of sequence obtained from the 5'-flanking region and first exon of GPD1 was compared with the corresponding mouse sequence. Both sequences share a HindIII site located in what has proven to be the highly conserved 3' untranslated region of an upstream gene of unknown function, D15Kzl. The 3.6-kilobase segment of mouse DNA located between D15Kzl and Gdc-1 was provisionally termed the GPDH promoter. Alignment of the mouse promoter with the corresponding human sequence revealed two conserved domains. An upstream distal promoter region is approximately 900 base pairs in length. A downstream or proximal promoter region consists of approximately 300 base pairs immediately upstream of a TATA-like box and contains the fat-specific elements 1 and 2. Analysis of the chromatin structure of the Gdc-1 promoter revealed four DNase I-hypersensitive sites. They were present in DNA of liver and brown fat, in which GPDH expression is high, but were absent in DNA of spleen, in which GPDH expression is low. Methylation studies of the promoter showed it to be heavily methylated in sperm. However, the DNA from each adult somatic tissue had a unique distribution of nonmethylated sites and could easily be identified by its methylation pattern. These data suggest a structural model of the promoter that explains how Gdc-1 expression is differentially regulated in many types of cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arapinis C., Elion J., Labie D., Krishnamoorthy R. Differences in DNase I sensitivity and methylation within the human beta-globin gene domain and correlation with expression. Eur J Biochem. 1986 Apr 1;156(1):123–129. doi: 10.1111/j.1432-1033.1986.tb09556.x. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Bennett A., Friedman J. M., Darnell J. E., Jr DNase I-hypersensitive sites in the 5'-flanking region of the rat serum albumin gene: correlation between chromatin structure and transcriptional activity. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6504–6508. doi: 10.1073/pnas.83.17.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Benvenisty N., Reshef L. Developmental acquisition of DNase I sensitivity of the phosphoenolpyruvate carboxykinase (GTP) gene in rat liver. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1132–1136. doi: 10.1073/pnas.84.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari B., Beckwith K. D., Miller R. E. Cloning, nucleotide sequence, and potential regulatory elements of the glutamine synthetase gene from murine 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5789–5793. doi: 10.1073/pnas.85.16.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H. DNA methylation and gene activity. Cell. 1988 Apr 8;53(1):3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- Cook J. R., Kozak L. P. Sn-glycerol-3-phosphate dehydrogenase gene expression during mouse adipocyte development in vivo. Dev Biol. 1982 Aug;92(2):440–448. doi: 10.1016/0012-1606(82)90189-0. [DOI] [PubMed] [Google Scholar]
- Cook J. S., Lucas J. J., Sibley E., Bolanowski M. A., Christy R. J., Kelly T. J., Lane M. D. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A. 1988 May;85(9):2949–2953. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Hunt C. R., Spiegelman B. M. Developmentally regulated mRNAs in 3T3-adipocytes: analysis of transcriptional control. J Cell Biol. 1985 Feb;100(2):514–520. doi: 10.1083/jcb.100.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Djian P., Phillips M., Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985 Sep;124(3):554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- Dobson D. E., Groves D. L., Spiegelman B. M. Nucleotide sequence and hormonal regulation of mouse glycerophosphate dehydrogenase mRNA during adipocyte and muscle cell differentiation. J Biol Chem. 1987 Feb 5;262(4):1804–1809. [PubMed] [Google Scholar]
- Fisher M., Mullen R. J. Neuronal influence on glial enzyme expression: evidence from chimeric mouse cerebellum. Neuron. 1988 Apr;1(2):151–157. doi: 10.1016/0896-6273(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Fisher M. Neuronal influence on glial enzyme expression: evidence from mutant mouse cerebella. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4414–4418. doi: 10.1073/pnas.81.14.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Grainger R. M., Hazard-Leonards R. M., Samaha F., Hougan L. M., Lesk M. R., Thomsen G. H. Is hypomethylation linked to activation of delta-crystallin genes during lens development? Nature. 1983 Nov 3;306(5938):88–91. doi: 10.1038/306088a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Heuckeroth R. O., Birkenmeier E. H., Levin M. S., Gordon J. I. Analysis of the tissue-specific expression, developmental regulation, and linkage relationships of a rodent gene encoding heart fatty acid binding protein. J Biol Chem. 1987 Jul 15;262(20):9709–9717. [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. C., Kotarski M. A., Johnston L. A., Stadler U., Birkenmeier E., Kozak L. P. Primary structure of the mouse glycerol-3-phosphate dehydrogenase gene. J Biol Chem. 1986 Sep 5;261(25):11779–11785. [PubMed] [Google Scholar]
- Johnston L. A., Kotarski M. A., Jerry D. J., Kozak L. P. An ubiquitously expressed gene 3.5 kilobases upstream of the glycerol-3-phosphate dehydrogenase gene in mice. Mol Cell Biol. 1989 Mar;9(3):935–945. doi: 10.1128/mcb.9.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Ntambi J. M., Kelly T. J., Jr, Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1989 Sep 5;264(25):14755–14761. [PubMed] [Google Scholar]
- Kaye J. S., Pratt-Kaye S., Bellard M., Dretzen G., Bellard F., Chambon P. Steroid hormone dependence of four DNase I-hypersensitive regions located within the 7000-bp 5'-flanking segment of the ovalbumin gene. EMBO J. 1986 Feb;5(2):277–285. doi: 10.1002/j.1460-2075.1986.tb04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Jackson P. D., Felsenfeld G. Protein-binding sites within the 5' DNase I-hypersensitive region of the chicken alpha D-globin gene. Mol Cell Biol. 1987 Jun;7(6):2059–2069. doi: 10.1128/mcb.7.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Povey S., Hopkinson D. A. Regulation of expression of liver-specific enzymes. III. Further analysis of a series of rat hepatoma X human somatic cell hybrids. Ann Hum Genet. 1982 Oct;46(Pt 4):307–327. doi: 10.1111/j.1469-1809.1982.tb01582.x. [DOI] [PubMed] [Google Scholar]
- Kozak L. P. Interacting genes control glycerol-3-phosphate dehydrogenase expression in developing cerebellum of the mouse. Genetics. 1985 May;110(1):123–143. doi: 10.1093/genetics/110.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak L. P., Jensen J. T. Genetic and developmental control of multiple forms of L-glycerol 3-phosphate dehydrogenase. J Biol Chem. 1974 Dec 25;249(24):7775–7781. [PubMed] [Google Scholar]
- Kunnath L., Locker J. DNaseI sensitivity of the rat albumin and alpha-fetoprotein genes. Nucleic Acids Res. 1985 Jan 11;13(1):115–129. doi: 10.1093/nar/13.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi J. M., Buhrow S. A., Kaestner K. H., Christy R. J., Sibley E., Kelly T. J., Jr, Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1988 Nov 25;263(33):17291–17300. [PubMed] [Google Scholar]
- Phillips M., Djian P., Green H. The nucleotide sequence of three genes participating in the adipose differentiation of 3T3 cells. J Biol Chem. 1986 Aug 15;261(23):10821–10827. [PubMed] [Google Scholar]
- Ratner P. L., Fisher M., Burkart D., Cook J. R., Kozak L. P. The role of mRNA levels and cellular localization in controlling sn-glycerol-3-phosphate dehydrogenase expression in tissues of the mouse. J Biol Chem. 1981 Apr 10;256(7):3576–3579. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Rifkind R. A., Marks P. A. Murine erythroleukemia cell differentiation: DNase I hypersensitivity and DNA methylation near the globin genes. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1180–1184. doi: 10.1073/pnas.79.4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Sciaky-Gallili N., Razin A., Cedar H. Pattern of methylation of two genes coding for housekeeping functions. Proc Natl Acad Sci U S A. 1983 May;80(9):2422–2426. doi: 10.1073/pnas.80.9.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Klisak I. J., Zollman S., Sparkes R. S., Mohandas T., Lusis A. J., Gordon J. I. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J Biol Chem. 1987 Nov 25;262(33):16060–16071. [PubMed] [Google Scholar]
- Tratner I., Nahon J. L., Sala-Trepat J. M., Venetianer A. Albumin and alpha-fetoprotein gene transcription in rat hepatoma cell lines is correlated with specific DNA hypomethylation and altered chromatin structure in the 5' region. Mol Cell Biol. 1987 May;7(5):1856–1864. doi: 10.1128/mcb.7.5.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Yang V. W., Christy R. J., Cook J. S., Kelly T. J., Lane M. D. Mechanism of regulation of the 422(aP2) gene by cAMP during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1989 May;86(10):3629–3633. doi: 10.1073/pnas.86.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]